Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers
Abstract
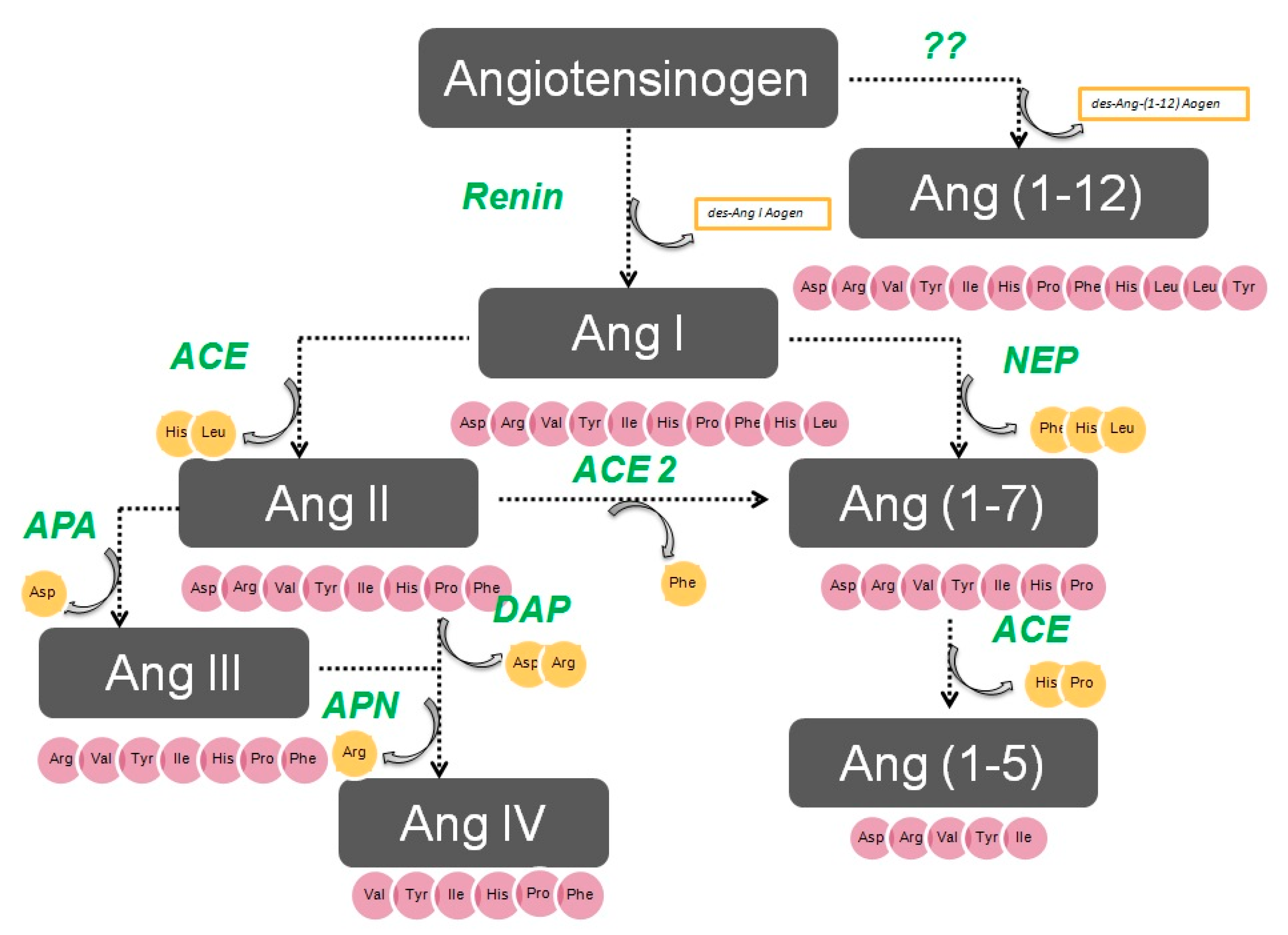
1. Aminopeptidases in the Renin–Angiotensin System
2. Aminopeptidases in Arterial Hypertension
Aminopeptidases after Antihypertensive Therapy
3. Brain APA
Systemic and Renal APA
4. APN
4.1. Brain APN in BP Regulation
4.2. Renal APN in BP Control
APN as a Regulator of Salt Sensitivity
4.3. Other Actions of APN Related to the Cardiovascular System
5. Therapeutic Strategies to Treat Arterial Hypertension with Aminopeptidases
5.1. Inhibition of APA
5.2. APN Blockade in the Treatment of Hypertension
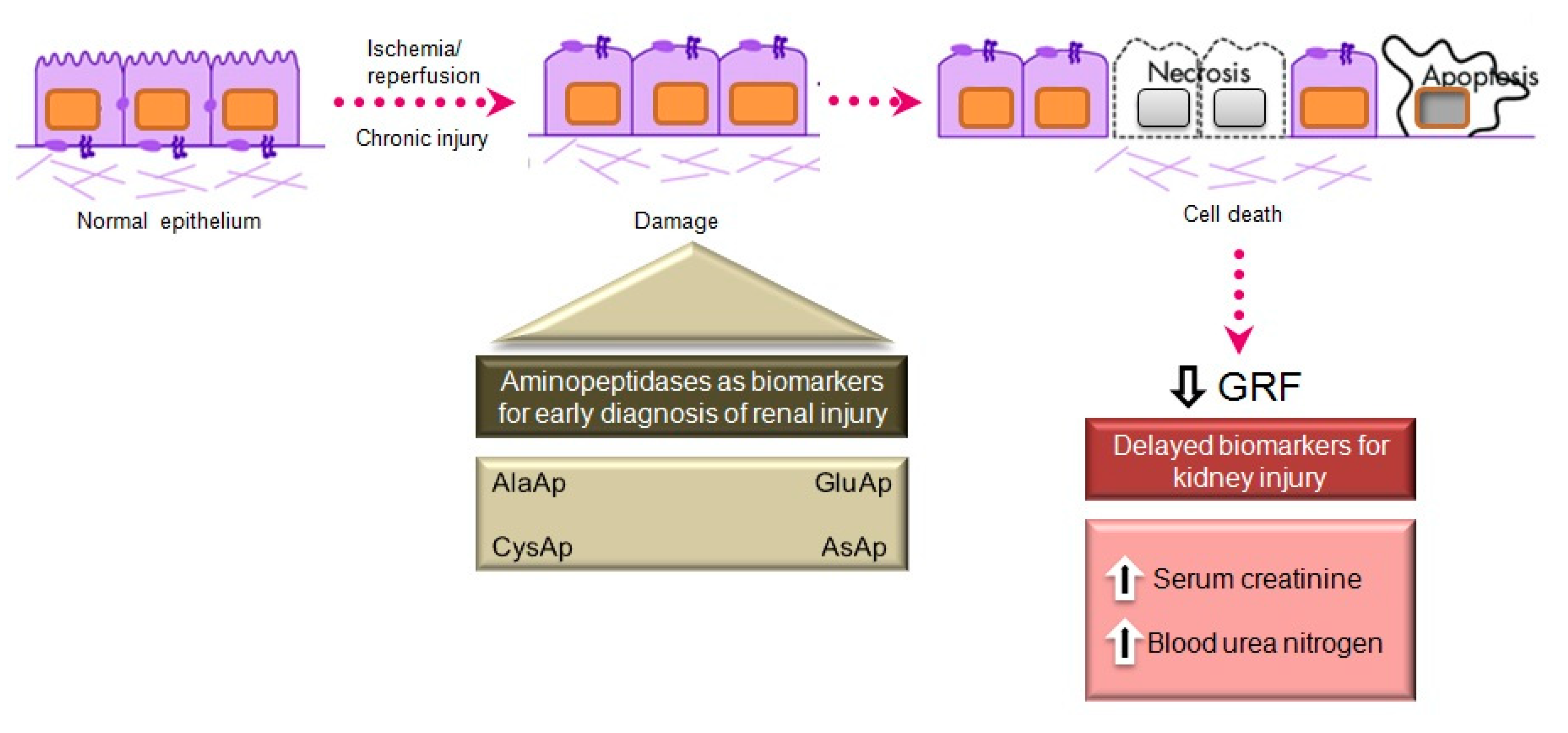
6. APs as Urinary Biomarkers of Renal Injury
Acute Kidney Injury
7. Quantification of Urinary APs
7.1. Aminopeptidases in Urinary Vesicles and Exosomes
7.2. AKI Induced by Surgical Procedures
7.3. Urinary Aminopeptidases as Biomarkers of Renal Dysfunction in Chronic Diseases
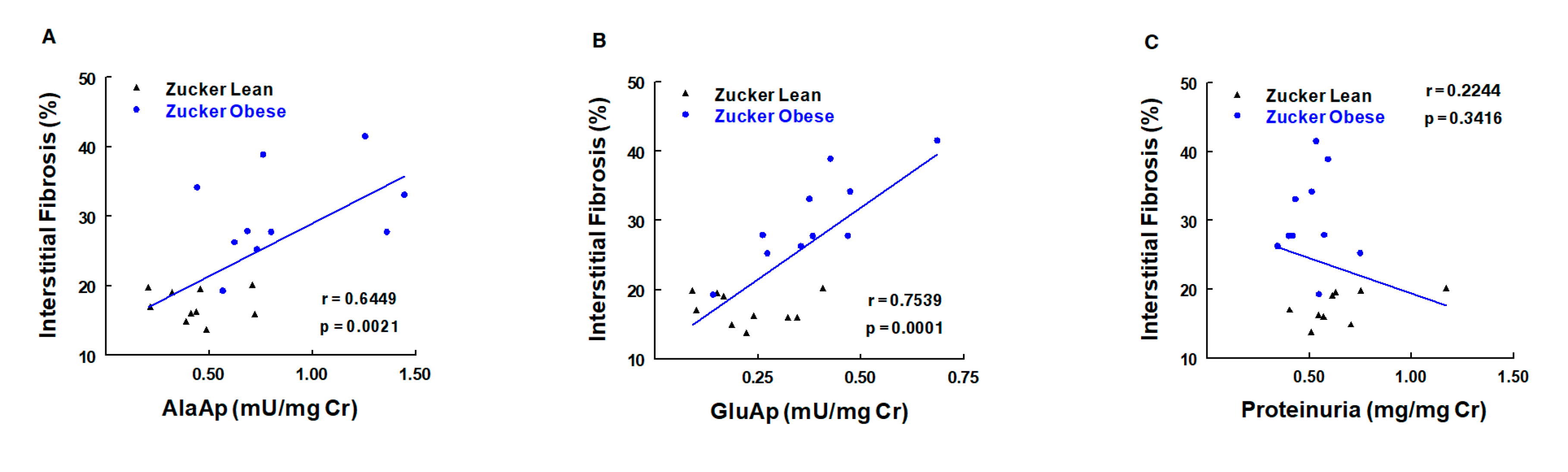
7.3.1. Obesity
7.3.2. Hypertension
7.3.3. Hyperthyroidism
7.3.4. Diabetes Mellitus
7.3.5. Urinary APs in Other Chronic Diseases
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| AKI | Acute kidney injury |
| AngI | Angiotensin I |
| AngII | Angiotensin II |
| AngIII | Angiotensin III |
| AngIV | Angiotensin IV |
| APs | Aminopeptidases |
| APA | Aminopeptidase A |
| APN | Aminopeptidase N |
| AT1 | Angiotensin receptor type 1 |
| AT2 | Angiotensin receptor type 2 |
| BP | Blood pressure |
| CAP | Cystinil AP |
| DOCA | Deoxycorticosterone |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| RAS | Renin–angiotensin system |
| SHR | Spontaneous hypertensive rat |
| WKY | Wistar Kyoto rat |
| ZO | Zucker obese rats |
References
- Johnston, C.I. Biochemistry and pharmacology of the renin-angiotensin system. Drugs 1990, 39, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Reudelhuber, T.L. The renin-angiotensin system: Peptides and enzymes beyond angiotensin II. Curr. Opin. Nephrol. Hypertens. 2005, 14, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Chappell, M.C. Novel Angiotensin peptides. Cell. Mol. Life. Sci. 2004, 61, 2720–2727. [Google Scholar] [CrossRef]
- Vauquelin, G.; Michotte Smolders, Y.I.; Sarre, S.; Ebinger, G.; Dupont, A.; Vanderheyden, P. Cellular targets for angiotensin II fragments: Pharmacological and molecular evidence. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Enzyme Nomenclature. Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzymes by the Reactions they Catalyse. Available online: https://www.qmul.ac.uk/sbcs/iubmb/enzyme/ (accessed on 18 June 2020).
- De Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar] [PubMed]
- Dinh, D.T.; Frauman, A.G.; Johnston, C.I.; Fabiani, M.E. Angiotensin receptors: Distribution, signalling and function. Clin. Sci. 2001, 100, 481–492. [Google Scholar] [CrossRef]
- Chappell, M.C. Nonclassical renin-angiotensin system and renal function. Compr. Physiol. 2012, 2, 2733–2735. [Google Scholar] [PubMed]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Banegas, I.; Wangensteen, R.; Martinez-Cañamero, M.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. Brain, heart and kidney correlate for the control of blood pressure and water balance: Role of angiotensinases. Neuroendocrinology 2014, 100, 198–208. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Alba, F.; Vives, F.; Banegas, I.; de Gasparo, M. Role of central and peripheral aminopeptidase activities in the control of blood pressure: A working hypothesis. Heart Fail. Rev. 2008, 13, 339–353. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Martinez, J.M.; Vargas, F.; Alba, F. Renal aminopeptidase activities in animal models of hypertension. Regul. Pept. 1997, 72, 155–159. [Google Scholar] [CrossRef]
- Prieto, I.; Martinez, A.; Martinez, J.M.; Ramírez, M.J.; Vargas, F.; Alba, F.; Ramírez, M. Activities of aminopeptidases in a rat saline model of volume hypertension. Horm. Metab. Res. 1998, 30, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, M.; Goto, Y.; Maruyama, M.; Hattori, A. Biochemical and enzymatic properties of the m1 family of aminopeptidases involved in the regulation of blood pressure. Heart Fail. Rev. 2008, 13, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Kitatani, K.; Matsumoto, H.; Miyazawa, S.; Rogi, T.; Tsuruoka, N.; Mizutani, S.; Natori, Y.; Tsujimoto, M. Characterization of recombinant human adipocyte-derived leucine aminopeptidase expressed in Chinese hamster ovary cells. J. Biochem. 2000, 128, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, T.; Hattori, A.; Masuda, S.; Nomura, Y.; Nakayama, H.; Mizutani, S.; Tsujimoto, M. Human leukocytederived arginine aminopeptidase. The third member of the oxytocinase subfamily of aminopeptidases. J. Biol. Chem. 2003, 278, 32275–32283. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, C.; Ebisui, E.; Usui, M.; Ogawa, N.; Suzuki, A.; Mataga, N.; Hiromi Takahashi-Iwanaga, H.; Katsuhiko Mikoshiba, K. ERp44 exerts redox dependent control of blood pressure at the ER. Mol. Cell 2015, 58, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nakayama, J.; Yamakawa-Kobayashi, K.; Hamaguchi, H.; Miyazaki, R.; Arinami, T. Identification of 33 polymorphisms in the adipocyte-derived leucine aminopeptidase (ALAP) gene and possible association with hypertension. Hum. Mutat. 2002, 19, 251–257. [Google Scholar] [CrossRef]
- Robert, Y.; Zee, L.; Rivera, A.; Inostroza, Y.; Ridker, P.M.; Daniel, I.; Chasman, D.I.; Romero, J.R. Gene variation of endoplasmic reticulum aminopeptidases 1 and 2, and risk of blood pressure progression and incident hypertension among 17,255 initially healthy women. Int. J. Genom. 2018, 2018, 2308585. [Google Scholar]
- Hallberg, P.; Lind, L.; Michaelsson, K.; Kurland, L.; Kahan, T.; Malmqvist, K.; Ohman, K.P.; Nystrom, F.; Liljedahl, U.; Syvanen, A.C.; et al. Adipocyte-derived leucine aminopeptidase genotype and response to antihypertensive therapy. BMC Cardiovasc. Disord. 2003, 3, 11. [Google Scholar] [CrossRef][Green Version]
- Johnson, M.P.; Roten, L.T.; Dyer, T.D.; East, C.E.; Forsmo, S.; Blangero, J.; Brennecke, S.P.; Austgulen, R.; Moses, E.K. The ERAP2 gene is associated with preeclampsia in Australian and Norwegian populations. Hum. Genet. 2009, 126, 655–666. [Google Scholar] [CrossRef]
- Taranta, A.; Gianviti, A.; Palma, A.; De Luca, V.; Mannucci, L.; Procaccino, M.A.; Ghiggeri, G.M.; Caridi, G.; Fruci, D.; Ferracuti, S.; et al. Genetic risk factors in typical haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 2009, 24, 1851–1857. [Google Scholar] [CrossRef][Green Version]
- Banegas, I.; Ramírez, M.; Vives, F.; Alba, F.; Segarra, A.B.; Duran, R.; De Gasparo, M.; Prieto, I. Aminopeptidase activity in the nigrostriatal system and prefrontal cortex of rats with experimental hemiparkinsonism. Horm. Metab. Res. 2005, 37, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Segarra, A.B.; de Gasparo, M.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Segarra, A.B.; Villarejo, A.B.; de Gasparo, M.; Martínez-Cañamero, M.M.; Ramírez-Sánchez, M. Neuropeptidase activity in the frontal cortex of Wistar-Kyoto and spontaneously hypertensive rats treated with vasoactive drugs: A bilateral study. J. Hypertens. 2019, 37, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.B.; Ramírez-Sánchez, M.; Segarra, A.B.; Martínez-Cañamero, M.; Prieto, I. Influence of extra virgin olive oil on blood pressure and kidney angiotensinase activities in spontaneously hypertensive rats. Planta Med. 2015, 81, 664–669. [Google Scholar] [CrossRef]
- Wright, J.W.; Mizutani, S.; Harding, J.W. Focus on Brain Angiotensin III and Aminopeptidase A in the Control of Hypertension. Int. J. Hypertens. 2012, 2012, 124758. [Google Scholar] [CrossRef]
- Wright, J.W.; Mizutani, S.; Murray, C.E.; Amir, H.Z.; Harding, J.W. Aminopeptidase-induced elevations and reductions in blood pressure in the spontaneously hypertensive rat. J. Hypertens. 1990, 8, 969–974. [Google Scholar] [CrossRef]
- Jensen, L.L.; Harding, J.W.; Wright, J.W. Increased blood pressure induced by central application of aminopeptidase inhibitors is angiotensinergic-dependent in normotensive and hypertensive rat strains. Brain Res. 1989, 490, 48–55. [Google Scholar] [CrossRef]
- Wright, J.W.; Roberts, K.A.; Cook, V.I.; Murray, C.E.; Sardinia, M.F.; Harding, J.W. Intracerebroventricularly infused [D-Arg1]angiotensin III, is superior to [D-Asp1]angiotensin II, as a pressor agent in rats. Brain Res. 1990, 514, 5–10. [Google Scholar] [CrossRef]
- Wright, J.W.; Tamura-Myers, E.; Wilson, W.L.; Roques, B.P.; Llorens-Cortes, C.; Speth, R.C.; Harding, J.W. Conversion of brain angiotensin II to angiotensin III is critical for pressor response in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R725–R733. [Google Scholar] [CrossRef]
- Lodja, Z.; Gossrau, R. Study on aminopeptidase A. Histochemistry 1980, 67, 237–290. [Google Scholar]
- Wright, J.W.; Harding, J.W. Brain angiotensin receptor subtypes AT1, AT2 and AT4 and their functions. Regul. Pept. 1995, 59, 269–295. [Google Scholar] [CrossRef]
- Speth, R.C.; Thompson, S.M.; Johns, S.J. Angiotensin II receptors: Structural and functional considerations. Adv. Exp. Med. Biol. 1995, 377, 169–192. [Google Scholar] [PubMed]
- Mizutani, S.; Akiyama, H.; Kurauchi, O.; Taira, H.; Narita, O.; Tomoda, Y. In vitro degradation of angiotensin II (A-II) by human placental subcellular fractions, pregnancy sera and purified placental aminopeptidases. Acta Endocrinol. 1985, 110, 135–139. [Google Scholar] [CrossRef]
- Yamada, R.; Mizutani, S.; Kurauchi, O.; Okano, K.; Imaizumi, H.; Narita, O.; Tomoda, Y. Purification and characterization of human placental aminopeptidase A. Enzyme 1988, 40, 223–230. [Google Scholar] [CrossRef]
- Chauvel, E.N.; Llorens-Cortes, C.; Coric, P.; Wilk, S.; Roques, B.P.; Fournie-Zaluski, M.C. Differential inhibition of aminopeptidase A and aminopeptidase N by new-amino thiols. J. Med. Chem. 1994, 37, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Nomura, S.; Okada, M.; Ohno, Y.; Kobayashi, H.; Nakashima, Y.; Murata, Y.; Takeuchi, M.; Kuno, N.; Nagasaka, T.; et al. Hypertension and Angiotensin II hypersensitivity in aminopeptidase A-deficient mice. Mol. Med. 2003, 9, 57–62. [Google Scholar] [CrossRef]
- Mizutani, S.; Furuhashi, M.; Imaizumi, H.; Ito, Y.; Kurauchi, O.; Tomoda, Y. Effects of human placental aminopeptidases in spontaneously hypertensive rats. Med. Sci. Res. 1987, 15, 1203–1204. [Google Scholar]
- Goto, Y.; Hattori, A.; Ishii, Y.; Mizutani, S.; Tsujimoto, M. Enzymatic properties of aminopeptidase A: Regulation of its enzymatic activity by calcium and angiotensin IV. J. Biol. Chem. 2006, 281, 23503–23513. [Google Scholar] [CrossRef]
- Bivol, L.M.; Vagnes, O.B.; Iversen, B.M. The renal vascular response to ANG II injection is reduced in the nonclipped kidney of two-kidney, one-clip hypertension. Am. J. Physiol. Ren. Physiol. 2005, 289, F393–F400. [Google Scholar] [CrossRef]
- Prieto, I.; Hermoso, F.; Gaspara, M.; Vargas, F.; Alba, F.; Segarra, A.B.; Banegas, I.; Ramirez, M. Angiotensinase activities in the kidney of renovascular hypertensive rats. Peptides 2003, 24, 755–760. [Google Scholar] [CrossRef][Green Version]
- Kobori, H.; Nishiyama, A.; Abe, Y.; Navar, G. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 2003, 41, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Nomura, S.; Mitsui, T.; Suzuki, M.; Kobayashi, H.; Ito, T.; Itakura, A.; Kikkawa, F.; Mizutani, S. (2005) Possible involvement of aminopeptidase A in hypertension and renal damage in Dahl saltsensitive rats. Am. J. Hypertens. 2005, 18, 538–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, J.M.; Prieto, I.; Ramirez, M.J.; de Gasparo, M.; Hermoso, F.; Arias, J.M.; Alba, F.; Ramirez, M. Sex differences and agerelated changes in human serum aminopeptidase A activity. Clin. Chim. Acta 1998, 274, 53–61. [Google Scholar] [CrossRef]
- Baylis, C.; Engels, K.; Hymel, A.; Navar, L.G. Plasma rennin activity and metabolic rate of angiotensin II in the unstressed aging rat. Mech. Aging Dev. 1997, 97, 163–172. [Google Scholar] [CrossRef]
- Velez, J.C.; Arif, E.; Rodgers, J.; Hicks, M.P.; Arthur, J.M.; Nihalani, D.; Bruner, E.T.; Budisavljevic, M.N.; Atkinson, C.; Fitzgibbon, W.R.; et al. Deficiency of the Angiotensinase Aminopeptidase A Increases Susceptibility to Glomerular Injury. J. Am. Soc. Nephrol. 2017, 28, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, H.; Noren, O.; Olsen, J. Structure and function of aminopeptidase N. Adv. Exp. Med. Biol. 2000, 477, 25–34. [Google Scholar]
- Paul, M.; Poyan, M.A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Robert, S.D. Aminopeptidase N in arterial hypertension. Heart Fail. Rev. 2008, 13, 293–298. [Google Scholar]
- Amin, S.A.; Adhikari, N.; Jha, T. Design of aminopeptidase N inhibitors as anti-cancer agents. J. Med. Chem. 2018, 61, 6468–6490. [Google Scholar]
- Dan, H.; Tani, K.; Hase, K.; Shimizu, T.; Tamiya, H.; Biraa, Y.; Huang, L.; Yanagawa, H.; Sone, S. CD13/aminopeptidase N in collagen vascular diseases. Rheumatol. Int. 2003, 23, 271–276. [Google Scholar] [CrossRef]
- Khatun, A.; Kang, K.H.; Ryu, D.Y.; Rahman, M.S.; Kwon, W.S.; Pang, M.G. Effect of Aminopeptidase N on functions and fertility of mouse spermatozoa in vitro. Theriogenology 2018, 118, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef]
- Banegas, I.; Prieto, I.; Vives, F.; Alba, F.; de Gasparo, M.; Segarra, A.B.; Hermoso, F.; Durán, R.; Ramírez, M. Brain aminopeptidases and hypertension. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Jensen, L.L.; Cushing, L.L.; Harding, J.W. Leucine aminopeptidase M-induced reductions in blood pressure in spontaneously hypertensive rats. Hypertension 1989, 13, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Batt, C.M.; Jensen, L.L.; Harding, J.W.; Wright, J.W. Microinfusion of aminopeptidase M into the paraventricular nucleus of the hypothalamus in normotensive and hypertensive rats. Brain Res. Bull. 1996, 39, 235–240. [Google Scholar] [CrossRef]
- Abhold, R.H.; Sullivan, M.J.; Wright, J.W.; Harding, J.W. Binding, degradation and pressor activity of angiotensins II and III after aminopeptidase inhibition with amastatin and bestatin. J. Pharmacol. Exp. Ther. 1987, 242, 957–962. [Google Scholar]
- Kenny, A.J.; Maroux, S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol. Rev. 1982, 62, 91–128. [Google Scholar] [CrossRef]
- Albiston, A.L.; McDowall, S.G.; Matsacos, D.; Sim, P.; Clune, E.; Mustafa, T.; Lee, J.; Mendelsohn, F.A.; Simpson, R.J.; Connolly, L.M.; et al. Evidence that the angiotensin IV [AT(4)] receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001, 276, 48623–48626. [Google Scholar] [CrossRef]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.Y.; Mendelsohn, F.A.; Jenkins, T.A.; Albiston, A.L. The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef]
- Hamilton, T.A.; Handa, R.K.; Harding, J.W.; Wright, J.W. A role for the angiotensin IV/AT4 system in mediating natriuresis in the rat. Peptides 2001, 22, 935–944. [Google Scholar] [CrossRef]
- Handa, R.K.; Krebs, L.T.; Harding, J.W.; Handa, S.E. Angiotensin IV AT4-receptor system in the rat kidney. Am. J. Physiol. Ren. Physiol. 1998, 274, F290–F299. [Google Scholar] [CrossRef] [PubMed]
- Kotlo, K.; Shukla, S.; Tawar, U.; Skidgel, R.A.; Danziger, R.S. Aminopeptidase N reduces basolateral Na_-K_-ATPase in proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2007, 293, F1047–F1053. [Google Scholar] [CrossRef] [PubMed]
- Farjah, M.; Roxas, B.; Danziger, R.S. Dietary NaCl regulates in renal APN transcript/protein abundance and activity: Relevance to hypertension in the Dahl rat. Hypertension 2004, 43, 282–285. [Google Scholar] [CrossRef]
- Williams, J.S.; Raji, A.; Williams, G.H.; Conlin, P.R. Nonmodulating hypertension is associated with insulin resistance and the Lys528Arg variant human adipocyte-derived leucine aminopeptidase. Hypertension 2006, 48, 331–336. [Google Scholar]
- Ogimoto, G.; Yudowski, G.A.; Barker, C.J.; Kohler, M.; Katz, A.I.; Feraille, E.; Pedemonte, C.H.; Berggren, P.O.; Bertorello, A.M. G protein-coupled receptors regulate Na,K-ATPase activity and endocytosis by modulating the recruitment of adaptor protein 2 and clathrin. Proc. Natl. Acad. Sci. USA 2000, 97, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.M.; Liu, J.; Tanta, F.; Kabak, B.; Wakefield, B.; Malhotra, D.; Kennedy, D.J.; Nadoor, A.; Fedorova, O.V.; Gunning, W.; et al. Salt loading induces redistribution of the plasmalemmal Na-K-ATPase in proximal tubule cells. Kidney Int. 2005, 67, 1868–1877. [Google Scholar] [CrossRef]
- Linardi, A.; Panunto, P.C.; Ferro, E.S.; Hyslop, S. Peptidase activities in rats treated chronically with N (omega)-nitro-L-arginine methyl ester (L-NAME). Biochem. Pharmacol. 2004, 68, 205–214. [Google Scholar] [CrossRef]
- Röhnert, P.; Schmidt, W.; Emmerlich, P.; Goihl, A.; Wrenger, S.; Bank, U.; Nordhoff, K.; Täger, M.; Ansorge, S.; Reinhold, D.; et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J. Neuroinflamm. 2012, 9, 44. [Google Scholar] [CrossRef]
- Pereira, F.E.; Cronin, C.; Ghosh, M.; Zhou, S.Y.; Agosto, M.; Subramani, J.; Wang, R.; Shen, J.B.; Schacke, W.; Liang, B.; et al. CD13 is essential for inflammatory trafficking and infarct healing following permanent coronary artery occlusion in mice. Cardiovasc. Res. 2013, 100, 74–83. [Google Scholar] [CrossRef]
- Khakoo, A.Y.; Sidman, R.L.; Pasqualini, R.; Arap, W. Does the renin-angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res. 2008, 68, 9112–9115. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase Nis a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Rangel, R.; Sun, Y.; Guzman-Rojas, L.; Ozawa, M.G.; Sun, J.; Giordano, R.J.; Van Pelt, C.S.; Tinkey, P.T.; Behringer, R.R.; Sidman, R.L.; et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc. Natl. Acad. Sci. USA 2007, 104, 4588–4593. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.L.; Smith, B.D. Molecular Imaging of Aminopeptidase N in Cancer and Angiogenesis. Contrast Media Mol. Imaging 2018, 2018, 5315172. [Google Scholar] [CrossRef] [PubMed]
- Marc, Y.; Llorens-Cortes, C. The role of the brain renin-angiotensin system in hypertension: Implications for new treatment. Prog. Neurobiol. 2011, 95, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.J.; Casals-Stenzel, J.; Lever, A.F. Inhibitors of the renin-angiotensin system in experimental hypertension, with a note on the measurement of angiotensin I, II and III during infusion of converting-enzyme inhibitor. Br. J. Clin. Pharmacol. 1979, 2, 233S–241S. [Google Scholar] [CrossRef]
- Fournie-Zaluski, M.C.; Fassot, C.; Valentin, B.; Djordjijevic, D.; Reaux-Le Goazigo, A.; Corvol, P.; Roques, B.P.; Llorens-Cortes, C. Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: A potential treatment of salt-dependent hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 7775–7780. [Google Scholar] [CrossRef]
- Song, L.; Wilk, S.; Healy, D.P. Aminopeptidase A antiserum inhibits intracerebroventricular angiotensin IIinduced dipsogenic and pressor responses. Brain Res. 1997, 744, 1–6. [Google Scholar] [CrossRef]
- Bodineau, L.; Frugière, A.; Marc, Y.; Inguimbert, N.; Fassot, C.; Balavoine, F.; Roques, B.; Llorens-Cortes, C. Orally active aminopeptidase A inhibitors reduce blood pressure: A new strategy for treating hypertension. Hypertension 2008, 51, 1318–1325. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Brain renin-angiotensin-A new look at an old system. Prog. Neurobiol. 2011, 95, 49–67. [Google Scholar] [CrossRef]
- Goto, Y.; Hattori, A.; Ishii, Y.; Tsujimoto, M. Reduced activity of the hypertension-associated Lys528Arg mutant of human adipocyte-derived leucine aminopeptidase (ALAP)/ ER-aminopeptidase-1. FEBS Lett. 2006, 580, 1833–1838. [Google Scholar] [CrossRef]
- Ishii, M.; Hattori, A.; Numaguchi, Y.; Tsujimoto, M.; Ishiura, S.; Kobayashi, H.; Murohara, T.; Wrght, J.W.; Mizutani, S. The effect of recombinant aminopeptidase a on hypertension in spontaneously hypertensive rats: Its effect in comparison with candesartan. Horm. Metab. Res. 2008, 40, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Okano, K.; Hasegawa, E. Human placental leucine aminopeptidase (P-LAP) as a hypotensive agent. Experientia 1982, 38, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohno, Y.; Itakura, A.; Takeuchi, M.; Murata, Y.; Kuno, N.; Mizutani, S. Possible involvement of aminopeptidase A in hypertension in spontaneously hypertensive rats (SHRs) and change of refractoriness in response to angiotensin II in pregnant SHRs. J. Hypertens. 2002, 20, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Wright, J.; Kobayashi, H. A new approach regarding the treatment of preeclampsia and preterm labor. Life Sci. 2011, 88, 17–23. [Google Scholar] [CrossRef]
- Kobayashi, H.; Mizutani, S.; Wright, J.W. Placental leucine aminopeptidase- and aminopeptidase A-deficient mice offer insight concerning the mechanisms underlying preterm labor and preeclampsia. J. Biomed. Biotechnol. 2011, 2011, 286947. [Google Scholar]
- Carey, R.M.; Padia, S.H. Role of angiotensin at2 receptors in natriuresis: Intrarenal mechanisms and therapeutic potential. Clin. Exp. Pharmacol. Physiol. 2013, 40, 527–534. [Google Scholar] [CrossRef]
- Padia, S.H.; Howell, N.L.; Kemp, B.A.; Fournie-Zaluski, M.C.; Roques, B.P.; Carey, R.M. Intrarenal aminopeptidase N inhibition restores defective angiontesin II type 2-mediated natriuresis in spontaneously hypertensive rats. Hypertension 2010, 55, 474–480. [Google Scholar] [CrossRef]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Carey, R.M. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension 2009, 53, 338–343. [Google Scholar] [CrossRef][Green Version]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Vaidya, V.S.; Schmouder, R.; Feig, P.; Dieterle, F. Nextgeneration biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010, 28, 436–440. [Google Scholar] [CrossRef]
- Devarajan, P. Emerging biomarkers of acute kidney injury. Contrib. Nephrol. 2007, 156, 203–212. [Google Scholar] [PubMed]
- Lisowska-Myjak, B. Serum and urinary biomarker of acute kidney injury. Blood. Purif. 2010, 29, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Holdt, B.; Peters, E.; Nagel, H.R.; Steiner, M. An automated assay of urinary alanine aminopeptidase activity. Clin. Chem. Lab. Med. 2008, 46, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ye, M.; Troyanovskaya, M.; Wilk, E.; Wilk, S.; Healy, D.P. Rat kidney glutamyl aminopeptidase (aminopeptidase A): Molecular identity and cellular localization. Am. J. Physiol. 1994, 267, F546–F557. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Ramírez, M.; Banegas, I.; Hermoso, F.; Vargas, F.; Vives, F.; Alba, F.; de Gasparo, M.; Prieto, I. Influence of thyroid disorders on kidney angiotensinase activity. Horm. Metab. Res. 2006, 38, 48–52. [Google Scholar] [CrossRef]
- Peters, J.E.; Mampel, E.; Schneider, I.; Burchardt, U.; Fukala, E.; Ahrens, I.; Haschen, R.J. Alanine aminopeptidase in urine in renal diseases. Clin. Chim. Acta 1972, 37, 213–224. [Google Scholar] [CrossRef]
- Marchewka, Z.; Długosz, A.; Kúzniar, J. Diagnostic application of AAP isoenzyme separation. Int. Urol. Nephrol. 1999, 31, 409–416. [Google Scholar] [CrossRef]
- Marchewka, Z.; Kúzniar, J.; Długosz, A. Enzymuria and β2-Mikroglobulinuria in the assessment of the influence of proteinuria on the progression of glomerulopathies. Int. Urol. Nephrol. 2001, 33, 673–676. [Google Scholar] [CrossRef]
- Idasiak-Piechocka, I.; Krzymánski, M. The role of tubulointerstitial changes in progression of chronic glomerulonephritis (GN). Przegl. Lek. 1996, 53, 443–453. [Google Scholar]
- Naghibi, B.; Ghafghazi, T.; Hajhashemi, V.; Talebi, A. Vancomycin-induced nephrotoxicity in rats: Is enzyme elevation a consistent finding in tubular injury? J. Nephrol. 2007, 20, 482–488. [Google Scholar]
- Jung, K.; Hempel, A.; Grutzmann, K.D.; Hempel, R.D.; Schreiber, G. Age-dependent excretion of alanine aminopeptydase, alkaline phosphatase, γ -glutamyltransferase and N-acetyl-β-D-glucosaaminidase in human urine. Enzyme 1990, 43, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Inselmann, G.; Balaschke, M.; Heidemann, H.T. Enzymuria following amphotericin B application in the rat. Mycoses 2003, 46, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Mitic, B.; Lazarevic, G.; Vlahovic, P.; Rajic, M.; Stefanovic, V. Diagnostic value of the aminopeptidase N, N-acetyl-beta-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren. Fail. 2008, 30, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, C.D.; Park, S.H.; Hwang, D.; et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2011, 11, 2459–2475. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, G.; Antic, S.; Vlahovic, P.; Djordjevic, V.; Zvezdanovic, L.; Stefanovic, V. Effects of aerobic exercise on microalbuminuria and enzymuria in type 2 diabetic patients. Ren. Fail. 2007, 29, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kuzniar, J.; Marchewka, Z.; Krasnowski, R.; Boratynska, M.; Długosz, A.; Klinger, M. Enzymuria and low molecular weight protein excretion as the differentiating marker of complications in the early post kidney transplantation period. Int. Urol. Nephrol. 2006, 38, 753–758. [Google Scholar] [CrossRef]
- Marchewka, Z.; Kuzniar, J.; Zynek-Litwin, M.; Falkiewicz, K.; Szymanska, B.; Roszkowska, A.; Klinger, M. Kidney graft function in long-term cyclosporine and tacrolimus treatment: Comparative study with nephrotoxicity markers. Transplant. Proc. 2009, 41, 1660–1665. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Levin, A.; Warnock, D.G.; Joannidis, M.; Mehta, R.L.; Kellum, J.A.; Ronco, C.; Shah, S. Improving outcomes from acute kidney injury. J. Am. Soc. Nephrol. 2007, 18, 1992–1994. [Google Scholar] [CrossRef]
- Safirstein, R.; Winston, J.; Moel, D.; Dikman, S.; Guttenplan, J. Cisplatin nephrotoxicity-insights into mechanism. Int. J. Androl. 1987, 10, 325–346. [Google Scholar] [CrossRef]
- Winston, J.A.; Safirstein, R. Reduced renal blood-flow in early cisplatin induced acute renal failure in the rat. Am. J. Physiol. 1985, 249, F490–F496. [Google Scholar] [CrossRef]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Vargas, F.; Montoro-Molina, S.; O’Valle, F.; Rodríguez-Martínez, M.D.; Osuna, A.; Prieto, I.; Ramírez, M.; Wangensteen, F. Urinary Aminopeptidase Activities as Early and Predictive Biomarkers of Renal Dysfunction in Cisplatin-Treated Rats. PLoS ONE 2012, 7, e40402. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Molina, S.; Quesada, A.; Zafra-Ruiz, P.V.; O’Valle, F.; Vargas, F.; de Gracia, M.C.; Osuna, A.; Wangensteen, F. Immunological detection of glutamyl aminopeptidase in urine samples from cisplatin-treated rats. Proteom. Clin. Appl. 2015, 9, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol. Ren. Physiol. 2014, 306, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Lozano-Ramos, S.I.; Bancu, I.; Lauzurica-Valdemoros, R.; Borras, F.E. Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front. Inmunol. 2015, 6, 6. [Google Scholar]
- Pisitkun, T.; Johnstone, R.; Knepper, M.A. Discovery of urinary biomarkers. Mol. Cell. Proteom. 2006, 5, 1760–1771. [Google Scholar] [CrossRef]
- Ohno, S.; Ishikawa, A.; Kuroda, M. Roles of exosomes and microvesicles in disease pathogenesis. Adv. Drug. Deliv. Rev. 2013, 65, 398–401. [Google Scholar] [CrossRef]
- Dimov, I.; Jankovic, V.L.; Stefanovic, V. Urinary exosomes. Sci. World J. 2009, 9, 1107–1118. [Google Scholar] [CrossRef][Green Version]
- Jayachandran, M.; Lugo, G.; Heiling, H.; Miller, V.M.; Rule, A.D.; Lieske, J.C. Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: A case control study. Biol. Sex. Differ. 2015, 6, 2. [Google Scholar] [CrossRef]
- Lv, L.L.; Cao, Y.; Liu, D.; Xu, M.; Liu, H.; Tang, R.N.; Ma, K.L.; Liu, B.C. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int. J. Biol. Sci. 2013, 9, 1021–1031. [Google Scholar] [CrossRef]
- Murakami, T.; Oakes, M.; Ogura, M.; Tovar, V.; Yamamoto, C.; Mitsuhashi, M. Development of glomerulus-, tubule-, and collecting duct-specific mRNA assay in human urinary exosomes and microvesicles. PLoS ONE 2014, 2, e109074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Álvarez, S.; Suazo, C.; Boltansky, A.; Ursu, M.; Carvajal, D.; Innocenti, G.; Vukusich, A.; Hurtado, M.; Villanueva, S.; Carreño, J.E. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 2013, 45, 3719–3723. [Google Scholar] [CrossRef] [PubMed]
- Dear, J.W.; Street, J.M.; Bailey, M.A. Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 2013, 13, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Segarra, A.B.; Montoro-Molina, S.; de Gracia, M.D.C.; Osuna, A.; O’Valle, F.; Gómez-Guzmán, M.; Vargas, F.; Wangensteen, R. Glutamyl aminopeptidase in microvesicular and exosomal fractions of urine is related with renal dysfunction in cisplatin-treated rats. PLoS ONE 2017, 12, e0175462. [Google Scholar] [CrossRef]
- Hultström, M. Development of structural kidney damage in spontaneously hypertensive rats. J. Hypertens. 2012, 30, 1087–1091. [Google Scholar] [CrossRef]
- Kim, N.; Dai, S.Y.; Pang, V.; Mazer, C.D. Vasopressinase Activity: A Potential Early Biomarker for Detecting Cardiopulmonary Bypass-Associated Acute Kidney Injury? Thorac. Cardiovasc. Surg. 2016, 64, 555–560. [Google Scholar] [CrossRef]
- Munshi, R.; Hsu, C.; Himmelfarb, J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011, 9, 11. [Google Scholar] [CrossRef]
- Osuna, A.; de Gracia, M.C.; Quesada, A.; Manzano, F.; Wangensteen, R. Alanil aminopeptidase como marcador temprano del daño renal agudo persistente en pacientes sometidos a cirugía cardíaca. In Proceedings of the XLIX Congreso de la Soc Esp Nefrol, A Coruña, España, 5 October 2019. [Google Scholar]
- de Artinano, A.A.; Castro, M.M. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 2009, 102, 1246–1253. [Google Scholar] [CrossRef]
- Montoro-Molina, S.; López-Carmona, A.; Quesada, A.; O’Valle, F.; Martín-Morales, N.; Osuna, A.; Vargas, F.; Wangensteen, R. Klotho and Aminopeptidases as Early Biomarkers of Renal Injury in Zucker Obese Rats. Front. Physiol. 2018, 9, 1599. [Google Scholar] [CrossRef]
- Llorens, S.; Fernandez, A.P.; Nava, E. Cardiovascular and renal alterations on the nitric oxide path way in spontaneous hypertension and ageing. Clin. Hemorheol. Microcirc. 2007, 37, 149–156. [Google Scholar]
- Sun, Z.J.; Zhang, Z.E. Historic perspectives and recent advances in major animal models of hypertension. Acta Pharmacol. Sin. 2005, 26, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Molina, S.; Quesada, A.; O’Valle, F.; Osuna, A.; de Gracia, M.C.; Vargas, F.; Wangensteen, R. Urinary aminopeptidase activities in spontaneously hypertensive rats. In Proceedings of the Physiology 2016, Dublin, Ireland, 39–31 July 2016. PCA320. [Google Scholar]
- Vargas, F.; Moreno, J.M.; Rodríguez-Gómez, I.; Wangensteen, R.; Osuna, A.; Alvarez-Guerra, M.; García-Estañ, J. Vascular and renal function in experimental thyroid disorders. Eur. J. Endocrinol. 2006, 154, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ishiyama, M.; Kosaka, J.; Mutoh, J.; Umemura, N.; Harase, C. Urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in patients with Graves’ disease, subacute thyroiditis, and silent thyroiditis: A longitudinal study. Endocrinol. Jpn. 1991, 38, 303–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Hoek, I.; Meyer, E.; Duchateau, L.; Peremans, K.; Smets, P.; Daminet, S. Retinol-binding protein in serum and urine of hyperthyroid cats before and after treatment with radioiodine. J. Vet. Intern. Med. 2009, 23, 1031–1037. [Google Scholar] [CrossRef]
- Pérez-Abud, R.; Rodríguez-Gómez, I.; Villarejo, A.B.; Moreno, J.M.; Wangensteen, R.; Tassi, M.; O’Valle, F.; Osuna, A.; Vargas, F. Salt sensitivity in experimental thyroid disorders in rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E281–E287. [Google Scholar] [CrossRef][Green Version]
- Conger, J.D.; Falk, S.A.; Gillum, D.M. The protective mechanism of thyroidectomy in a rat model of chronic renal failure. Am. J. Kidney Dis. 1989, 13, 217–225. [Google Scholar] [CrossRef]
- Wangensteen, R.; Segarra, A.B.; Ramirez-Sanchez, M.; Gasparo, M.D.; Dominguez, G.; Banegas, I.; Vargas, F.; Vives, F.; Prieto, I. Influence of thyroid disorders on the kidney expression and plasma activity of aminopeptidase A. Endocr. Regul. 2015, 49, 68–72. [Google Scholar] [CrossRef]
- Jung, K.; Pergande, M.; Schimie, E.; Ratzmann, K.P.; Illus, A. Urinary enzymes and low-molecular-mass proteins as indicator of diabetes nephropathy. Clin. Chem. 1988, 34, 544–547. [Google Scholar] [CrossRef]
- Sorokman, T.; Sokolnyk, S.; Popelyuk, O.; Makarova, O.; Kopchuk, T. Biomarkers of renal injury risk in children with pyelonephritis. Georgian Med. News 2018, 280–281, 98–103. [Google Scholar]
- Spasovski, D.; Masin-Spasovska, J.; Nada, M.; Calovski, J.; Sandevska, E.; Osmani, B.; Sotirova, T.; Balkanov, S.; Dukovski, D.; Ljatifi, A.; et al. Diagnostic value of brush border enzymes of the proximal renal tubules in rheumatoid arthritis. Clin. Lab. 2011, 57, 305–314. [Google Scholar]



| Enzyme | EC Number | Abbreviations |
|---|---|---|
| Leucyl AP | 3.4.11.1 | LAP |
| Membrane alanyl AP | 3.4.11.2 | APN, AlaAP |
| Cystinyl AP | 3.4.11.3 | CysAP, CAP |
| Prolyl AP | 3.4.11.5 | PIP |
| Aminopeptidase B | 3.4.11.6 | APB, ArgAP |
| Glutamyl AP | 3.4.11.7 | APA, GluAP, EAP |
| Aminopeptidase P | 3.4.11.9 | APP |
| Cytosol alanyl AP | 3.4.11.14 | AAP, AlaAP |
| Methionyl AP | 3.4.11.18 | eMetAP |
| Aspartyl AP | 3.4.11.21 | AspAP, DNPEP |
| Arginyl AP | 3.4.22.16 | iRAP, APR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, F.; Wangesteen, R.; Rodríguez-Gómez, I.; García-Estañ, J. Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers. Int. J. Mol. Sci. 2020, 21, 5615. https://doi.org/10.3390/ijms21165615
Vargas F, Wangesteen R, Rodríguez-Gómez I, García-Estañ J. Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers. International Journal of Molecular Sciences. 2020; 21(16):5615. https://doi.org/10.3390/ijms21165615
Chicago/Turabian StyleVargas, Félix, Rosemary Wangesteen, Isabel Rodríguez-Gómez, and Joaquín García-Estañ. 2020. "Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers" International Journal of Molecular Sciences 21, no. 16: 5615. https://doi.org/10.3390/ijms21165615
APA StyleVargas, F., Wangesteen, R., Rodríguez-Gómez, I., & García-Estañ, J. (2020). Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers. International Journal of Molecular Sciences, 21(16), 5615. https://doi.org/10.3390/ijms21165615









