Roles of Organellar RNA-Binding Proteins in Plant Growth, Development, and Abiotic Stress Responses
Abstract
1. Introduction
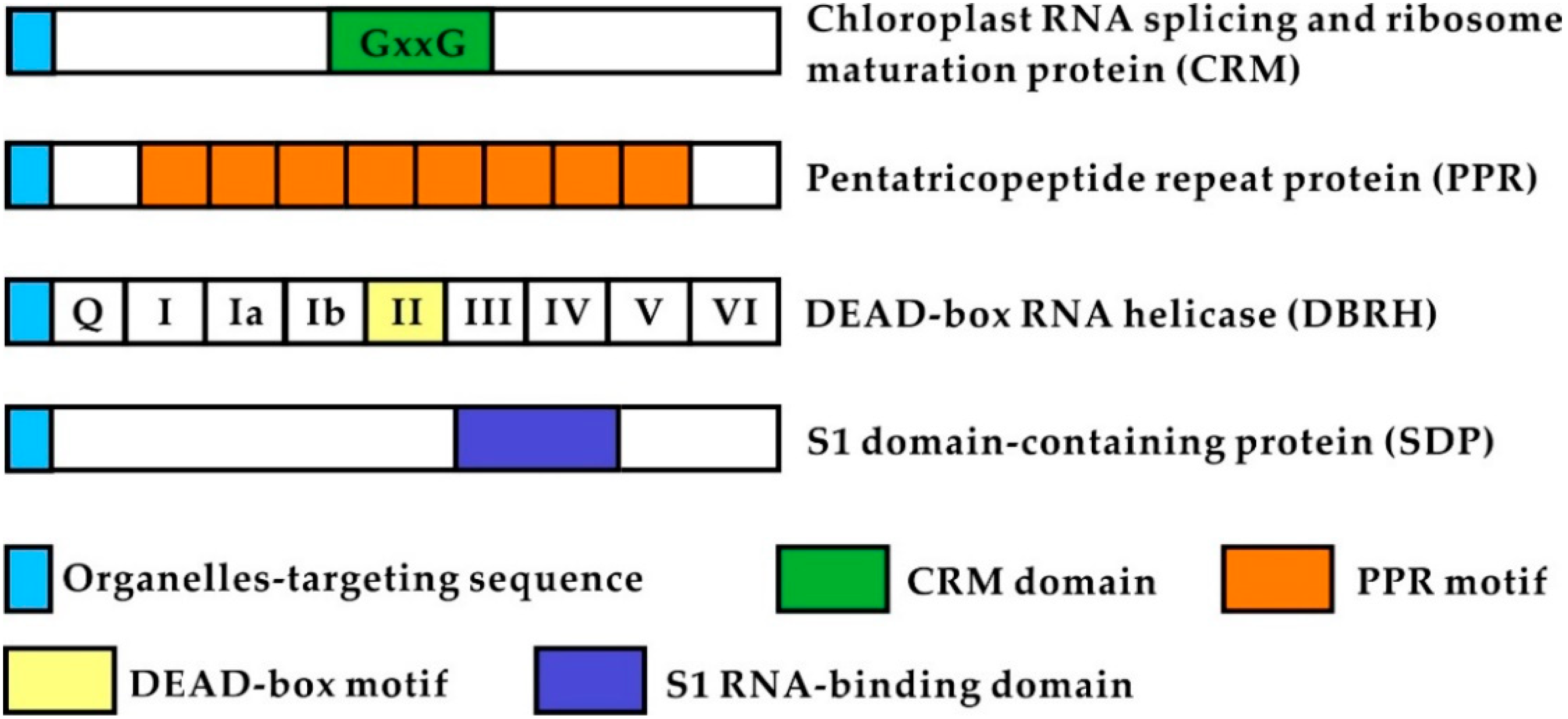
2. Domain or Motif Features of CRM, PPR, DBRH, and SDP Proteins
2.1. CRM Proteins
2.2. PPR Proteins
2.3. DEAD-Box RH Proteins (DBRH)
2.4. SDP Proteins
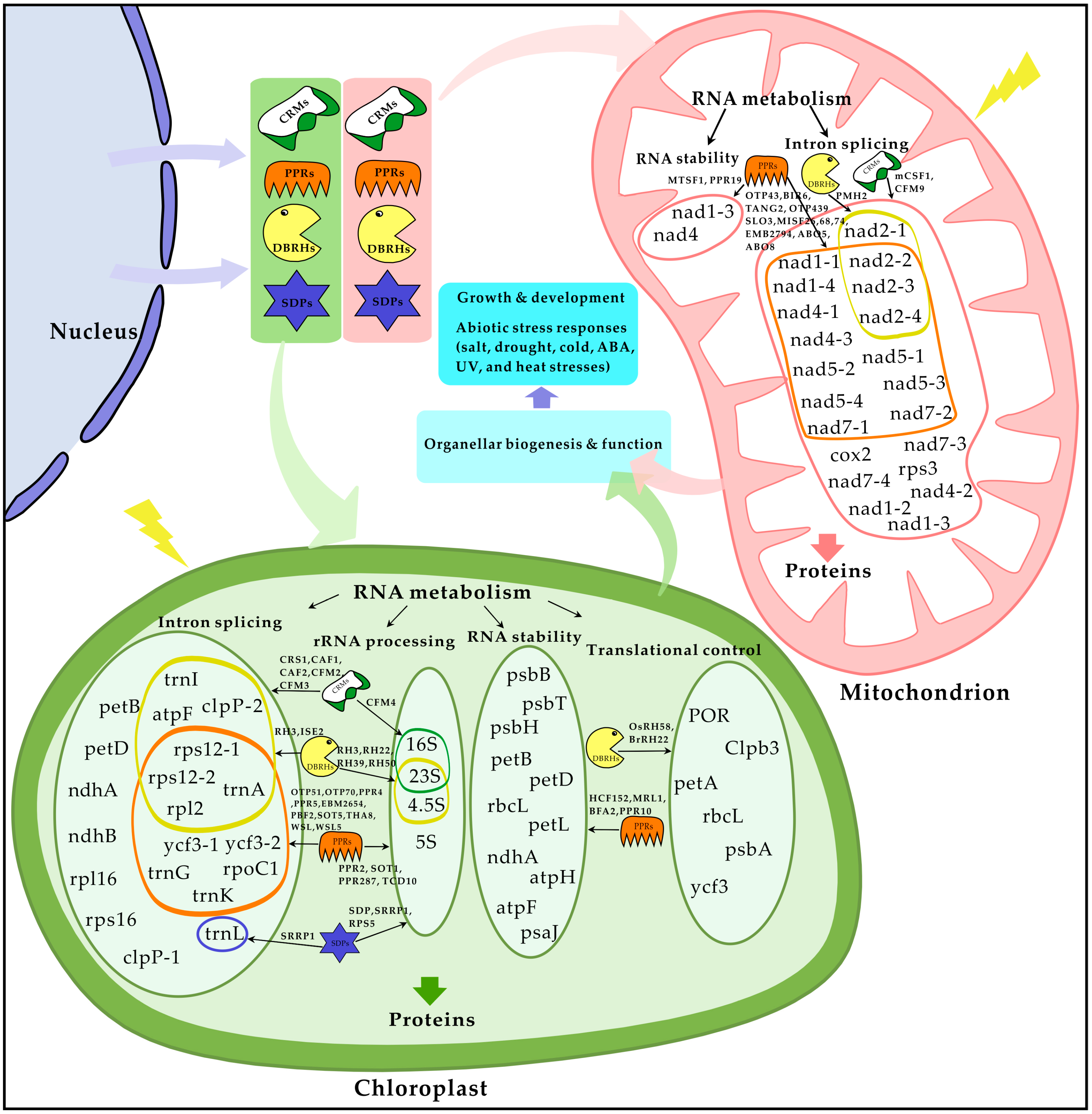
3. Functions of nCMRBPs in Plant Growth and Development
| Plant | Gene Name | Gene Number | Location | Molecular Function | Mutant Phenotype | Ref. |
|---|---|---|---|---|---|---|
| A. thaliana | CRM family | |||||
| AtCRS1 | At5g16180 | C | Splicing of group II intron (atpF) | Small and albino seedling | [28,56] | |
| AtCAF1 | At2g20020 | C | Splicing of group II introns (petD, rpl16, rps16, ndhA, rpoC1, ycf3-1, clpP-1, and trnG) | Albino seedling | [56] | |
| AtCAF2 | At1g23400 | C | Splicing of group II introns (ndhA, ndhB, petB, ycf3-1, and rps12-1) | Small and pale green seedling | [56] | |
| AtCFM2 | At3g01370 | C | Splicing of group I (trnL) and group II introns (ndhA, ycf3-1, and clpP-2) | Small and albino seedling | [28] | |
| AtCFM3a | At3g23070 | C/M | Splicing of group II intron (ndhB) | Stunted growth | [57] | |
| CFM4 | At4g39040 | C | 16S and 23S rRNA processing | Retarded growth | [24] | |
| mCSF1 | At4g31010 | M | Splicing of multiple mitochondrial introns | Embryo lethal Retarded growth | [60] | |
| CFM9 | At3g27550 | M | Splicing of multiple mitochondrial introns | Retarded growth | [61] | |
| O. sativa | OsCAF1 | Os01g 0495900 | C | Splicing of group II introns (atpF, rpl2, rps12, ndhA, ndhB, and ycf3) | Albino seedling | [58] |
| OsCFM2 | Os04g 0464800 | C | Splicing of group I (trnL) and group II introns (atpF, rpl2, rps12, ndhA, and ycf3-1) | Albino seedling | [59] | |
| OsCFM3 | Os11g 37990 | C | Splicing of group II introns (ndhB, petD, rpl16, rps16, trnG, and petB) | Albino seedling | [57] | |
| A. thaliana | PPR family | |||||
| OTP51 | At2g15820 | C | Splicing of ycf3 intron2 | Pale yellow seedling | [64] | |
| OTP70 | At4g25270 | C | Splicing of rpoC1 intron | Virescent seedling | [65] | |
| AtPPR4 | At5g04810 | C | Trans-splicing of rps12 intron1 | Embryo lethal, pale green, or albino seedling | [25] | |
| EMB2654 | At2g41720 | C | Trans-splicing of rps12 intron1 | Embryo lethal, pale green, or albino seedling | [66] | |
| PBF2 | At3g42630 | C | Splicing of ycf3 intron1 | Small and pale yellowish seedling | [67] | |
| SOT5/EMB2279 | At1g30610 | C | Splicing of rpl2 and trnK intron | Virescent seedling | [68] | |
| HCF152 | At3g09660 | C | Stabilization or processing of psbB-psbT-psbH-petB-petD | High chlorophyll fluorescence | [69] | |
| MRL1 | At4g34830 | C | Stabilization of rbcL | Pale green seedling | [70] | |
| PGR3 | At4g31850 | C | Stabilization of petL and probably ndhA | High chlorophyll fluorescence | [71] | |
| BFA2 | At4g30825 | C | Stabilization of atpH/F | Stunted growth | [72] | |
| AtPPR2 | At3g06430 | C | Chloroplast 23S rRNA processing | Embryo lethal or albino seedling | [75] | |
| SOT1 | At5g46580 | C | Chloroplast 23S-4.5 rRNA processing | Small and pale green seedling | [76] | |
| PPR287 | At4g59040 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Yellowish seedling | [77] | |
| OTP43 | At1g74900 | M | Trans-splicing of nad1 intron1 | Small and delayed development | [78] | |
| BIR6 | At3g48250 | M | Splicing of nad7 intron1 | Small and retarded growth | [79] | |
| TANG2 OTP439 | At1g19290 At3g48810 | M | Splicing of nad5 intron2 and 3 | Retarded growth | [80] | |
| SLO3 | At3g61360 | M | Splicing of nad7 intron2 | Delayed growth and development | [81] | |
| MISF26 MISF68 MISF74 | At1g66345 At3g16010 At4g01400 | M | Splicing of nad2 intron3 (MISF26) Splicing of nad2 intron2, nad4 intron1, and nad5 intron4 (MISF68) Splicing of nad1 intron4 and nad2 intron4 (MISF74) | Delayed growth | [82] | |
| EMB2794 | At2g02150 | M | Trans-splicing of nad2 intron2 | Retarded growth and developmental defect | [83] | |
| MTSF1 | At1g06710 | M | Stabilization of nad4 | Retarded growth | [84] | |
| PPR19 | At1g52620 | M | Stabilization of nad1 intron3 | Retarded growth and developmental defect | [85] | |
| Z. mays | PPR4 | Zm00001d026654 | C | Trans-splicing of rps12 intron1 | Seedling lethal pale green, or albino seedling | [62] |
| THA8 | GRMZM2G466032 | C | Splicing of ycf3 intron2 and trnA intron | Pale green seedling | [63] | |
| ZmPPR5 | GRMZM2G025409 | C | Splicing of trnG intron | Seedling lethal or pale green seedling | [73] | |
| PPR10 | GRMZM2G177169 | C | Stabilization of atpH and psaJ | Seedling lethal or yellowish green seedling | [74] | |
| A. thaliana | DBRH family | |||||
| RH3 | At5g26742 | C | Splicing of group II introns (trnI, trnA, rps12-1, rps12-2, and rpl2) and chloroplast 23S rRNA processing | Embryo lethal or pale green seedling | [88] | |
| ISE2 | At1g70070 | C | Splicing of group II introns (rpl2, atpF, rps12, and clpP) | Chlorotic seedling | [92] | |
| PMH2 | At3g22330 | M | Splicing of nad2 introns | Similar to wild-type | [93] | |
| RH22 | At1g59990 | C | Chloroplast 23S-4.5S rRNA processing | Embryo lethal or virescent seedling | [94] | |
| RH39 | At4g09730 | C | Chloroplast 23S rRNA processing | Retarded growth | [95] | |
| RH50 | At3g06980 | C | Chloroplast 23S-4.5S rRNA maturation | Similar to wild-type | [89] | |
| A. thaliana | SDP family | |||||
| SDP | At1g12800 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Pale green seedling | [21] | |
| RLSB | At1g71720 | C | Regulation of rbcL mRNA | Reduced seedling size | [96] | |
| N. benthamiana | STF | HM012811 | C | Regulation of plastid transcription | Yellowish leaves | [97] |
4. Physiological Functions of nCMRBPs in Abiotic Stress Responses
| Plant | Gene Name | Gene Number | Location | Molecular Function | Mutant Phenotype | Ref. |
|---|---|---|---|---|---|---|
| A. thaliana | CRM family | |||||
| CFM9 | At3g27550 | M | Splicing of multiple mitochondrial introns | Sensitive to salt, drought, or ABA | [61] | |
| CFM4 | At4g39040 | C | 16S and 23S rRNA processing | Sensitive to salt or cold stress | [24] | |
| A. thaliana | PPR family | |||||
| ABO5 | At1g51965 | M | Splicing of nad2 intron3 | Sensitive to ABA | [111] | |
| PPR40 | At3g16890 | M | Sensitive to salt, ABA, or oxidative stress Tolerant to salt stress in overexpression plants | [108,109] | ||
| GUN1 | At2g31400 | C | Sensitive to sucrose or ABA | [103] | ||
| ABO8 | At4g11690 | M | Splicing of nad4 intron3 | Sensitive to ABA | [112] | |
| PPR96 | At2g03380 | M | Probably mitochondrial RNA editing | Tolerant to salt, ABA, or oxidative stress | [113] | |
| PGN | At1g56570 | M | Regulation of NAD1, RPL2, NAD9, and MATR genes | Sensitive to salt, glucose, or ABA | [110] | |
| O. sativa | OsV4 | Os04g39970 | C | Plastid gene expression associated with plastid translation machinery | Sensitive to cold stress | [105] |
| WSL | Os01g37870 | C | Splicing of chloroplast rpl2 intron | Sensitive to salt, sucrose, or ABA | [104] | |
| TCD10 | Os10g28600 | C | Regulation of OsV4, OsRpoTp, V1, V2, RNRL, RNRS, 16S rRNA, rpl21, and OsDG2 genes | Sensitive to cold stress | [106] | |
| WSL5 | Os04g0684500 | C | RNA editing of rpl2 and atpA, and splicing of rpl2 and rps12 intron2 | Sensitive to cold stress | [107] | |
| A. thaliana | DBRH family | |||||
| RH3 | At5g26742 | C | Splicing of ndhA and ndhB introns | Sensitive to salt or cold stress | [26] | |
| O. sativa | TCD33 | Os03g01830 | C | Probably chloroplast ribosome assembly | Sensitive to cold stress | [91] |
| OsRH58 | Os01g73900 | C | Translational control of chloroplast POR, rbcL, Clpb3, PsbA, and PetA transcripts | Tolerant to salt or drought stress | [90] | |
| B. rapa | BrRH22 | Bra035413 | C | Translational control of chloroplast rbcL, psbA, and ycf3 transcripts | Tolerant to salt or drought stress | [114] |
| A. thaliana | SDP family | |||||
| SRRP1 | At3g23700 | C | Splicing of chloroplast trnL intron and 5S rRNA processing | Sensitive to ABA | [115] | |
| RPS5 | At2g33800 | C | Chloroplast 16S rRNA processing | Tolerant to cold stress in overexpression plants | [116] | |
| SDP | At1g12800 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Sensitive to UV, salt, heat, or freezing stress | [117] |
5. Cellular Roles of nCMRBPs in Organellar RNA Metabolism
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersson, S.G.E.; Karlberg, O.; Canback, B.; Kurland, C.G. On the origin of mitochondria: A genomics perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 165–177. [Google Scholar] [CrossRef]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kleine, T.; Maier, U.G.; Leister, D. DNA transfer from organelles to the nucleus: The idiosyncratic genetics of endosymbiosis. Annu. Rev. Plant Biol. 2009, 60, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Maier, U.G.; Zauner, S.; Woehle, C.; Bolte, K.; Hempel, F.; Allen, J.F.; Martin, W.F. Massively convergent evolution for ribosomal protein gene content in plastid and mitochondrial genomes. Genome Biol. Evol. 2013, 5, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Liere, K.; Weihe, A.; Borner, T. The transcription machineries of plant mitochondria and chloroplasts: Composition, function, and regulation. J. Plant Physiol. 2011, 168, 1345–1360. [Google Scholar] [CrossRef]
- Barkan, A. Expression of plastid genes: Organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011, 155, 1520–1532. [Google Scholar] [CrossRef]
- Millar, A.H.; Heazlewood, J.L.; Kristensen, B.K.; Braun, H.P.; Moller, I.M. The plant mitochondrial proteome. Trends Plant Sci. 2005, 10, 36–43. [Google Scholar] [CrossRef]
- Millar, A.H.; Whelan, J.; Small, I. Recent surprises in protein targeting to mitochondria and plastids. Curr. Opin. Plant Biol. 2006, 9, 610–615. [Google Scholar] [CrossRef]
- Kleine, T.; Leister, D. Retrograde signaling: Organelles go networking. Biochim. Biophy. Acta 2016, 1857, 1313–1325. [Google Scholar] [CrossRef]
- Del Campo, E.M. Post-transcriptional control of chloroplast gene expression. Gene Regul. Syst. Biol. 2009, 3, 31. [Google Scholar] [CrossRef]
- Stern, D.B.; Goldschmidt-Clermont, M.; Hanson, M.R. Chloroplast RNA metabolism. Annu. Rev. Plant Biol. 2010, 61, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Hammani, K.; Giege, P. RNA metabolism in plant mitochondria. Trends Plant Sci. 2014, 19, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Lorkovic, Z.J. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 2009, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Quesada, V. The roles of mitochondrial transcription termination factors (MTERFs) in plants. Physiol. Plant 2016, 157, 389–399. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.J.; Kwak, K.J. Plant RNA chaperones in stress response. Trends Plant Sci. 2013, 18, 100–106. [Google Scholar] [CrossRef]
- Lee, K.; Kang, H. Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol. Cells 2016, 39, 179–185. [Google Scholar]
- Robles, P.; Quesada, V. Transcriptional and post-transcriptional regulation of organellar gene expression (OGE) and its roles in plant salt tolerance. Int. J. Mol. Sci. 2019, 20, 1056. [Google Scholar] [CrossRef]
- Leister, D.; Wang, L.; Kleine, T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017, 8, 387. [Google Scholar] [CrossRef]
- Nawaz, G.; Kang, H. Chloroplast- or mitochondria-targeted DEAD-box RNA helicases play essential roles in organellar RNA metabolism and abiotic stress responses. Front. Plant Sci. 2017, 8, 871. [Google Scholar] [CrossRef]
- Barkan, A.; Klipcan, L.; Ostersetzer, O.; Kawamura, T.; Asakura, Y.; Watkins, K.P. The CRM domain: An RNA binding module derived from an ancient ribosome-associated protein. RNA 2007, 13, 55–64. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, K.; Lee, K.H.; Jung, S.; Jeon, Y.; Pai, H.S.; Kang, H. A nuclear-encoded chloroplast-targeted S1 RNA-binding domain protein affects chloroplast rRNA processing and is crucial for the normal growth of Arabidopsis thaliana. Plant J. 2015, 83, 277–289. [Google Scholar] [CrossRef]
- Shi, X.; Bentolila, S.; Hanson, M.R. Organelle RNA recognition motif-containing (ORRM) proteins are plastid and mitochondrial editing factors in Arabidopsis. Plant Signal. Behav. 2016, 11, e1167299. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.J.; Kim, D.H.; Jeon, Y.; Pai, H.S.; Kang, H. A nuclear-encoded chloroplast protein harboring a single CRM domain plays an important role in the Arabidopsis growth and stress response. BMC Plant Biol. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, S.J.; Colas des Francs-Small, C.; Whitby, M.; Small, I.; Kang, H. The coordinated action of PPR4 and EMB2654 on each intron half mediates trans-splicing of rps12 transcripts in plant chloroplasts. Plant J. 2019, 100, 1193–1207. [Google Scholar] [CrossRef]
- Gu, L.; Xu, T.; Lee, K.; Lee, K.H.; Kang, H. A chloroplast-localized DEAD-box RNA helicase AtRH3 is essential for intron splicing and plays an important role in the growth and stress response in Arabidopsis thaliana. Plant Physiol. Biochem. 2014, 82, 309–318. [Google Scholar] [CrossRef]
- Xu, T.; Lee, K.; Gu, L.; Kim, J.I.; Kang, H. Functional characterization of a plastid-specific ribosomal protein PSRP2 in Arabidopsis thaliana under abiotic stress conditions. Plant Physiol. Biochem. 2013, 73, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y.; Barkan, A. A CRM domain protein functions dually in group I and group II intron splicing in land plant chloroplasts. Plant Cell 2007, 19, 3864–3875. [Google Scholar] [CrossRef]
- Jacobs, J.; Kuck, U. Function of chloroplast RNA-binding proteins. Cell. Mol. Life Sci. 2011, 68, 735–748. [Google Scholar] [CrossRef]
- Ostheimer, G.J.; Barkan, A.; Matthews, B.W. Crystal structure of E. coli YhbY: A representative of a novel class of RNA binding proteins. Structure 2002, 10, 1593–1601. [Google Scholar] [CrossRef]
- Keren, I.; Klipcan, L.; Bezawork-Geleta, A.; Kolton, M.; Shaya, F.; Ostersetzer-Biran, O. Characterization of the molecular basis of group II intron RNA recognition by CRS1-CRM domains. J. Biol. Chem. 2008, 283, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Peeters, N. The PPR motif–a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000, 25, 45–47. [Google Scholar] [CrossRef]
- Schmitz-Linneweber, C.; Small, I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 2008, 13, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Gutmann, B.; Zhong, X.; Ye, Y.; Fisher, M.F.; Bai, F.; Castleden, I.; Song, Y.; Song, B.; Huang, J.; et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016, 85, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Schallenberg-Rudinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V. Helicases: Amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993, 3, 419–429. [Google Scholar] [CrossRef]
- Bird, L.E.; Subramanya, H.S.; Wigley, D.B. Helicases: A unifying structural theme? Curr. Opin. Struct. Biol. 1998, 8, 14–18. [Google Scholar] [CrossRef]
- Singleton, M.R.; Dillingham, M.S.; Wigley, D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef]
- Cordin, O.; Banroques, J.; Tanner, N.K.; Linder, P. The DEAD-box protein family of RNA helicases. Gene 2006, 367, 17–37. [Google Scholar] [CrossRef]
- De la Cruz, J.; Kressler, D.; Linder, P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem. Sci. 1999, 24, 192–198. [Google Scholar] [CrossRef]
- Rocak, S.; Linder, P. Dead-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.; Jankowsky, E. From unwinding to clamping—the DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 2011, 12, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.K.; Linder, P. DExD/H box RNA helicases: From generic motors to specific dissociation functions. Mol. Cell 2001, 8, 251–262. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; Russell, R. RNA helicase proteins as chaperones and remodelers. Annu. Rev. Biochem. 2014, 83, 697–725. [Google Scholar] [CrossRef]
- Subramanian, A.R. Structure and functions of ribosomal protein S1. Prog. Nucleic Acid Res. Mol. Biol. 1983, 28, 101–142. [Google Scholar]
- Bycroft, M.; Hubbard, T.J.; Proctor, M.; Freund, S.M.; Murzin, A.G. The solution structure of the S1 RNA binding domain: A member of an ancient nucleic acid–binding fold. Cell 1997, 88, 235–242. [Google Scholar] [CrossRef]
- Deryusheva, E.I.; Machulin, A.V.; Matyunin, M.A.; Galzitskaya, O.V. Investigation of the Relationship between the S1 Domain and Its Molecular Functions Derived from Studies of the Tertiary Structure. Molecules 2019, 24, 3681. [Google Scholar] [CrossRef]
- Draper, D.E.; Pratt, C.W.; Von Hippel, P.H. Escherichia coli ribosomal protein S1 has two polynucleotide binding sites. Proc. Natl. Acad. Sci. USA 1977, 74, 4786–4790. [Google Scholar] [CrossRef]
- Jacques, N.; Dreyfus, M. Translation initiation in Escherichia coli: Old and new questions. Mol. Microbiol. 1990, 4, 1063–1067. [Google Scholar] [CrossRef]
- Aliprandi, P.; Sizun, C.; Perez, J.; Mareuil, F.; Caputo, S.; Leroy, J.-L.; Odaert, B.; Laalami, S.; Uzan, M.; Bontems, F. S1 ribosomal protein functions in translation initiation and ribonuclease RegB activation are mediated by similar RNA-protein interactions: An NMR and SAXS analysis. J. Biol. Chem. 2008, 283, 13289–13301. [Google Scholar] [CrossRef]
- Young, C.L.; Karbstein, K. The roles of S1 RNA-binding domains in Rrp5’s interactions with pre-rRNA. RNA 2011, 17, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Regnier, P.; Grunberg-Manago, M.; Portier, C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J. Biol. Chem. 1987, 262, 63–68. [Google Scholar] [PubMed]
- Yehudai-Resheff, S.; Portnoy, V.; Yogev, S.; Adir, N.; Schuster, G. Domain analysis of the chloroplast polynucleotide phosphorylase reveals discrete functions in RNA degradation, polyadenylation, and sequence homology with exosome proteins. Plant Cell 2003, 15, 2003–2019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gribskov, M. Translational initiation factors IF-1 and eIF-2α share an RNA-binding motif with prokaryotic ribosomal protein S1 and polynucleotide phosphorylase. Gene 1992, 119, 107–111. [Google Scholar] [CrossRef]
- Company, M.; Arenas, J.; Abelson, J. Requirement of the RNA helicase-like protein PRP22 for release of messenger RNA from spliceosomes. Nature 1991, 349, 487–493. [Google Scholar] [CrossRef]
- Asakura, Y.; Barkan, A. Arabidopsis orthologs of maize chloroplast splicing factors promote splicing of orthologous and species-specific group II introns. Plant Physiol. 2006, 142, 1656–1663. [Google Scholar] [CrossRef]
- Asakura, Y.; Bayraktar, O.A.; Barkan, A. Two CRM protein subfamilies cooperate in the splicing of group IIB introns in chloroplasts. RNA 2008, 14, 2319–2332. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, L.; Wang, Z.; Hu, G.; Ren, D.; Hu, J.; Zhu, L.; Gao, Z.; Zhang, G.; Guo, L.; et al. OsCAF1, a CRM domain containing protein, influences chloroplast development. Int. J. Mol. Sci. 2019, 20, 4386. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, L.; Ren, D.; Hu, J.; Zhu, L.; Gao, Z.; Zhang, G.; Guo, L.; Zeng, D.; Qian, Q. Characterization of the CRM gene family and elucidating the function of OsCFM2 in rice. Biomolecules 2020, 10, 327. [Google Scholar] [CrossRef]
- Zmudjak, M.; Colas des Francs-Small, C.; Keren, I.; Shaya, F.; Belausov, E.; Small, I.; Ostersetzer-Biran, O. mCSF1, a nucleus-encoded CRM protein required for the processing of many mitochondrial introns, is involved in the biogenesis of respiratory complexes I and IV in Arabidopsis. New Phytol. 2013, 199, 379–394. [Google Scholar] [CrossRef]
- Lee, K.; Park, S.J.; Park, Y.I.; Kang, H. CFM9, a mitochondrial CRM protein, is crucial for mitochondrial intron splicing, mitochondria function and Arabidopsis growth and stress responses. Plant Cell Physiol. 2019, 60, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Linneweber, C.; Williams-Carrier, R.E.; Williams-Voelker, P.M.; Kroeger, T.S.; Vichas, A.; Barkan, A. A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 2006, 18, 2650–2663. [Google Scholar] [CrossRef] [PubMed]
- Khrouchtchova, A.; Monde, R.A.; Barkan, A. A short PPR protein required for the splicing of specific group II introns in angiosperm chloroplasts. RNA 2012, 18, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- de Longevialle, A.F.; Hendrickson, L.; Taylor, N.L.; Delannoy, E.; Lurin, C.; Badger, M.; Millar, A.H.; Small, I. The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J. 2008, 56, 157–168. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.L.; des Francs-Small, C.C.; Delannoy, E.; Kahlau, S.; Tanz, S.K.; de Longevialle, A.F.; Fujii, S.; Small, I. OTP70 is a pentatricopeptide repeat protein of the E subgroup involved in splicing of the plastid transcript rpoC1. Plant J. 2011, 65, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Aryamanesh, N.; Ruwe, H.; Sanglard, L.V.; Eshraghi, L.; Bussell, J.D.; Howell, K.A.; Small, I.; des Francs-Small, C.C. The pentatricopeptide repeat protein EMB2654 is essential for trans-splicing of a chloroplast small ribosomal subunit transcript. Plant Physiol. 2017, 173, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Z.; Zhang, Y.; Zhou, W.; Zhang, A.; Lu, C. Pentatricopeptide repeat protein PHOTOSYSTEM I BIOGENESIS FACTOR2 is required for splicing of ycf3. J. Integr. Plant Biol. 2020, (in press). [CrossRef]
- Huang, W.; Zhu, Y.; Wu, W.; Li, X.; Zhang, D.; Yin, P.; Huang, J. The pentatricopeptide repeat protein SOT5/EMB2279 is required for plastid rpl2 and trnK intron splicing. Plant Physiol. 2018, 177, 684–697. [Google Scholar] [CrossRef]
- Meierhoff, K.; Felder, S.; Nakamura, T.; Bechtold, N.; Schuster, G. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 2003, 15, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Johnson, X.; Wostrikoff, K.; Finazzi, G.; Kuras, R.; Schwarz, C.; Bujaldon, S.; Nickelsen, J.; Stern, D.B.; Wollman, F.A.; Vallon, O. MRL1, a conserved pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in chlamydomonas and Arabidopsis. Plant Cell 2010, 22, 234–248. [Google Scholar] [CrossRef]
- Cai, W.H.; Okuda, K.; Peng, L.W.; Shikanai, T. PROTON GRADIENT REGULATION 3 recognizes multiple targets with limited similarity and mediates translation and RNA stabilization in plastids. Plant J. 2011, 67, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, W.; Che, L.; Rochaix, J.D.; Lu, C.; Li, W.; Peng, L. PPR protein BFA2 is essential for the accumulation of the atpH/F transcript in chloroplasts. Front. Plant Sci. 2019, 10, 446. [Google Scholar] [CrossRef]
- Beick, S.; Schmitz-Linneweber, C.; Williams-Carrier, R.; Jensen, B.; Barkan, A. The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol. Cell Biol. 2008, 28, 5337–5347. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, J.; Bayraktar, O.A.; Prikryl, J.; Barkan, A. Site-specific binding of a PPR protein defines and stabilizes 5’ and 3’ mRNA termini in chloroplasts. EMBO J. 2009, 28, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Q.; Li, C.; Wang, H.; Chen, H.; Berg, H.; Xia, Y.J. AtPPR2, an Arabidopsis pentatricopeptide repeat protein, binds to plastid 23S rRNA and plays an important role in the first mitotic division during gametogenesis and in cell proliferation during embryogenesis. Plant J. 2011, 67, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, S.; Ruwe, H.; Zhang, D.; Melonek, J.; Zhu, Y.; Hu, X.; Gusewski, S.; Yin, P.; Small, I.D.; et al. SOT1, a pentatricopeptide repeat protein with a small MutS-related domain, is required for correct processing of plastid 23S-4.5S rRNA precursors in Arabidopsis thaliana. Plant J. 2016, 85, 607–621. [Google Scholar] [CrossRef]
- Lee, K.; Park, S.J.; Han, J.H.; Jeon, Y.; Pai, H.S.; Kang, H. A chloroplast-targeted pentatricopeptide repeat protein PPR287 is crucial for chloroplast function and Arabidopsis development. BMC Plant Biol. 2019, 19, 244. [Google Scholar] [CrossRef]
- de Longevialle, A.F.; Meyer, E.H.; Andres, C.; Taylor, N.L.; Lurin, C.; Millar, A.H.; Small, I.D. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 intron 1 in Arabidopsis thaliana. Plant Cell 2007, 19, 3256–3265. [Google Scholar] [CrossRef]
- Koprivova, A.; des Francs-Small, C.C.; Calder, G.; Mugford, S.T.; Tanz, S.; Lee, B.R.; Zechmann, B.; Small, I.; Kopriva, S. Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J. Biol. Chem. 2010, 285, 32192–32199. [Google Scholar] [CrossRef]
- Des Francs-Small, C.C.; de Longevialle, A.F.; Li, Y.; Lowe, E.; Tanz, S.K.; Smith, C.; Bevan, M.W.; Small, I. The pentatricopeptide repeat proteins TANG2 and ORGANELLE TRANSCRIPT PROCESSING439 are involved in the splicing of the multipartite nad5 transcript encoding a subunit of mitochondrial complex I. Plant Physiol. 2014, 165, 1409–1416. [Google Scholar] [CrossRef]
- Hsieh, W.Y.; Liao, J.C.; Chang, C.Y.; Harrison, T.; Boucher, C.; Hsieh, M.H. The SLOW GROWTH3 pentatricopeptide repeat protein is required for the splicing of mitochondrial NADH dehydrogenase subunit7 intron 2 in Arabidopsis. Plant Physiol. 2015, 168, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Aube, F.; Quadrado, M.; Dargel-Graffin, C.; Mireau, H. Three new pentatricopeptide repeat proteins facilitate the splicing of mitochondrial transcripts and complex I biogenesis in Arabidopsis. J. Exp. Bot. 2018, 69, 5131–5140. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Cainzos, M.; Shevtsov, S.; Cordoba, J.P.; Sultan, L.D.; Brennicke, A.; Takenaka, M.; Pagnussat, G.; Ostersetzer-Biran, O.; Zabaleta, E. Mitochondrial pentatricopeptide repeat protein, EMB2794, plays a pivotal role in NADH dehydrogenase subunit nad2 mRNA maturation in Arabidopsis thaliana. Plant Cell Physiol. 2020, (in press). [CrossRef] [PubMed]
- Haili, N.; Arnal, N.; Quadrado, M.; Amiar, S.; Tcherkez, G.; Dahan, J.; Briozzo, P.; Colas des Francs-Small, C.; Vrielynck, N.; Mireau, H. The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res. 2013, 41, 6650–6663. [Google Scholar] [CrossRef]
- Lee, K.; Han, J.H.; Park, Y.I.; Colas des Francs-Small, C.; Small, I.; Kang, H. The mitochondrial pentatricopeptide repeat protein PPR19 is involved in the stabilization of NADH dehydrogenase 1 transcripts and is crucial for mitochondrial function and Arabidopsis thaliana development. New Phytol. 2017, 215, 202–216. [Google Scholar] [CrossRef]
- Mingam, A.; Toffano-Nioche, C.; Brunaud, V.; Boudet, N.; Kreis, M.; Lecharny, A. DEAD-box RNA helicases in Arabidopsis thaliana: Establishing a link between quantitative expression, gene structure and evolution of a family of genes. Plant Biotechnol. J. 2004, 2, 401–415. [Google Scholar] [CrossRef]
- Umate, P.; Tuteja, R.; Tuteja, N. Genome-wide analysis of helicase gene family from rice and Arabidopsis: A comparison with yeast and human. Plant Mol. Biol. 2010, 73, 449–465. [Google Scholar] [CrossRef]
- Asakura, Y.; Galarneau, E.; Watkins, K.P.; Barkan, A.; van Wijk, K.J. Chloroplast RH3 DEAD box RNA helicases in maize and Arabidopsis function in splicing of specific group II introns and affect chloroplast ribosome biogenesis. Plant Physiol. 2012, 159, 961–974. [Google Scholar] [CrossRef]
- Paieri, F.; Tadini, L.; Manavski, N.; Kleine, T.; Ferrari, R.; Morandini, P.; Pesaresi, P.; Meurer, J.; Leister, D. The DEAD-box RNA helicase RH50 is a 23S-4.5S rRNA maturation factor that functionally overlaps with the plastid signaling factor GUN1. Plant Physiol. 2018, 176, 634–648. [Google Scholar] [CrossRef]
- Nawaz, G.; Kang, H. Rice OsRH58, a chloroplast DEAD-box RNA helicase, improves salt or drought stress tolerance in Arabidopsis by affecting chloroplast translation. BMC Plant Biol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Xiaomei, W.; Rongrong, K.; Ting, Z.; Yuanyuan, G.; Jianlong, X.; Zhongze, P.; Gangseob, L.; Dongzhi, L.; Yanjun, D. A DEAD-box RNA helicase TCD33 that confers chloroplast development in rice at seedling stage under cold stress. J. Plant Physiol. 2020, 248, 153138. [Google Scholar] [CrossRef] [PubMed]
- Bobik, K.; Fernandez, J.C.; Hardin, S.R.; Ernest, B.; Ganusova, E.E.; Staton, M.E.; Burch-Smith, T.M. The essential chloroplast ribosomal protein uL15c interacts with the chloroplast RNA helicase ISE2 and affects intercellular trafficking through plasmodesmata. New Phytol. 2019, 221, 850–865. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.; Schmidt-Gattung, S.; Binder, S. The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol. Biol. 2010, 72, 459–467. [Google Scholar] [CrossRef]
- Chi, W.; He, B.; Mao, J.; Li, Q.; Ma, J.; Ji, D.; Zou, M.; Zhang, L. The function of RH22, a DEAD RNA helicase, in the biogenesis of the 50S ribosomal subunits of Arabidopsis chloroplasts. Plant Physiol. 2012, 158, 693–707. [Google Scholar] [CrossRef]
- Nishimura, K.; Ashida, H.; Ogawa, T.; Yokota, A. A DEAD box protein is required for formation of a hidden break in Arabidopsis chloroplast 23S rRNA. Plant J. 2010, 63, 766–777. [Google Scholar] [CrossRef]
- Yerramsetty, P.; Stata, M.; Siford, R.; Sage, T.L.; Sage, R.F.; Wong, G.K.; Albert, V.A.; Berry, J.O. Evolution of RLSB, a nuclear-encoded S1 domain RNA binding protein associated with post-transcriptional regulation of plastid-encoded rbcL mRNA in vascular plants. BMC Evol. Biol. 2016, 16, 141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeon, Y.; Jung, H.J.; Kang, H.; Park, Y.I.; Lee, S.H.; Pai, H.S. S1 domain-containing STF modulates plastid transcription and chloroplast biogenesis in Nicotiana benthamiana. New Phytol. 2012, 193, 349–363. [Google Scholar] [CrossRef]
- Nouri, M.Z.; Moumeni, A.; Komatsu, S. Abiotic stresses: Insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 2015, 16, 20392–20416. [Google Scholar] [CrossRef]
- Kumar, A.A.; Mishra, P.; Kumari, K.; Panigrahi, K.C. Environmental stress influencing plant development and flowering. Front. Biosci. 2012, 4, 1315–1324. [Google Scholar]
- Wang, Y.; Berkowitz, O.; Selinski, J.; Xu, Y.; Hartmann, A.; Whelan, J. Stress responsive mitochondrial proteins in Arabidopsis thaliana. Free Radic. Biol. Med. 2018, 122, 28–39. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Joshi, P.; Raval, M.; Biswal, U. Photosynthesis, a global sensor of environmental stress in green plants: Stress signalling and adaptation. Curr. Sci. 2011, 47–56. [Google Scholar]
- Cottage, A.; Mott, E.K.; Kempster, J.A.; Gray, J.C. The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J. Exp. Bot. 2010, 61, 3773–3786. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tan, Z.; Wu, F.; Sheng, P.; Heng, Y.; Wang, X.; Ren, Y.; Wang, J.; Guo, X.; Zhang, X. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Mol. Plant 2014, 7, 1329–1349. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.D.; Su, Q.Q.; Lin, D.Z.; Jiang, Q.; Xu, J.L.; Zhang, J.H.; Teng, S.; Dong, Y.J. The rice OsV4 encoding a novel pentatricopeptide repeat protein is required for chloroplast development during the early leaf stage under cold stress. J. Integr. Plant Biol. 2014, 56, 400–410. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.; Liu, Y.; Gong, X.; Xu, J.; Lin, D.; Dong, Y. The rice pentatricopeptide repeat gene TCD10 is needed for chloroplast development under cold stress. Rice 2016, 9, 67. [Google Scholar] [CrossRef]
- Liu, X.; Lan, J.; Huang, Y.; Cao, P.; Zhou, C.; Ren, Y.; He, N.; Liu, S.; Tian, Y.; Nguyen, T.; et al. WSL5, a pentatricopeptide repeat protein, is essential for chloroplast biogenesis in rice under cold stress. J. Exp. Bot. 2018, 69, 3949–3961. [Google Scholar] [CrossRef]
- Zsigmond, L.; Rigo, G.; Szarka, A.; Szekely, G.; Otvos, K.; Darula, Z.; Medzihradszky, K.F.; Koncz, C.; Koncz, Z.; Szabados, L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008, 146, 1721–1737. [Google Scholar] [CrossRef]
- Zsigmond, L.; Szepesi, A.; Tari, I.; Rigo, G.; Kiraly, A.; Szabados, L. Overexpression of the mitochondrial PPR40 gene improves salt tolerance in Arabidopsis. Plant Sci. 2012, 182, 87–93. [Google Scholar] [CrossRef]
- Laluk, K.; AbuQamar, S.; Mengiste, T. The Arabidopsis mitochondria-localized pentatricopeptide repeat protein PGN functions in defense against necrotrophic fungi and abiotic stress tolerance. Plant Physiol. 2011, 156, 2053–2068. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Chen, Z.; Ren, X.; Hong, X.; Gong, Z. ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J. 2010, 63, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; He, J.; Qin, Y.; Hua, D.; Duan, Y.; Chen, Z.; Gong, Z. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 2014, 10, e1004791. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Zhao, J.Y.; Lu, P.P.; Chen, M.; Guo, C.H.; Xu, Z.S.; Ma, Y.Z. The E-subgroup pentatricopeptide repeat protein family in Arabidopsis thaliana and confirmation of the responsiveness PPR96 to abiotic stresses. Front. Plant Sci. 2016, 7, 1825. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, G.; Lee, K.; Park, S.J.; Kim, Y.O.; Kang, H. A chloroplast-targeted cabbage DEAD-box RNA helicase BrRH22 confers abiotic stress tolerance to transgenic Arabidopsis plants by affecting translation of chloroplast transcripts. Plant Physiol. Biochem. 2018, 127, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Jung, H.J.; Kim, B.M.; Xu, T.; Lee, K.; Kim, Y.O.; Kang, H. A chloroplast-localized S1 domain-containing protein SRRP1 plays a role in Arabidopsis seedling growth in the presence of ABA. J. Plant Physiol. 2015, 189, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, H.; Yang, Y.; Fish, T.; Lyi, S.M.; Thannhauser, T.W.; Zhang, L.; Li, L. Plastid ribosomal protein S5 is involved in photosynthesis, plant development, and cold stress tolerance in Arabidopsis. J. Exp. Bot. 2016, 67, 2731–2744. [Google Scholar] [CrossRef]
- Dinh, S.N.; Park, S.J.; Han, J.H.; Kang, H. A chloroplast-targeted S1 RNA-binding domain protein plays a role in Arabidopsis response to diverse abiotic stresses. J. Plant Biol. 2019, 62, 74–81. [Google Scholar] [CrossRef]
- Bigot, S.; Buges, J.; Gilly, L.; Jacques, C.; Le Boulch, P.; Berger, M.; Delcros, P.; Domergue, J.B.; Koehl, A.; Ley-Ngardigal, B.; et al. Pivotal roles of environmental sensing and signaling mechanisms in plant responses to climate change. Global Change Biol. 2018, 24, 5573–5589. [Google Scholar] [CrossRef]
- Dourmap, C.; Roque, S.; Morin, A.; Caubriere, D.; Kerdiles, M.; Beguin, K.; Perdoux, R.; Reynoud, N.; Bourdet, L.; Audebert, P.A.; et al. Stress signalling dynamics of the mitochondrial electron transport chain and oxidative phosphorylation system in higher plants. Ann. Bot. 2020, 125, 721–736. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Huang, S.C.; Jupe, F.; Sasaki, E.; Schmitz, R.J.; Urich, M.A.; Castanon, R.; Nery, J.R.; Barragan, C.; He, Y.; et al. Epigenomic Diversity in a Global Collection of Arabidopsis thaliana Accessions. Cell 2016, 166, 492–505. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Furtauer, L.; Kustner, L.; Weckwerth, W.; Heyer, A.G.; Nagele, T. Resolving subcellular plant metabolism. Plant J. 2019, 100, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Kustner, L.; Furtauer, L.; Weckwerth, W.; Nagele, T.; Heyer, A.G. Subcellular dynamics of proteins and metabolites under abiotic stress reveal deferred response of the Arabidopsis thaliana hexokinase-1 mutant gin2-1 to high light. Plant J. 2019, 100, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Salvato, F.; Thal, B.; Eubel, H.; Thelen, J.J.; Moller, I.M. The proteome of higher plant mitochondria. Mitochondrion 2017, 33, 22–37. [Google Scholar] [CrossRef]
- Kleffmann, T.; Russenberger, D.; von Zychlinski, A.; Christopher, W.; Sjolander, K.; Gruissem, W.; Baginsky, S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 2004, 14, 354–362. [Google Scholar] [CrossRef]
- Herschlag, D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995, 270, 20871–20874. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.A. Taming free energy landscapes with RNA chaperones. RNA Biol. 2010, 7, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rajkowitsch, L.; Chen, D.; Stampfl, S.; Semrad, K.; Waldsich, C.; Mayer, O.; Jantsch, M.F.; Konrat, R.; Bläsi, U.; Schroeder, R. RNA chaperones, RNA annealers and RNA helicases. RNA Biol. 2007, 4, 118–130. [Google Scholar] [CrossRef]
- Ivanyi-Nagy, R.; Davidovic, L.; Khandjian, E.; Darlix, J.-L. Disordered RNA chaperone proteins: From functions to disease. Cell. Mol. Life Sci. 2005, 62, 1409–1417. [Google Scholar] [CrossRef]
- Ivanyi-Nagy, R.; Lavergne, J.-P.; Gabus, C.; Ficheux, D.; Darlix, J.-L. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 2008, 36, 712–725. [Google Scholar] [CrossRef]
- Chambers, J.R.; Bender, K.S. The RNA chaperone Hfq is important for growth and stress tolerance in Francisella novicida. PLoS ONE 2011, 6, e19797. [Google Scholar] [CrossRef] [PubMed]
- Chaulk, S.G.; Smith−Frieday, M.N.; Arthur, D.C.; Culham, D.E.; Edwards, R.A.; Soo, P.; Frost, L.S.; Keates, R.A.; Glover, J.M.; Wood, J.M. ProQ is an RNA chaperone that controls ProP levels in Escherichia coli. Biochemistry 2011, 50, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Stryker, J.M.; Lambowitz, A.M. A DEAD-box protein functions as an ATP-dependent RNA chaperone in group I intron splicing. Cell 2002, 109, 769–779. [Google Scholar] [CrossRef]
- Mohr, S.; Matsuura, M.; Perlman, P.S.; Lambowitz, A.M. A DEAD-box protein alone promotes group II intron splicing and reverse splicing by acting as an RNA chaperone. Proc. Natl. Acad. Sci. USA 2006, 103, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-R.; Rowe, C.E.; Mohr, S.; Jiang, Y.; Lambowitz, A.M.; Perlman, P.S. The splicing of yeast mitochondrial group I and group II introns requires a DEAD-box protein with RNA chaperone function. Proc. Natl. Acad. Sci. USA 2005, 102, 163–168. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jung, H.J.; Kwak, K.J.; Kim, M.K.; Oh, S.H.; Han, Y.S.; Kang, H. The Arabidopsis U12-type spliceosomal protein U11/U12-31K is involved in U12 intron splicing via RNA chaperone activity and affects plant development. Plant Cell 2010, 22, 3951–3962. [Google Scholar] [CrossRef]
- Kwak, K.J.; Jung, H.J.; Lee, K.H.; Kim, Y.S.; Kim, W.Y.; Ahn, S.J.; Kang, H. The minor spliceosomal protein U11/U12-31K is an RNA chaperone crucial for U12 intron splicing and the development of dicot and monocot plants. PLoS ONE 2012, 7, e43707. [Google Scholar] [CrossRef][Green Version]
- Castiglioni, P.; Warner, D.; Bensen, R.J.; Anstrom, D.C.; Harrison, J.; Stoecker, M.; Abad, M.; Kumar, G.; Salvador, S.; D’Ordine, R.; et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol. 2008, 147, 446–455. [Google Scholar] [CrossRef]
- Chaikam, V.; Karlson, D. Functional characterization of two cold shock domain proteins from Oryza sativa. Plant Cell Environ. 2008, 31, 995–1006. [Google Scholar] [CrossRef]
- Karlson, D.; Imai, R. Conservation of the cold shock domain protein family in plants. Plant Physiol. 2003, 131, 12–15. [Google Scholar] [CrossRef]
- Karlson, D.; Nakaminami, K.; Toyomasu, T.; Imai, R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J. Biol. Chem. 2002, 277, 35248–35256. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Kang, H. The role of a zinc finger-containing glycine-rich RNA-binding protein during the cold adaptation process in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Kim, J.S.; Kang, H. Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J. 2005, 42, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jung, H.J.; Lee, H.J.; Kim, K.; Goh, C.H.; Woo, Y.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding protein7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 2008, 55, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Sasaki, K.; Imai, R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 23454–23460. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010, 61, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Zinc finger-containing glycine-rich RNA-binding protein in Oryza sativa has an RNA chaperone activity under cold stress conditions. Plant Cell Environ. 2010, 33, 759–768. [Google Scholar]
- Hu, J.; Manduzio, S.; Kang, H. Epitranscriptomic RNA methylation in plant development and abiotic stress responses. Front. Plant Sci. 2019, 10, 500. [Google Scholar] [CrossRef]
- Yue, H.; Nie, X.; Yan, Z.; Weining, S. N6-methyladenosine regulatory machinery in plants: Composition, function and evolution. Plant Biotech. J. 2019, 17, 1194–1208. [Google Scholar] [CrossRef]
- Arribas-Hernández, L.; Brodersen, P. Occurrence and functions of m6A and other covalent modifications in plant mRNA. Plant Physiol. 2020, 182, 79–96. [Google Scholar] [CrossRef]
- Shen, L.; Liang, Z.; Wong, C.E.; Yu, H. Messenger RNA modifications in plants. Trends Plant Sci. 2019, 24, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, K.; Zhang, D.; Wan, Y.; Wen, Y.; Lu, Q.; Wang, L. High-throughput m6A-seq reveals RNA m6A methylation patterns in the chloroplast and mitochondria transcriptomes of Arabidopsis thaliana. PLoS ONE 2017, 12, e0185612. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kang, H. Roles of Organellar RNA-Binding Proteins in Plant Growth, Development, and Abiotic Stress Responses. Int. J. Mol. Sci. 2020, 21, 4548. https://doi.org/10.3390/ijms21124548
Lee K, Kang H. Roles of Organellar RNA-Binding Proteins in Plant Growth, Development, and Abiotic Stress Responses. International Journal of Molecular Sciences. 2020; 21(12):4548. https://doi.org/10.3390/ijms21124548
Chicago/Turabian StyleLee, Kwanuk, and Hunseung Kang. 2020. "Roles of Organellar RNA-Binding Proteins in Plant Growth, Development, and Abiotic Stress Responses" International Journal of Molecular Sciences 21, no. 12: 4548. https://doi.org/10.3390/ijms21124548
APA StyleLee, K., & Kang, H. (2020). Roles of Organellar RNA-Binding Proteins in Plant Growth, Development, and Abiotic Stress Responses. International Journal of Molecular Sciences, 21(12), 4548. https://doi.org/10.3390/ijms21124548






