In Search of New Therapeutics—Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond
Abstract
1. Introduction
- Difficulties in inducing ovulation in some patients, despite stimulation by pharmacological agents (letrozole, clomiphene citrate, or gonadotropins).
- Unsatisfactory reduction of insulin resistance and hyperinsulinemia, in spite of implementing lifestyle changes and undergoing metformin therapy.
- Frequent unsatisfactory results of attempts to counteract hirsutism, acne and androgenic alopecia by application of, e.g., oral Contraceptives—thus, the sensitivity of androgen receptors within the skin to testosterone and dihydrotestosterone (DHT) should be lowered.
2. Leading Factors in PCOS Pathogenesis
2.1. Developmental Programming of PCOS
2.2. Genetics in PCOS
2.3. Hyperandrogenemia
2.4. Insulin Resistance and Hyperinsulinemia
2.5. Environmental Factors
3. Potential Drug Targets and Active Substances in Novel Therapies
3.1. Attenuation of Developmental Programming of PCOS
3.2. Approaches to Alleviate Hyperandrogenism
3.3. Sensitization to Insulin/Reduction of Hyperinsulinemia
3.4. Handling Other PCOS-Related Health Complications
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKR | aldo-keto reductase |
| AMH | anti-müllerian hormone |
| AMP | adenosine monophosphate |
| AMPK | adenosine monophosphate-activated protein kinase |
| AQP | aquaporin |
| BAT | brown adipose tissue |
| CYP | cytochrome P450 |
| DCI | D-chiro-inositol |
| DHT | dihydrotestosterone |
| FSH | follicle stimulating hormone |
| GABA | -aminobutyric acid |
| GLUT | glucose transporter (protein) |
| GnRH | gonadotropin-releasing hormone |
| hCG | human chorionic gonadotropin |
| HNF-4 | hepatocyte nuclear factor 4 alpha |
| HSD | hydroxysteroid dehydrogenase |
| IGF-1 | insulin-like growth factor-1 |
| IPG | inositol phosphoglycan |
| LH | luteinizing hormone |
| MI | myo-inositol |
| PCOS | polycystic ovary syndrome |
| SHBG | sex hormone-binding globulin |
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Cardoso, R.C.; Padmanabhan, V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol. Cell. Endocrinol. 2016, 435, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Makrinou, E.; Drong, A.; Christopoulos, G.; Lerner, A.; Chapa-Chorda, I.; Karaderi, T.; Lavery, S.; Hardy, K.; Lindgren, C.; Franks, S. Genome-wide methylation profiling in granulosa lutein cells of women with polycystic ovary syndrome (PCOS). Mol. Cell. Endocrinol. 2020, 500, 110611. [Google Scholar] [CrossRef] [PubMed]
- Prapas, N.; Karkanaki, A.; Prapas, I.; Kalogiannidis, I.; Katsikis, I.; Panidis, D. Genetics of polycystic ovary syndrome. Hippokratia 2009, 13, 216. [Google Scholar] [PubMed]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. Appl. Clin. Genet. 2019, 12, 249. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.; Cappelli, V.; Massaro, M.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Merhi, Z. Relationship between advanced glycation end products and steroidogenesis in PCOS. Reprod. Biol. Endocrinol. 2016, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Prati, A.; Despini, G.; Marini, G.; Ricchieri, F. PCOS from Lifestyle to the Use of Inositol and Insulin Sensitizers. In Frontiers in Gynecological Endocrinology; Springer International Publishing: Cham, Switzerland, 2014; pp. 59–67. [Google Scholar]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Kim, C.H.; Chon, S.J.; Lee, S.H. Effects of lifestyle modification in polycystic ovary syndrome compared to metformin only or metformin addition: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Franik, S.; Eltrop, S.M.; Kremer, J.A.; Kiesel, L.; Farquhar, C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Tanbo, T.; Mellembakken, J.; Bjercke, S.; Ring, E.; Åbyholm, T.; Fedorcsak, P. Ovulation induction in polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2018, 97, 1162–1167. [Google Scholar]
- Hu, S.; Yu, Q.; Wang, Y.; Wang, M.; Xia, W.; Zhu, C. Letrozole versus clomiphene citrate in polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2018, 297, 1081–1088. [Google Scholar] [PubMed]
- Coutinho, E.A.; Kauffman, A.S. The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med. Sci. 2019, 7, 84. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.; Middleton, L.; Jimenez, M.; Desai, R.; McMahon, A.; Allan, C.; Handelsman, D.; Walters, K. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014, 155, 3146–3159. [Google Scholar] [CrossRef] [PubMed]
- Kosova, G.; Urbanek, M. Genetics of the polycystic ovary syndrome. Mol. Cell. Endocrinol. 2013, 373, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gharani, N.; Waterworth, D.M.; Batty, S.; White, D.; Gilling-Smith, C.; Conway, G.S.; McCarthy, M.; Franks, S.; Williamson, R. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum. Mol. Genet. 1997, 6, 397–402. [Google Scholar]
- Diamanti-Kandarakis, E.; Bartzis, M.I.; Bergiele, A.T.; Tsianateli, T.C.; Kouli, C.R. Microsatellite polymorphism (tttta) n at- 528 base pairs of gene CYP11α influences hyperandrogenemia in patients with polycystic ovary syndrome. Fertil. Steril. 2000, 73, 735–741. [Google Scholar] [CrossRef]
- Chua, A.K.; Azziz, R.; Goodarzi, M.O. Association study of CYP17 and HSD11B1 in polycystic ovary syndrome utilizing comprehensive gene coverage. MHR Basic Sci. Reprod. Med. 2012, 18, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Barnes, R.B.; Jose, F.C.; Lucky, A.W. Dysregulation of cytochrome P450c17α as the cause of polycystic ovarian syndrome. Fertil. Steril. 1990, 53, 785–791. [Google Scholar] [CrossRef]
- Wickenheisser, J.K.; Quinn, P.G.; Nelson, V.L.; Legro, R.S.; Strauss, J.F., III; McAllister, J.M. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J. Clin. Endocrinol. Metab. 2000, 85, 2304–2311. [Google Scholar] [PubMed]
- Mehdizadeh, A.; Kalantar, S.M.; Sheikhha, M.H.; Aali, B.S.; Ghanei, A. Association of SNP rs. 2414096 CYP19 gene with polycystic ovarian syndrome in Iranian women. Int. J. Reprod. Biomed. 2017, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, S.; Tan, Y.; Xia, D.; Xia, Y.; Cao, Y.; Wang, W.; Wu, X.; Wang, H.; Yi, L.; et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 11. [Google Scholar] [CrossRef]
- Witchel, S.F.; Lee, P.A.; Suda-Hartman, M.; Hoffman, E.P. Hyperandrogenism and manifesting heterozygotes for 21-hydroxylase deficiency. Biochem. Mol. Med. 1997, 62, 151–158. [Google Scholar] [CrossRef]
- Witchel, S.F.; Kahsar-Miller, M.; Aston, C.E.; White, C.; Azziz, R. Prevalence of CYP21 mutations and IRS1 variant among women with polycystic ovary syndrome and adrenal androgen excess. Fertil. Steril. 2005, 83, 371–375. [Google Scholar] [CrossRef]
- Liu, N.; Ma, Y.; Wang, S.; Zhang, X.; Zhang, Q.; Zhang, X.; Fu, L.; Qiao, J. Association of the genetic variants of luteinizing hormone, luteinizing hormone receptor and polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2012, 10, 36. [Google Scholar] [CrossRef]
- Thathapudi, S.; Kodati, V.; Erukkambattu, J.; Addepally, U.; Qurratulain, H. Association of luteinizing hormone chorionic gonadotropin receptor gene polymorphism (rs2293275) with polycystic ovarian syndrome. Genet. Test. Mol. Biomarkers 2015, 19, 128–132. [Google Scholar] [CrossRef]
- Yan, J.; Tian, Y.; Gao, X.; Cui, L.; Ning, Y.; Cao, Y.; Chen, Y.; Peng, F.; You, L.; Liu, F.; et al. A genome-wide association study identifies FSHR rs2300441 associated with follicle-stimulating hormone levels. Clin. Genet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baban, A.S.S.; Korsheed, S.H.; Al Hayawi, A.Y. The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn AL Haitham J. Pure Appl. Sci. 2018, 257–272. [Google Scholar] [CrossRef]
- Garg, D.; Tal, R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod. Biomed. Online 2016, 33, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.X.; Li, Y.; Hu, R.; Wang, F.M.; Zhang, X.M.; Guan, B. Anti-Müllerian hormone gene polymorphism is associated with androgen levels in Chinese polycystic ovary syndrome patients with insulin resistance. J. Assist. Reprod. Genet. 2016, 33, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, M.; Woodroffe, A.; Ewens, K.; Diamanti-Kandarakis, E.; Legro, R.; Strauss, J., III; Dunaif, A.; Spielman, R. Candidate gene region for polycystic ovary syndrome on chromosome 19p13. 2. J. Clin. Endocrinol. Metab. 2005, 90, 6623–6629. [Google Scholar] [CrossRef]
- Gonzalez, A.; Abril, E.; Roca, A.; Aragón, M.J.; Figueroa, M.J.; Velarde, P.; Royo, J.L.; Real, L.M.; Ruiz, A. CAPN10 alleles are associated with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Dilek, S.; Ertunc, D.; Tok, E.C.; Erdal, E.M.; Aktas, A. Association of Gly972Arg variant of insulin receptor substrate-1 with metabolic features in women with polycystic ovary syndrome. Fertil. Steril. 2005, 84, 407–412. [Google Scholar] [CrossRef]
- Deswal, R.; Yadav, A.; Dang, A.S. Sex hormone binding globulin-an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst. Biol. Reprod. Med. 2018, 64, 12–24. [Google Scholar] [CrossRef]
- Deepika, M.; Reddy, K.R.; Yashwanth, A.; Rani, V.U.; Latha, K.P.; Jahan, P. TNF-α haplotype association with polycystic ovary syndrome—A South Indian study. J. Assist. Reprod. Genet. 2013, 30, 1493–1503. [Google Scholar] [CrossRef][Green Version]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F., III. Functional genomics of PCOS: From GWAS to molecular mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.S. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front. Endocrinol. 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Novaira, H.J.; Sonko, M.L.; Hoffman, G.; Koo, Y.; Ko, C.; Wolfe, A.; Radovick, S. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol. Endocrinol. 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.; Ryden, M.; Näslund, E.; Muehlen, I.E.; Wiren, M.; Lafontan, M.; Arner, P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004, 47, 420–428. [Google Scholar] [CrossRef]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81. [Google Scholar] [CrossRef]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. (Landmark Ed.) 2019, 24, 890–934. [Google Scholar]
- Stepto, N.K.; Moreno-Asso, A.; McIlvenna, L.C.; Walters, K.A.; Rodgers, R.J. Molecular mechanisms of insulin resistance in polycystic ovary syndrome: Unraveling the conundrum in skeletal muscle? J. Clin. Endocrinol. Metab. 2019, 104, 5372–5381. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, E3. [Google Scholar] [CrossRef]
- Shorakae, S.; Jona, E.; de Courten, B.; Lambert, G.W.; Lambert, E.A.; Phillips, S.E.; Clarke, I.J.; Teede, H.J.; Henry, B.A. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clin. Endocrinol. 2019, 90, 425–432. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, T.; Zhao, H.; Huang, Y.; Ye, R.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Zhou, H.; et al. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 2708–2713. [Google Scholar] [PubMed]
- Xiao, W.; Huarong, W.; Wei, L.; Zhiyuan, Z.; Yanhao, Z.; Wenqiang, Z.; Zijiang, C.; Guoliang, X.; Chao, W. High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin. Sci. 2019, 132, 759–776. [Google Scholar]
- Mirczuk, S.M.; Lessey, A.J.; Catterick, A.R.; Perrett, R.M.; Scudder, C.J.; Read, J.E.; Lipscomb, V.J.; Niessen, S.J.; Childs, A.J.; McArdle, C.A.; et al. Regulation and Function of C-Type Natriuretic Peptide (CNP) in Gonadotrope-Derived Cell Lines. Cells 2019, 8, 1086. [Google Scholar]
- Azhary, J.M.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Takahashi, N.; Nose, E.; Oi, N.; Wada-Hiraike, O.; Urata, Y.; Hirata, T.; et al. Androgens increase accumulation of advanced glycation end products in granulosa cells by activating ER stress in PCOS. Endocrinology 2020, 161, bqaa015. [Google Scholar] [PubMed]
- Lin, P.H.; Chang, C.C.; Wu, K.H.; Shih, C.K.; Chiang, W.; Chen, H.Y.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M.; et al. Dietary glycotoxins, advanced glycation end products, inhibit cell proliferation and progesterone secretion in ovarian granulosa cells and mimic PCOS-like symptoms. Biomolecules 2019, 9, 327. [Google Scholar]
- Merhi, Z.; Buyuk, E.; Cipolla, M. Advanced glycation end products alter steroidogenic gene expression by granulosa cells: An effect partially reversible by vitamin D. MHR Basic Sci. Reprod. Med. 2018, 24, 318–326. [Google Scholar]
- Diamanti-Kandarakis, E.; Chatzigeorgiou, A.; Papageorgiou, E.; Koundouras, D.; Koutsilieris, M. Advanced glycation end-products and insulin signaling in granulosa cells. Exp. Biol. Med. 2016, 241, 1438–1445. [Google Scholar]
- Kinyua, A.W.; Doan, K.V.; Yang, D.J.; Huynh, M.K.Q.; Choi, Y.H.; Shin, D.M.; Kim, K.W. Insulin regulates adrenal steroidogenesis by stabilizing SF-1 activity. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Van Leckwyck, M.; Kong, W.; Burton, K.J.; Amati, F.; Vionnet, N.; Pralong, F.P. Decreasing insulin sensitivity in women induces alterations in LH pulsatility. J. Clin. Endocrinol. Metab. 2016, 101, 3240–3249. [Google Scholar]
- Yki-Järvinen, H.; Mäkimattila, S.; Utriainen, T.; Rutanen, E.M. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J. Clin. Endocrinol. Metab. 1995, 80, 3227–3232. [Google Scholar]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clin. Med. 2016, 16, 262. [Google Scholar]
- Monte, A.; Barros, V.; Santos, J.; Menezes, V.; Cavalcante, A.; Gouveia, B.; Bezerra, M.; Macedo, T.; Matos, M. Immunohistochemical localization of insulin-like growth factor-1 (IGF-1) in the sheep ovary and the synergistic effect of IGF-1 and FSH on follicular development in vitro and LH receptor immunostaining. Theriogenology 2019, 129, 61–69. [Google Scholar]
- Martins, F.; Saraiva, M.; Celestino, J.; Bruno, J.; Almeida, A.; Cunha, R.; Silva, J.; Campello, C.; Lucci, C.; Matos, M.; et al. Expression of protein and mRNA encoding Insulin Growth Factor-I (IGF-I) in goat ovarian follicles and the influence of IGF-I on in vitro development and survival of caprine preantral follicles. Anim. Reprod. (AR) 2018, 7, 349–361. [Google Scholar]
- Campbell, B. The endocrine and local control of ovarian follicle development in the ewe. Anim. Reprod. (AR) 2018, 6, 159–171. [Google Scholar]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [PubMed]
- Zhang, B.; Zhou, W.; Shi, Y.; Zhang, J.; Cui, L.; Chen, Z.J. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr. Disord. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; McWhirter, L.; Diniz Behn, C.; Bubar, K.M.; Kaar, J.L.; Pyle, L.; Rahat, H.; Garcia-Reyes, Y.; Carreau, A.M.; Wright, K.P., Jr.; et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3525–3534. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Zhai, J.; Xu, J.; Li, S.; Li, W.; Chen, Z.J.; Du, Y. Continuous Light-Induced PCOS-Like Changes in Reproduction, Metabolism, and Gut Microbiota in Sprague-Dawley Rats. Front. Microbiol. 2020, 10, 3145. [Google Scholar] [PubMed]
- Szczuko, M.; Splinter, J.; Zapałowska-Chwyć, M.; Ziętek, M.; Maciejewska, D. Fluorine may intensify the mechanisms of polycystic ovary syndrome (PCOS) development via increased insulin resistance and disturbed thyroid-stimulating hormone (TSH) synthesis even at reference levels. Med. Hypotheses 2019, 128, 58–63. [Google Scholar] [CrossRef]
- Lestari, P. Plastics and its effect to women reproductive systems. Maj. Obstet. Dan Ginekol. 2020, 28, 1–2. [Google Scholar]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [PubMed]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol a in polycystic ovary syndrome: A case–control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, M.; Chow, E.; Aschengrau, A.; Mahalingaiah, S. Prenatal exposure to endocrine disruptors: A developmental etiology for polycystic ovary syndrome. Reprod. Sci. 2017, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.C.; Padmanabhan, V. Developmental programming of PCOS traits: Insights from the sheep. Med. Sci. 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Campbell, R.E. Pathological pulses in PCOS. Mol. Cell. Endocrinol. 2019, 498, 110561. [Google Scholar] [CrossRef] [PubMed]
- Kawwass, J.F.; Sanders, K.M.; Loucks, T.L.; Rohan, L.C.; Berga, S.L. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum. Reprod. 2017, 32, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A.S.; Thackray, V.G.; Ryan, G.E.; Tolson, K.P.; Glidewell-Kenney, C.A.; Semaan, S.J.; Poling, M.C.; Iwata, N.; Breen, K.M.; Duleba, A.J.; et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol. Reprod. 2015, 93, 69. [Google Scholar] [PubMed]
- Lundgren, J.A.; Kim, S.H.; Burt Solorzano, C.M.; McCartney, C.R.; Marshall, J.C. Progesterone suppression of luteinizing hormone pulse frequency in adolescent girls with hyperandrogenism: Effects of metformin. J. Clin. Endocrinol. Metab. 2018, 103, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Katulski, K.; Podfigurna, A.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 2018, 61, 149–157. [Google Scholar] [CrossRef]
- Turcu, A.; Smith, J.M.; Auchus, R.; Rainey, W.E. Adrenal androgens and androgen precursors—Definition, synthesis, regulation and physiologic actions. Compr. Physiol. 2011, 4, 1369–1381. [Google Scholar]
- Lu, M.; Zhang, R.; Yu, T.; Wang, L.; Liu, S.; Cai, R.; Guo, X.; Jia, Y.; Wang, A.; Jin, Y.; et al. CREBZF regulates testosterone production in mouse Leydig cells. J. Cell. Physiol. 2019, 234, 22819–22832. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Zhao, S.; Zhang, Y.; Ma, H.; Yang, Z.; Yang, W.; Zhao, C.; Wang, L.; Zhang, Q. Melatonin receptor depletion suppressed hCG-induced testosterone expression in mouse Leydig cells. Cell. Mol. Biol. Lett. 2019, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Kempegowda, P.; Walsh, M.; Taylor, A.E.; Manolopoulos, K.N.; Allwood, J.W.; Semple, R.K.; Hebenstreit, D.; Dunn, W.B.; Tomlinson, J.W.; et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270. [Google Scholar] [CrossRef]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Churchill, S.J.; Wang, E.T.; Pisarska, M.D. Metabolic consequences of polycystic ovary syndrome. Minerva Ginecol. 2015, 67, 545–555. [Google Scholar] [PubMed]
- Chaudhari, A.P.; Kaustubh, M.; Pooja, D.M. Anxiety, depression, and quality of life in women with polycystic ovarian syndrome. Indian J. Psychol. Med. 2018, 40, 239–246. [Google Scholar] [CrossRef]
- Hussain, A.; Chandel, R.; Ganie, M.; Dar, M.; Rather, Y.; Wani, Z.; Shiekh, J.; Shah, M.S. Prevalence of psychiatric disorders in patients with a diagnosis of polycystic ovary syndrome in kashmir. Indian J. Psychol. Med. 2015, 37, 66–70. [Google Scholar] [CrossRef]
- Cooney, L.; Dokras, A. Depression and Anxiety in Polycystic Ovary Syndrome: Etiology and Treatment. Curr. Psychiatry Rep. 2017, 19, 83. [Google Scholar] [CrossRef]
- Rodriguez-Paris, D.; Remlinger-Molenda, A.; Kurzawa, R.; Głowińska, A.; Spaczyński, R.; Rybakowski, F.; Pawełczyk, L.; Banaszewska, B. Psychiatric disorders in women with polycystic ovary syndrome. Psychiatr. Pol. 2019, 53, 955–966. [Google Scholar] [CrossRef]
- Franik, G.; Krysta, K.; Witkowska, A.; Dudek, A.; Krzystanek, M.; Madej, P. The impact of sex hormones and metabolic markers on depressive symptoms and cognitive functioning in PCOS patients. Gynecol. Endocrinol. 2019, 35, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Meier, R. Polycystic Ovary Syndrome. Nurs. Clin. N. Am. 2018, 53, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.C.; Chapman, L.W.; Mesinkovska, N.A. The efficacy and use of finasteride in women: A systematic review. Int. J. Dermatol. 2019, 58, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Tartagni, M.V.; Alrasheed, H.; Damiani, G.R.; Montagnani, M.; Maria, A.; De Pergola, G.; Tartagni, M.; Loverro, G. Intermittent low-dose finasteride administration is effective for treatment of hirsutism in adolescent girls: A pilot study. J. Pediatr. Adolesc. Gynecol. 2014, 27, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, R.; Di Muzio, M.; Giorgetti, A.; Girolami, D.; Borgia, L.; Tagliabracci, A. Flutamide-induced hepatotoxicity: Ethical and scientific issues. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 69–77. [Google Scholar]
- Fulghesu, A.M.; Melis, F.; Murru, G.; Canu, E.; Melis, G.B. Very low dose of flutamide in the treatment of hyperandrogenism. Gynecol. Endocrinol. 2018, 34, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777. [Google Scholar] [CrossRef]
- Tsuboi, R.; Tanaka, T.; Nishikawa, T.; Ueki, R.; Yamada, H.; Katsuoka, K.; Ogawa, H.; Takeda, K. A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur. J. Dermatol. 2007, 17, 37–44. [Google Scholar]
- Messenger, A.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Artini, P.G.; Obino, M.E.R.; Sergiampietri, C.; Pinelli, S.; Papini, F.; Casarosa, E.; Cela, V. PCOS and pregnancy: A review of available therapies to improve the outcome of pregnancy in women with polycystic ovary syndrome. Expert Rev. Endocrinol. Metab. 2018, 13, 87–98. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, H.; Wang, X.; Zhang, M.; Liu, B.; Chen, Z.; Yang, T.; Fang, J.; Zhang, Y.; Liu, W.; et al. HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, F.F.; Yin, R.; Ding, G.L.; El-prince, M.; Gao, Q.; Shi, B.W.; Pan, H.H.; Huang, Y.T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012, 90, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Stage, T.B.; Glintborg, D.; Andersen, M.; Christensen, M.M.H. The pharmacogenetics of metformin in women with polycystic ovary syndrome: A randomized trial. Basic Clin. Pharmacol. Toxicol. 2018, 122, 239–244. [Google Scholar] [CrossRef]
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Network, I.P.; Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Tapanainen, J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. Off. J. Am. Fertil. Soc. 2018, 110, 364–379. [Google Scholar]
- Wei, L.L.; Ren, X.; Zhao, Y.Y.; Wang, L.; Zhao, Y.F. Facilitative glucose transporters: Expression, distribution and the relationship to diseases. Sheng Li Xue Bao [Acta Physiol. Sin.] 2019, 71, 350–360. [Google Scholar]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of diet on insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, dgaa425. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, Y.; Shang, L.; Yang, L.; Huang, J.; Ma, J.; Liao, X.; Zhou, H.; Xian, J.; Liang, G.; et al. Effect of type 2 diabetes mellitus caveolin-3 K15N mutation on glycometabolism. Exp. Ther. Med. 2019, 18, 2531–2539. [Google Scholar] [PubMed]
- Hagiwara-Chatani, N.; Shirai, K.; Kido, T.; Horigome, T.; Yasue, A.; Adachi, N.; Hirai, Y. Membrane translocation of t-SNARE protein syntaxin-4 abrogates ground-state pluripotency in mouse embryonic stem cells. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.J.; Griesel, B.A.; King, C.D.; Josey, M.A.; Olson, A.L. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet–fed transgenic mice. Diabetes 2013, 62, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Yoder, S.M.; Oh, E.; Thurmond, D.C. Munc18c: A controversial regulator of peripheral insulin action. Trends Endocrinol. Metab. 2014, 25, 601–608. [Google Scholar] [CrossRef] [PubMed]
- McNay, E.C.; Pearson-Leary, J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp. Neurol. 2020, 323, 113076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, X.; Zhuang, L.; Liu, X.; Zhao, H.; Shan, Y.; Liu, Z.; Li, F.; Wang, Y.; Fang, J. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul. Toxicol. Pharmacol. 2020, 110, 104544. [Google Scholar] [CrossRef]
- Laganà, A.S.; Rossetti, P.; Sapia, F.; Chiofalo, B.; Buscema, M.; Valenti, G.; Rapisarda, A.M.C.; Vitale, S.G. Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: Changing the perspective of the disease. Int. J. Endocrinol. Metab. 2017, 15, e43695. [Google Scholar] [CrossRef]
- Monastra, G.; Unfer, V.; Harrath, A.H.; Bizzarri, M. Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol. Endocrinol. 2017, 33, 1–9. [Google Scholar] [CrossRef]
- Cabrera-Cruz, H.; Oróstica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E237–E248. [Google Scholar] [CrossRef]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O. Inositols in Polycystic Ovary Syndrome: An Overview on the Advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef]
- Facchinetti, F.; Appetecchia, M.; Aragona, C.; Bevilacqua, A.; Bezerra Espinola, M.S.; Bizzarri, M.; D’Anna, R.; Dewailly, D.; Diamanti-Kandarakis, E.; Hernández Marín, I.; et al. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: A further help for human reproduction and beyond. Expert Opin. Drug Metab. Toxicol. 2020, 16, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in polycystic ovary syndrome: Restoring fertility through a pathophysiology-based approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Olszanecka-Glinianowicz, M.; Banaś, M.; Zahorska-Markiewicz, B.; Janowska, J.; Kocełak, P.; Madej, P.; Klimek, K. Is the polycystic ovary syndrome associated with chronic inflammation per se? Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 133, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Merhi, Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Pluta, D.; Blukacz, Ł.; Madej, P. The role of aquaporins in polycystic ovary syndrome—A way towards a novel drug target in PCOS. Med. Hypotheses 2017, 102, 23–27. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, B.; Wang, L.; Zeng, X.; Li, B.; Sha, X.; Liu, H. AQP8 and AQP9 expression in patients with polycystic ovary syndrome and its association with in vitro fertilization-embryo transfer outcomes. Exp. Ther. Med. 2019, 18, 755–760. [Google Scholar] [CrossRef]
- Tian, C.; Song, W.; Hou, X. The expression and function of AQP7 and AQP9 in granulose cells and oocytes of patients with PCOS. Fertil. Steril. 2018, 110, e116. [Google Scholar] [CrossRef]
- Wang, D.; Di, X.; Wang, J.; Li, M.; Zhang, D.; Hou, Y.; Hu, J.; Zhang, G.; Zhang, H.; Sun, M.; et al. Increased formation of follicular antrum in aquaporin-8-deficient mice is due to defective proliferation and migration, and not steroidogenesis of granulosa cells. Front. Physiol. 2018, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yin, Y.; Tan, Y.; Hong, K.; Zhou, H. The Flavanone, Naringenin, Modifies Antioxidant and Steroidogenic Enzyme Activity in a Rat Model of Letrozole-Induced Polycystic Ovary Syndrome. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Kampa, R.P.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the mitochondrial BKCa channel by the citrus flavonoid naringenin as a potential means of preventing cell damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yuan, X.; Ye, R.; Zhou, H.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Dong, M.; Huang, Y.; et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Chandran, A.; Merlin, N.; Ammu, L.; Dharan, S.S. Fennel Treatment to PCOS: An Insilico Evaluation to explore the Therapeutic Efficacy of Anethole. Res. J. Pharm. Technol. 2019, 12, 4958–4962. [Google Scholar] [CrossRef]
- Zhu, J.l.; Chen, Z.; Feng, W.j.; Long, S.l.; Mo, Z.C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An international consortium update: Pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]

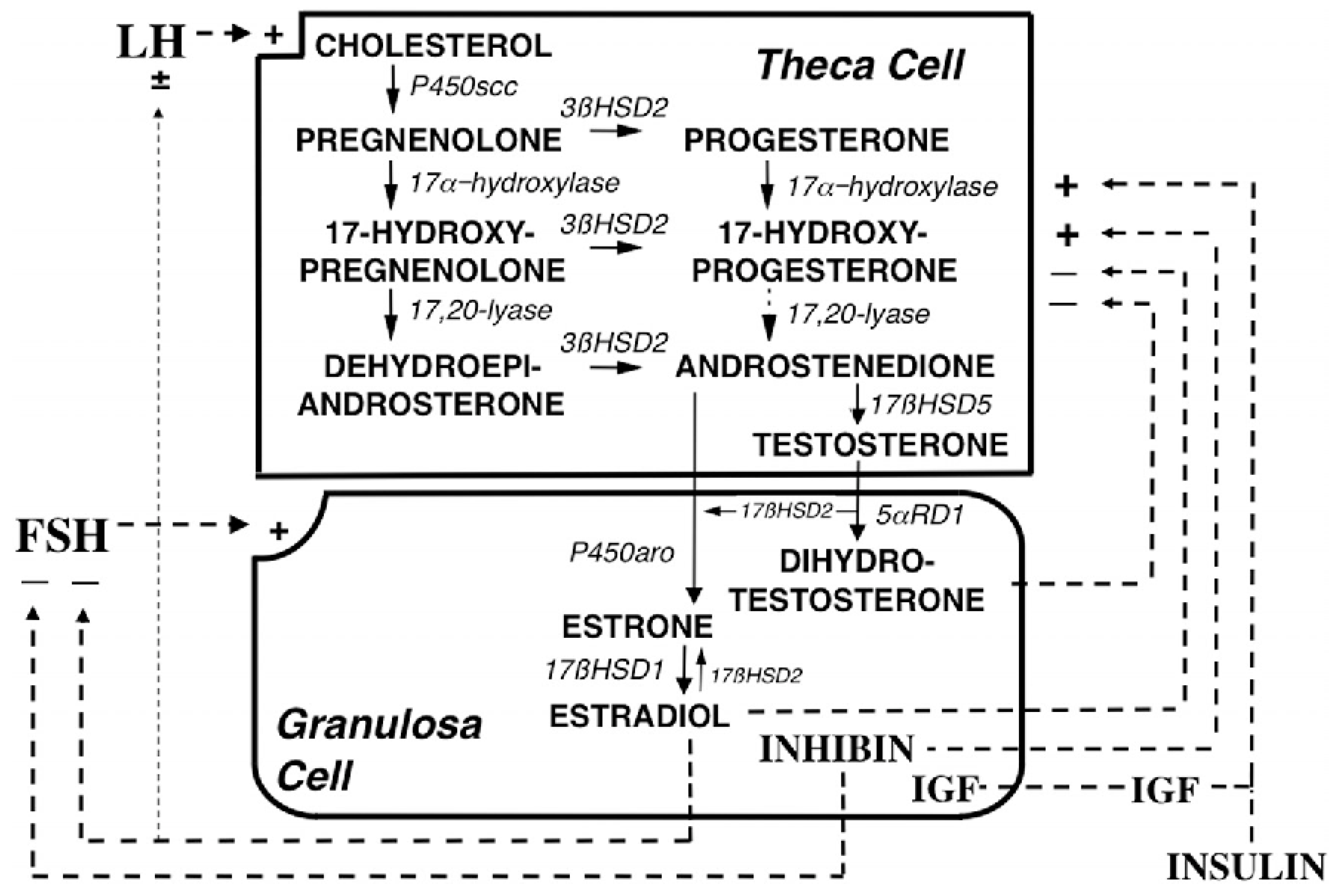
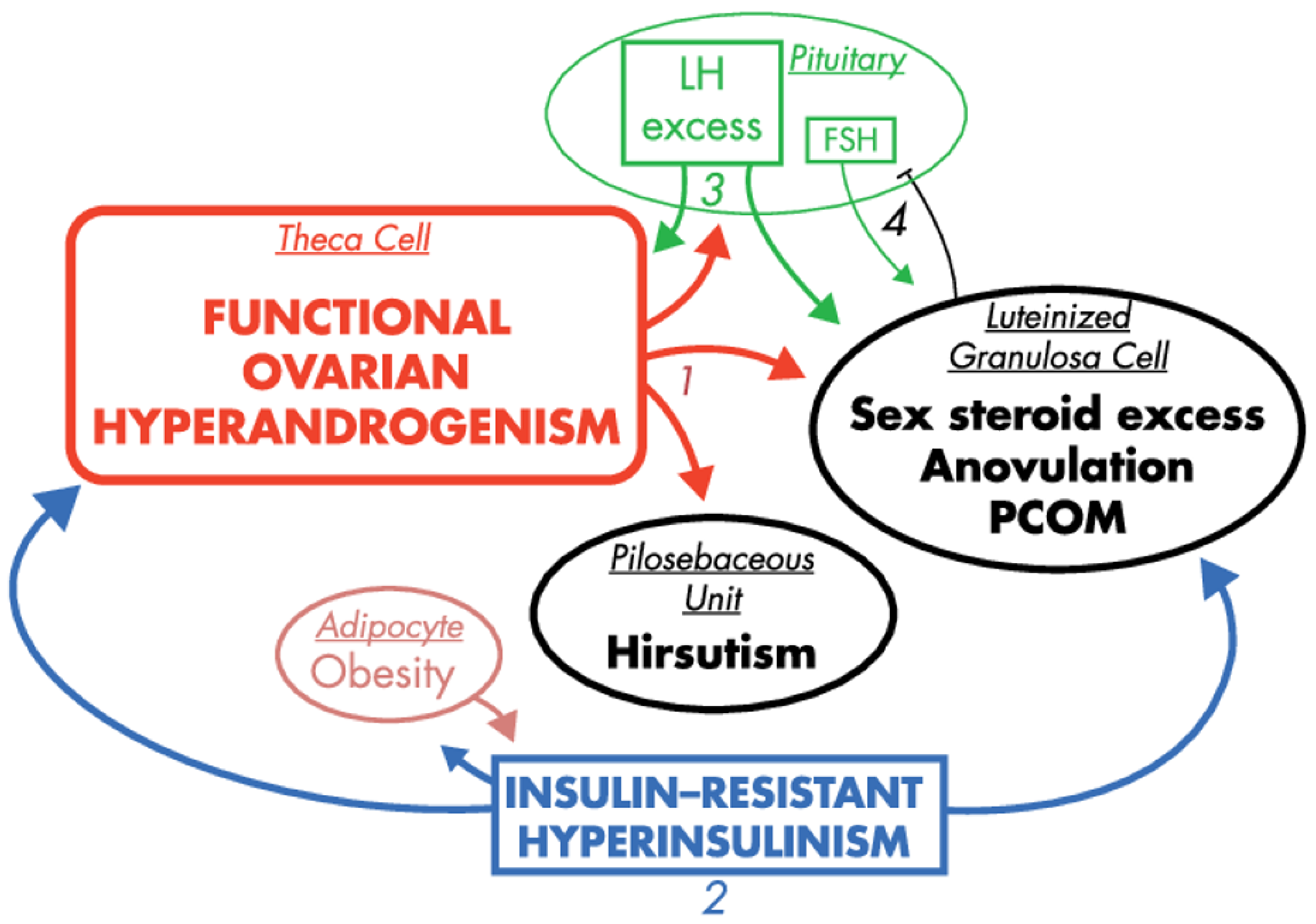
| Gene Type | Function | Group |
|---|---|---|
| CYP11a | Present in all steroid-producing tissues, encodes a cytochrome P450 enzyme that mediates the cleavage of the cholesterol side chain, which is a dominating process in the rate of formation steroid hormones; its role was also confirmed in etiology of hyperandrogenism and PCOS [21,22]. | Ovarian and adrenal steroidogenesis |
| CYP17 | Encodes an enzyme cytochrome P450-C17; component of the androgen synthesis pathway, which is dysfunctional in PCOS [23]. Its role in PCOS was also characterized in [24,25]. | |
| CYP19 | Encodes important enzymes in androgen synthesis pathways, including cytochrome P450 aromatase. Its role in PCOS was described in [26]. The changes in concentration of P450 was combined with PCOS; for details please see [27] | |
| CYP21 | Encodes an important 21-hydroxylase enzyme involved in synthesis pathways of steroid hormones; reported increased frequency of heterozygosity for mutations in the 21-hydroxylase gene in women with PCOS [28,29]. | |
| LH | Encodes beta subunit of luteinizing hormone. High level of circulating LH is a common biochemical indicator of PCOS [30]; point mutation-Trp8Arg and Ilg15Thr in the gene encoding beta subunit was identified in patients with PCOS [31]. | Gonadotropin release regulation |
| FSHR | Protein encoded by this gene – G-protein coupled receptor is involved in hormonal regulation of gonadal development. Mutation of this gene disrupts the structural conformation of protein and in result causes ovarian hyperstimulation syndrome; the association of follicle-stimulating hormone receptor (FSHR) and PCOS is characterized in [32,33] | |
| AMH | Encodes the anti-müllerian hormone, it is involved in gonadotropin secretion. The important role of AMH in the pathophysiology of PCOS was characterized in [34,35]. | |
| INSR | Insulin receptor gene on chromosome 19p13.2; Identified region D19S884 was characterized as a fragment of chromosome involved in PCOS [36]. | Insulin secretion and action |
| CAPN10 | Encodes Calpain 10 protein, belonging to calcium-dependent cysteine proteases family, which impacts on insulin metabolism and secretion what is the reason for association CAPN10 with PCOS etiology [5,37]. | |
| IRS1, IRS2 | Involved in insulin secretion and action; encodes insulin receptor substrates IRS1 and IRS2. Gly972Arg variant of IRS1 was identified more often in women with PCOS [38]. | |
| AR | Mutations in androgen receptors cause the disruption of the respective cellular pathways and in result- hyperandrogenism associated with PCOS. | Steroid hormone action |
| SHBG | SHBG gene is in 17p13-p12 chromosome and encodes Sex Hormone Binding Globulin, an important biomarker in PCOS risk assessment which binds androgens and other hormones, thus regulates the androgen level in the body [39]. | |
| TNF-alpha | Encodes a cytokine Tumor Necrosis Factor which modulates several biological processes, including immunity and inflammation, obesity and insulin resistance; its role in PCOS has been examined [40]. | Chronic inflammation |
| FTO | Gene encodes fat mass and obesity-associated protein. FTO rs9939609 polymorphism is significantly associated with risk of PCOS [30]. | Adipose tissue metabolism |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Trybek, P.; Jarosz, T.; Radosz, P.; Setlak, M.; Madej, P. In Search of New Therapeutics—Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond. Int. J. Mol. Sci. 2020, 21, 7054. https://doi.org/10.3390/ijms21197054
Wawrzkiewicz-Jałowiecka A, Kowalczyk K, Trybek P, Jarosz T, Radosz P, Setlak M, Madej P. In Search of New Therapeutics—Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond. International Journal of Molecular Sciences. 2020; 21(19):7054. https://doi.org/10.3390/ijms21197054
Chicago/Turabian StyleWawrzkiewicz-Jałowiecka, Agata, Karolina Kowalczyk, Paulina Trybek, Tomasz Jarosz, Patrycja Radosz, Marcin Setlak, and Paweł Madej. 2020. "In Search of New Therapeutics—Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond" International Journal of Molecular Sciences 21, no. 19: 7054. https://doi.org/10.3390/ijms21197054
APA StyleWawrzkiewicz-Jałowiecka, A., Kowalczyk, K., Trybek, P., Jarosz, T., Radosz, P., Setlak, M., & Madej, P. (2020). In Search of New Therapeutics—Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and Beyond. International Journal of Molecular Sciences, 21(19), 7054. https://doi.org/10.3390/ijms21197054






