Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders
Abstract
1. Methodology of Literature Search for Review
1.1. Data Sources and Search
1.2. Data Extraction
2. Introduction on Topic
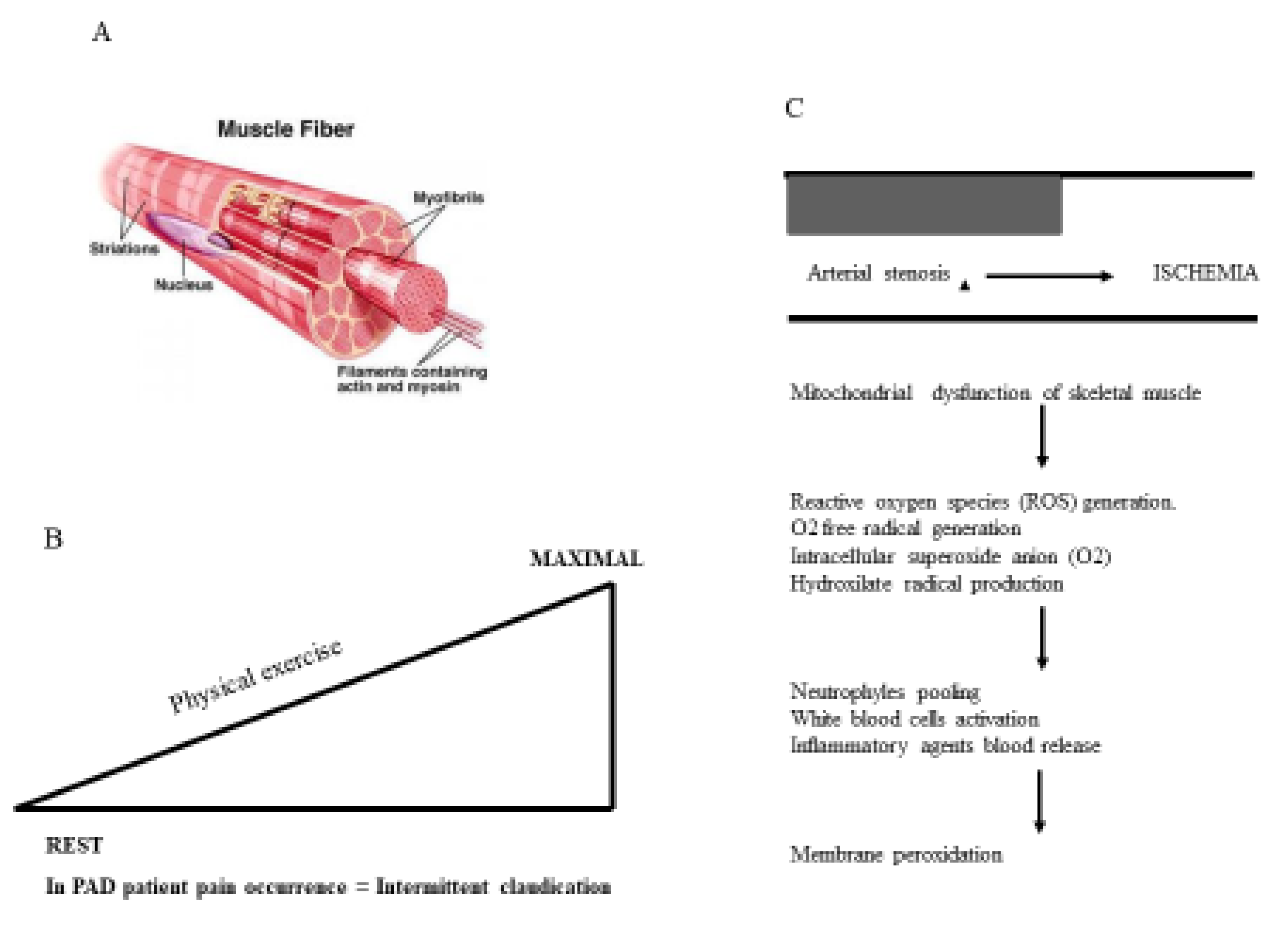
3. Biochemistry of the Physical Exercise: Pro and Anti Oxidative Effects
4. Oxidative Stress and Physical Exercise in Patients with Peripheral Arterial Disease
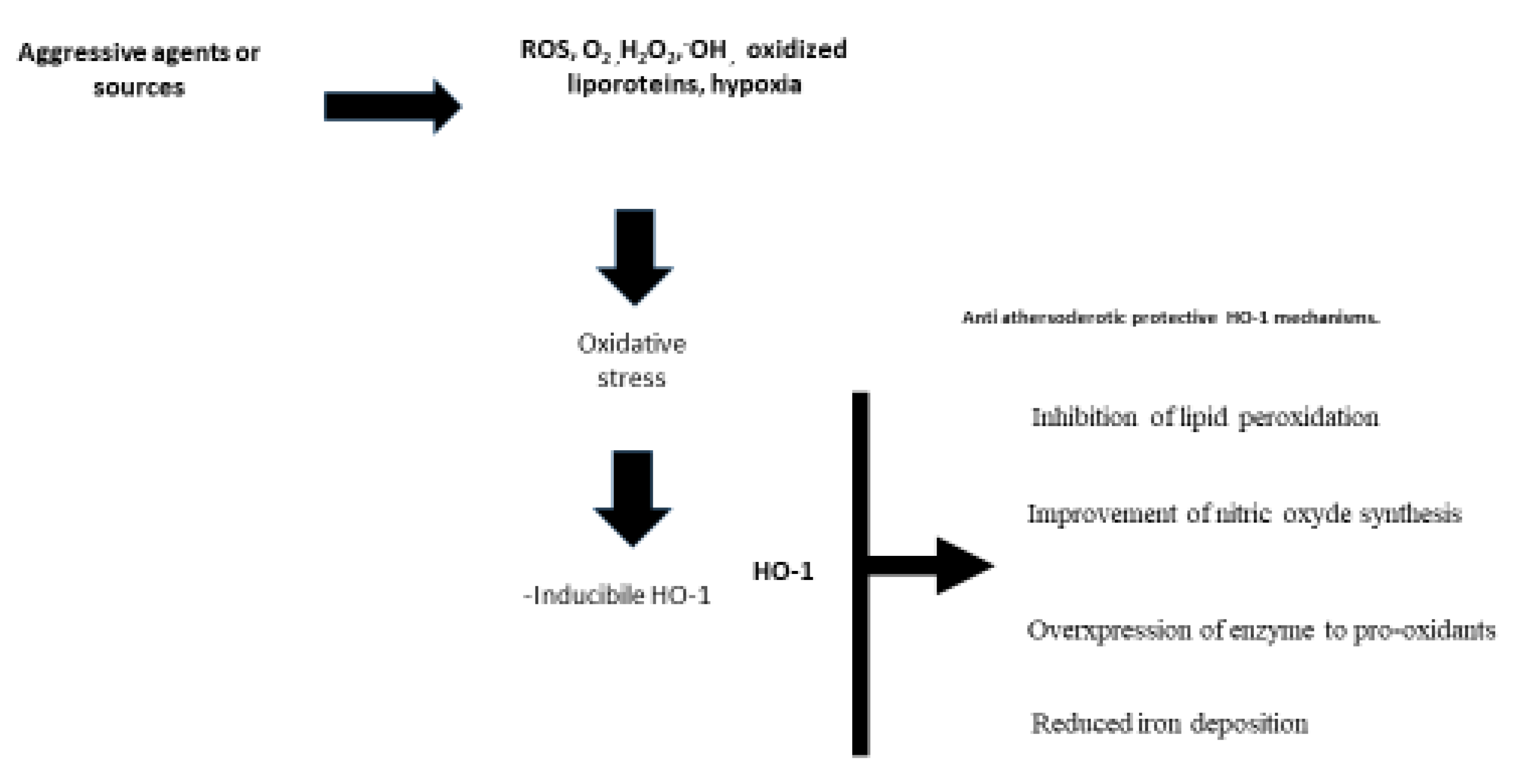
5. Antioxidants, and Heme Oxygenase 1 in Peripheral Artery Disease
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Aronow, H.; Hiatt, W.R. The burden of peripheral artery disease and the role of antiplatelet therapy. Postgrad. Med. 2009, 121, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Anzaldi, M.; Fiore, V.; Catanzaro, S.; Simili, M.; Torrisi, B.; Neri, S. Study on unrecognized peripheral arterial disease (PAD) by ankle/brachial index and arterial co-morbidity in Catania (Sicily, Italy). Angiology 2010, 61, 524–529. [Google Scholar] [CrossRef]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Katsiki, N. Oxidative Stress and Inflammation: Their Role in the Pathogenesis of Peripheral Artery Disease with or Without Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2018, 16, 547–554. [Google Scholar] [CrossRef]
- Leung, F.P.; Yung, L.M.; Laher, I.; Yao, X.; Chen, Z.Y.; Huang, Y. Exercise, vascular wall and cardiovascular diseases: An update. Sports Med. 2008, 38, 1009–1024. [Google Scholar] [CrossRef]
- López-Cruz, R.I.; Zenteno-Savín, T.; Galván-Magaña, F. Superoxide production, oxidative damage and enzymatic antioxidant defenses in shark skeletal muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 50–56. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Musiari, G.; Miceli, G.; Arnao, V.; Pinto, A. Preventive and Therapeutic Role of Muscle Contraction against Chronic Diseases. Curr. Pharm. Des. 2016, 22, 4686–4699. [Google Scholar] [CrossRef]
- Steven, S.; Daiber, A.; Dopheide, J.F.; Münzel, T.; Espinola-Klein, C. Peripheral artery disease, redox signaling, oxidative stress—Basic and clinical aspects. Redox Biol. 2017, 12, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Hoier, B.; Walker, P.J.; Schulze, K.; Bangsbo, J.; Hellsten, Y.; Askew, C.D. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 2016, 246, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Nyberg, M.; Hellsten, Y. Nitric oxide and reactive oxygen species in limb vascular function: What is the effect of physical activity? Free Radic. Res. 2014, 48, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.A.; Green, S.; Green, A.A.; Walker, P.J. Walking performance, oxygen uptake kinetics and resting muscle pyruvate dehydrogenase complex activity in peripheral arterial disease. Clin. Sci. 2004, 106, 241–249. [Google Scholar] [CrossRef]
- Wang, H.; Hiatt, W.R.; Barstow, T.J.; Brass, E.P. Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: Alterations with disease. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 22–27. [Google Scholar] [CrossRef]
- Brass, E.P. Skeletal muscle metabolism as a target for drug therapy in peripheral arterial disease. Vasc. Med. 1996, 1, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef]
- Semenza, G.L. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J. Cell Biochem. 2007, 102, 840–847. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Tuttolomondo, A.; Musiari, G.; Schimmenti, C.; D’Angelo, A.; Pinto, A. Are the Myokines the Mediators of Physical Activity-Induced Health Benefits? Curr. Pharm. Des. 2016, 22, 3622–3647. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Miceli, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A. New insights about the putative role of myokines in the context of cardiac rehabilitation and secondary cardiovascular prevention. Ann. Transl. Med. 2017, 5, 300. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Pedersen, B.K. Skeletal muscle as an immunogenic organ. Curr. Opin. Pharmacol. 2008, 8, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D. Editorial (Thematic Issue: Myokines and Exercise Training: More Shadows than Lights). Curr. Pharm. Des. 2016, 22, 3619–3621. [Google Scholar] [CrossRef] [PubMed]
- Finkler, M.; Lichtenberg, D.; Pinchuk, I. The relationship between oxidative stress and exercise. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H.; Wideman, L.; McKenzie, M.J.; Consitt, L.A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 15, 126–131. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef]
- McDermott, M.M. Lower extremity manifestations of peripheral artery disease: The pathophysiologic and functional implications of leg ischemia. Circ. Res. 2015, 116, 1540–1550. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Greenland, P.; Guralnik, J.M.; Criqui, M.H.; Chan, C.; Chan, C.; Pearce, W.H.; Schneider, J.R.; Ferrucci, L.; et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and legsymptoms. JAMA 2004, 292, 453–461. [Google Scholar] [CrossRef]
- Kiani, S.; Aasen, J.G.; Holbrook, M.; Khemka, A.; Sharmeen, F.; LeLeiko, R.M.; Tabit, C.E.; Farber, A.; Eberhardt, R.T.; Gokce, N.; et al. Peripheral artery disease is associated with severe impairment of vascular function. Vasc. Med. 2013, 18, 72–78. [Google Scholar] [CrossRef]
- de Silva, R.C.; Wolosker, N.; Yugar-Toledo, J.C.; Consolim-Colombo, F.M. Vascular reactivity is impaired and associated with walking ability in patients with intermittent claudication. Angiology 2015, 66, 680–686. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Greenland, P.; Liu, K.; Guralnik, J.M.; Criqui, M.H.; Dolan, N.C.; Chan, C.; Celic, L.; Pearce, W.H.; Schneider, J.R.; et al. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA 2001, 286, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis-an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Schiano, V.; Chiariello, M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 2008, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 1998, 97, 425–428. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur. Heart J. 2007, 28, 354–362. [Google Scholar] [CrossRef]
- Lin, C.W.; Hsu, L.A.; Chen, C.C.; Yeh, J.T.; Sun, J.H.; Lin, C.H.; Chen, S.T.; Hsu, B.R.; Huang, Y.Y. C-reactive protein as an outcome predictor for percutaneous transluminal angioplasty in diabetic patients with peripheral arterial disease and infected foot ulcers. Diabetes Res. Clin. Pract. 2010, 90, 167–172. [Google Scholar] [CrossRef]
- Jialal, I.; Verma, S.; Devaraj, S. Inhibition of endothelial nitric oxide synthase by C-reactive protein: Clinical relevance. Clin. Chem. 2009, 55, 206–208. [Google Scholar] [CrossRef]
- Pipinos, I.I.; Judge, A.R.; Selsby, J.T.; Zhu, Z.; Swanson, S.A.; Nella, A.A.; Dodd, S.L. The myopathy of peripheral arterial occlusive disease: Part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc. Endovasc. Surg. 2007, 41, 481–489. [Google Scholar] [CrossRef]
- Koutakis, P.; Weiss, D.J.; Miserlis, D.; Shostrom, V.K.; Papoutsi, E.; Ha, D.M.; Ha, D.M.; Carpenter, L.A.; McComb, R.D.; Casale, G.P.; et al. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol. 2014, 2, 921–928. [Google Scholar]
- Thompson, J.R.; Swanson, S.A.; Haynatzki, G.; Koutakis, P.; Johanning, J.M.; Reppert, P.R.; Papoutsi, E.; Miserlis, D.; Zhu, Z.; Casale, G.P.; et al. Protein Concentration and Mitochondrial Content in the Gastrocnemius Predicts Mortality Rates in Patients With Peripheral Arterial Disease. Ann. Surg. 2015, 261, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Pipinos, I.I.; Judge, A.R.; Zhu, Z.; Selsby, J.T.; Swanson, S.A.; Johanning, J.M.; Baxter, B.T.; Lynch, G.T.; Dodd, S.L. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic. Biol. Med. 2006, 41, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Wolfel, E.E.; Regensteiner, J.G.; Brass, E.P. Skeletal muscle carnitine metabolism in patients with unilateral peripheral arterial disease. J. Appl. Physiol. 1992, 73, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Pipinos, I.I.; Sharov, V.G.; Shepard, A.D.; Anagnostopoulos, P.V.; Katsamouris, A.; Todor, A.; Filis, K.A.; Sabbah, H.N. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J. Vasc. Surg. 2003, 38, 827–832. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfe, N.; Zoll, J.; Geny, B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am. J. Physiol. Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Marcus Cooper, M.; Dina Laznik, D.; Jessica Chinsomboon, J.; Shamina, M.; et al. HIF independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Koutakis, P.; Miserlis, D.; Myers, S.A.; Kim, J.K.; Zhu, Z.; Papoutsi, E.; Papoutsi, E.; Swanson, S.A.; Haynatzki, G.; Ha, D.M.; et al. Abnormal accumulation of desmin in gastrocnemiusmyofibers of patients with peripheral artery disease: Associations with altered myofiber morphology and density, mitochondrialdysfunction and impaired limb function. J. Histochem. Cytochem. 2015, 63, 256–269. [Google Scholar] [CrossRef]
- Ceci, R.; Beltran Valls, M.R.; Duranti, G.; Dimauro, I.; Quaranta, F.; Pittaluga, M.; Sabatini, S.; Caserotti, P.; Parisi, P.; Parisi, A.; et al. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019, 41. [Google Scholar] [CrossRef]
- Melikoglu, M.A.; Kaldirimci, M.; Katkat, D.; Sen, I.; Kaplan, I.; Senel, K. The effect of regular long term training on antioxidant enzymatic activities. J. Sports Med. Phys. Fitness 2008, 48, 388–390. [Google Scholar]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; La Torre, A.; Porcelli, S. Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate-intensity continuous training in masters runners. PLoS ONE 2014, 9, e87506. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.; Jackson, T.; Latimer, H. Role/s of ‘Antioxidant’ Enzymes in Ageing. Subcell. Biochem. 2018, 90, 425–450. [Google Scholar] [PubMed]
- Peake, J.M.; Markworth, J.F.; Nosaka, K.; Raastad, T.; Wadley, G.D.; Coffey, V.G. Modulating exercise-induced hormesis: Does less equal more? J. Appl. Physiol. 2015, 119, 172–189. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurano, M.; Hasegawa, T.; Takano, H.; Iida, H.; Yasuda, T.; Nakajima, T.; Kurano, M.; Hasegawa, T.; Takano, H.; et al. Pentraxin3 and high-sensitive C-reactive protein are independent inflammatory markers released during high-intensity exercise. Eur. J. Appl. Physiol. 2010, 110, 905–913. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Mazzarino, M.C.; Di Pino, L.; Malaponte, G.; Porto, C.; Pennisi, G.; Marchese, G.; Costa, M.P.; Digrandi, D.; Celotta, G.; et al. High Circulating Levels of Cytokines (IL-6 and TNFalpha), Adhesion Molecules (VCAM-1 and ICAM-1) and Selectins in Patients With Peripheral Arterial Disease at Rest and After a Treadmill Test. Vasc. Med. 2003, 8, 15–19. [Google Scholar] [CrossRef]
- Andreozzi, G.M.; Martini, R.; Cordova, R.; D’Eri, A.; Salmistraro, G.; Mussap, M. Plebani M Circulating levels of cytokines (IL-6 and IL-1beta) in patients with intermittent claudication, at rest, after maximal exercise treadmill test and during restore phase. Could they be progression markers of the disease? Int. Angiol. 2007, 26, 245–252. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, S65–S67. [Google Scholar] [CrossRef]
- McDermott, M.M.; Polonsky, T.S. Home-based exercise. A therapeutic option for peripheral arterial disease. Circulation 2016, 134, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Angelini, C.; Rosa, M.; Carrozzo, R.; Perna, S.; Corsi, M.; Matarazzo, A.; Marcialis, A. Muscle Carnitine Deficiency in Patients With Severe Peripheral Vascular Disease. Circulation 1991, 84, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Ruff, L.; Brass, E.P. Carnitine and acyl carnitine metabolism during exercise in humans: Dependence on skeletal muscle metabolic state. J. Clin. Investig. 1989, 84, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Chiariello, M.; Ferulano, G.; Policicchio, A.; Nevola, E.; Rossini, A.; Attisano, T.; Ambrosio, G.; Siliprandi, N.; Angelini, C. Increases in walking distance in patients with peripheral vascular disease treated with L-carnitine: A double-blind, cross-over study. Circulation 1988, 77, 767–773. [Google Scholar] [CrossRef]
- Pignatelli, P.; Lenti, L.; Sanguigni, V.; Frati, G.; Simeoni, I.; Gazzaniga, P.P.; Pulcinelli, F.M.; Violi, F. Carnitine inhibits arachidonic acid turnover, platelet function, and oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H41–H48. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Malaponte, G.; Di Pino, L.; Digrandi, D.; Pennisi, G.; Mazzarino, M.C. Effects of ischaemic stress on leukocyte activation processes in patients with chronic peripheral occlusive arterial disease: Role of L-propionyl carnitine administration. Pharmacol. Res. 2001, 44, 305–309. [Google Scholar] [CrossRef]
- Stasi, M.A.; Scioli, M.G.; Arcuri, G.; Mattera, G.G.; Lombardo, K.; Marcellini, M.; Riccioni, T.; De Falco, S.; Pisano, C.; Luigi Spagnoli, L.G.; et al. Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 426–435. [Google Scholar] [CrossRef]
- Silvestro, A.; Scopacasa, F.; Oliva, G.; de Cristofaro, T.; Iuliano, L.; Brevetti, G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 2002, 165, 277–283. [Google Scholar] [CrossRef]
- Carr, A.C.; Zhu, B.Z.; Frei, B. Potential anti-atherogenic mechanisms of ascorbate (vitamin C) and alpha-tocopherol (vitamin E). Circ. Res. 2000, 87, 349–354. [Google Scholar] [CrossRef]
- Esterbauer, H.; Dieber-Rotheneder, M.; Striegl, G.; Waeg, G. Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am. J. Clin. Nutr. 1991, 53 (Suppl. 1), 314S–321S. [Google Scholar] [CrossRef]
- Stephens, N.G.; Parsons, A.; Schofield, P.M.; Kelly, F.; Cheeseman, K.; Mitchinson, M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Arosio, E.; De Marchi, S.; Zannoni, M.; Prior, M.; Lechi, A. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease: A randomized, double-blind, placebo-controlled trial. Mayo Clin. Proc. 2002, 77, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Marcoccia, A.; Pignatelli, P.; Andreozzi, P.; Borgia, M.C.; Cangemi, R.; Chiarotti, F.; Violi, F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur. Heart J. 2007, 28, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Nocella, C.; Bartimoccia, S.; Bucci, T.; De Falco, E.; Peruzzi, M.; Chimenti, I.; Biondi-Zoccai, G.; Pignatelli, P.; et al. Epicatechin and catechin modulate endothelial activation induced by platelets of patients with peripheral artery disease. Oxid. Med. Cell Longev. 2014, 2014, 691015. [Google Scholar] [CrossRef] [PubMed]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.H.W.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Lenti, L.; Sanguigni, V.; Frati, G.; Simeoni, I.; Gazzaniga, P.P.; Pulcinelli, F.M.; Violi, F. Plasma heme oxygenase 1 is decreased in peripheral artery disease patients. Mol. Med. Rep. 2016, 14, 3459–3463. [Google Scholar]
- Gardner, A.W.; Parker, D.E.; Webb, N.; Montgomery, P.S.; Scott, K.J.; Blevins, S.M. Calf Muscle Hemoglobin Oxygen Saturation Characteristics and Exercise Performance in Patients With Intermittent Claudication. J. Vasc. Surg. 2008, 48, 644–649. [Google Scholar] [CrossRef]
- Hickman, P.; Harrison, D.K.; Hill, A.; McLaren, M.; Tamei, H.; McCollum, P.T.; Belch, J.J. Exercise in patients with intermittent claudication results in the generation of oxygen derived free radicals and endothelial damage. Adv. Exp. Med. Biol. 1994, 361, 565–570. [Google Scholar]
- Hamburg, N.M.; Balady, G.J. Exercise rehabilitation in peripheral artery disease: Functional impact and mechanisms of benefits. Circulation 2011, 123, 87–97. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Hiatt, W.R.; Coll, J.R.; Criqui, M.H.; Treat-Jacobson, D.; McDermott, M.M.; Hirsch, A.T.; Treat-Jacobson, D.; McDermott, M.M. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc. Med. 2008, 13, 15–22. [Google Scholar] [CrossRef]
- Garg, P.K.; Tian, L.; Criqui, M.H.; Liu, K.; Ferrucci, L.; Guralnik, J.M.; Tan, J.; NcDermott, M.M. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation 2006, 114, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Koutakis, P.; Ismaeel, A.; Farmer, P.; Purcell, S.; SSmith, R.S.; Eidson, J.L.; Bohannon, W.T. Oxidative stress and antioxidant treatment in patients with peripheral arterial disease. Physiol. Rep. 2018, 6, e13650. [Google Scholar] [CrossRef] [PubMed]
- Dopheide, J.F.; Scheer, M.; Doppler, C.; Obst, V.; Stein, P.; Vosseler, M.; Abegunewardene, N.; Gori, T.; Münzel, T.; Daiber, A.; et al. Change of walking distance in intermittent claudication: Impact on inflammation, oxidative stress and mononuclear cells: A pilot study. Clin. Res. Cardiol. 2015, 104, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Carnevale, R.; Cangemi, R.; Angelico, F.; Augelletti, T.; Di Santo, S.; Calabrese, C.M.; DellaVolpe, L.; Pignatelli, P.; Perri, L.; et al. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int. J. Cardiol. 2013, 165, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.D.; Drew, R.C.; Blaha, C.A.; Mast, J.L.; Cui, J.; Reed, A.; Sinoway, L.I. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J. Physiol. 2012, 590, 6237–6246. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, H.; Liu, J.; Zhao, H.; Wang, H. Is serum total bilirubin a predictor of prognosis in arteriosclerotic cardiovascular disease? A meta-analysis. Medicine 2019, 98, e17544. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An nrf2/small maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Ishikawa, K.; Maruyama, Y. Heme oxygenase as an intrinsic defense system in vascular wall: Implication against atherogenesis. J. Atheroscler. Thromb. 2001, 8, 63–70. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Kim, J.A.; Territo, M.C.; Wayner, E.; Carlos, T.M.; Parhami, F.; Smith, C.W.; Haberland, M.E.; Fogelman, A.M.; Berliner, J.A. Partial characterization of leukocyte binding molecules on endothelial cells induced byminimally oxidized ldl. Arterioscler. Thromb. 1994, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Duckers, H.J.; Boehm, M.; True, A.L.; Yet, S.F.; San, H.; Park, J.L.; Clinton Webb, R.; Lee, M.E.; Nabel, G.J.; Nabel, E.G. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001, 7, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin. Chem. 2000, 46, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Sugawara, D.; Wang, X.; Suzuki, K.; Itabe, H.; Maruyama, Y.; Lusis, A.J. Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL-receptor knockout mice. Circ. Res. 2001, 88, 506–512. [Google Scholar] [CrossRef]
- Ishikawa, K.; Sugawara, D.; Goto, J.; Watanabe, Y.; Kawamura, K.; Shiomi, M.; Itabe, H.; Maruyama, Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation 2001, 104, 1831–1836. [Google Scholar] [CrossRef]
- Hayashi, S.; Takamiya, R.; Yamaguchi, T.; Matsumoto, K.; Tojo, S.J.; Tamatani, T.; Kitajima, M.; Makino, N.; Ishimura, Y.; Suematsu, M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: Role of bilirubin generated by the enzyme. Circ. Res. 1999, 85, 663–671. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Ibe, S.; Saita, E.; Sasaki, K.; Hanako, N.; Miura, K.; Ikegami, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Plasma Heme Oxygenase-1 Levels in Patients with Coronary and Peripheral Artery Diseases. Dis. Markers 2018, 2018, 6138124. [Google Scholar] [CrossRef]
- Li Volti, G.; Seta, F.; Schwartzman, M.L.; Nasjletti, A.; Abraham, N.G. Heme oxygenase attenuates angiotensin II mediated increase in cyclooxygenase-2 activity in human femoral endothelial cells. Hypertension 2003, 41, 715–719. [Google Scholar] [CrossRef]
- Cao, J.; Peterson, S.J.; Sodhi, K.; Vanella, L.; Barbagallo, I.; Rodella, L.F.; Schwartzman, M.L.; Abraham, N.G.; Kappas, A. Heme Oxygenase Gene Targeting to Adipocytes Attenuates Adiposity and Vascular Dysfunction in Mice Fed a High-Fat Diet. Hypertension 2012, 60, 467–475. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappa, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Suzuki, M.; Iso-o, N.; Takeshita, S.; Tsukamoto, K.; Mori, I.; Sato, T.; Ohno, M.; Nagai, R.; Ishizaka, N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hind limb ischemia. Biochem. Biophys. Res. Commun. 2003, 302, 138–143. [Google Scholar] [CrossRef]
- Lee, T.S.; Chang, C.C.; Zhu, Y.; Shyy, J.Y. Simvastatin induces heme oxygenase-1: A novel mechanism of vessel protection. Circulation 2004, 110, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. Int. J. Mol. Sci. 2019, 20, 3628. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.M.; Stranieri, C.; Rigoni, A.M.; De Marchi, S.; Peserico, D.; Mozzini, C.; Cominacini, L.; Garbin, U. Physical Exercise reduces cytotoxicity and up-regulates Nrf2 and UPR expression in circulating cells of peripheral artery disease patients: An hypoxic Adaptation? J. Atheroscler. Thromb. 2018, 25, 808–820. [Google Scholar] [CrossRef] [PubMed]
| Antioxidants | Effects and Markers | References |
|---|---|---|
| Propionyl-l-Carnitine | FMD and brachial basal diameter significantly increased Increase in NOx bioavailability Decrease in 8-OHdG | [62,63,64,65,67] |
| Vitamin C | Reduces OxS walking induced Reduces arterial pressure response to physical exercise No reduction of flow mediated dilatation (FMD) by maximal physical exercise No elevation of TABRS OxS marker No elevation of soluble CMA-1 | [68,69,70] |
| Vitamin E | Reduces OxS walking induced | [71,72] |
| Gluthatione | Reduces pain free walking distance Improves macrocirculatory flow after physical exercise | [73,74] |
| Polyphenols: Epicatechin Catechin | Enhances platelet activation Increases the release of soluble cell adhesion molecules (sCAMs) Decreases eNOS activation Effects on NO bioavailability | [75,76] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorelli, S.S.; Marino, E.; Scuto, S.; Di Raimondo, D. Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders. Int. J. Mol. Sci. 2020, 21, 4393. https://doi.org/10.3390/ijms21124393
Signorelli SS, Marino E, Scuto S, Di Raimondo D. Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders. International Journal of Molecular Sciences. 2020; 21(12):4393. https://doi.org/10.3390/ijms21124393
Chicago/Turabian StyleSignorelli, Salvatore Santo, Elisa Marino, Salvatore Scuto, and Domenico Di Raimondo. 2020. "Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders" International Journal of Molecular Sciences 21, no. 12: 4393. https://doi.org/10.3390/ijms21124393
APA StyleSignorelli, S. S., Marino, E., Scuto, S., & Di Raimondo, D. (2020). Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders. International Journal of Molecular Sciences, 21(12), 4393. https://doi.org/10.3390/ijms21124393







