Characterization of Carbonic Anhydrase In Vivo Using Magnetic Resonance Spectroscopy
Abstract
1. Introduction
2. In Vivo Magnetic Resonance Spectroscopy (MRS) for Studying Carbonic Anhydrase
2.1. Theory of 13C Magnetization Transfer Catalyzed by Carbonic Anhydrase
2.2. 13C Magnetization Transfer MRS
2.2.1. Magnetic Resonance Hardware
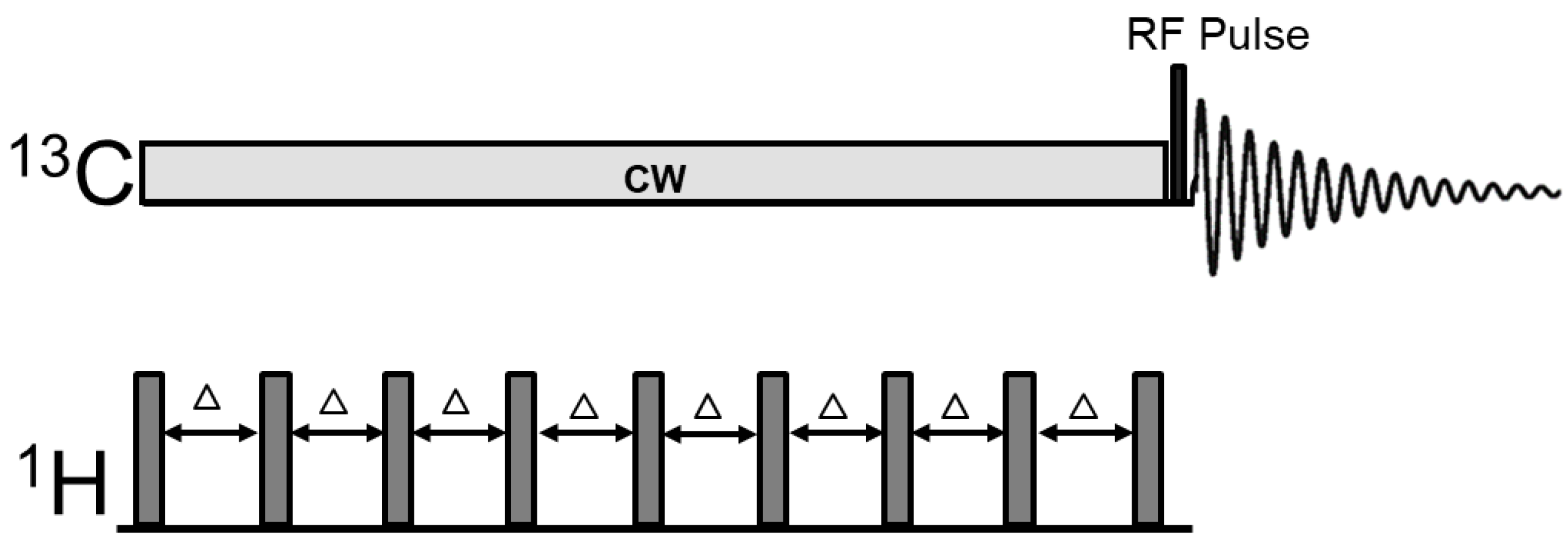
2.2.2. 13C Magnetization Transfer MRS Pulse Sequence
2.3. Isotope Labeling Strategies and 13C MRS of the Carboxylic/Amide Spectral Region
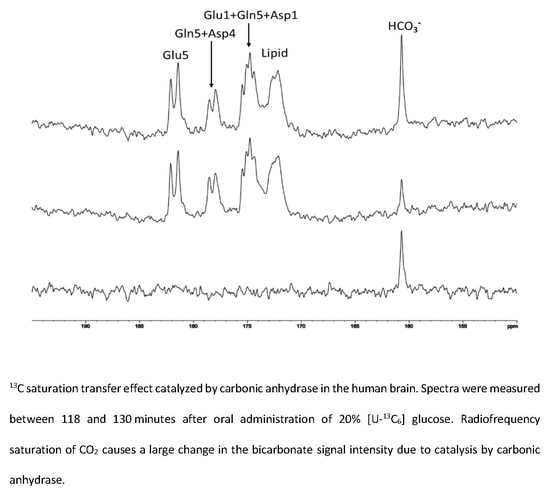
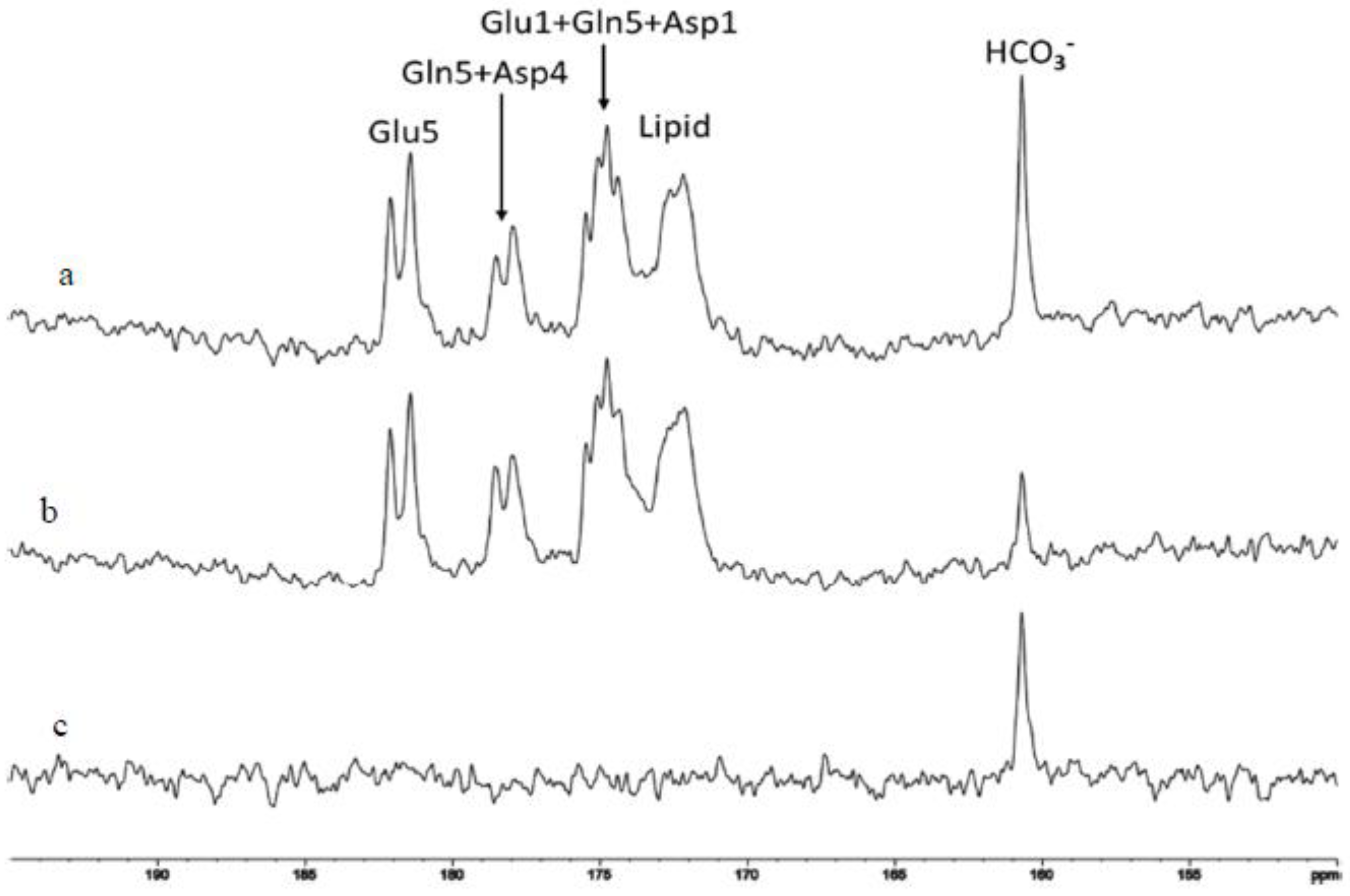
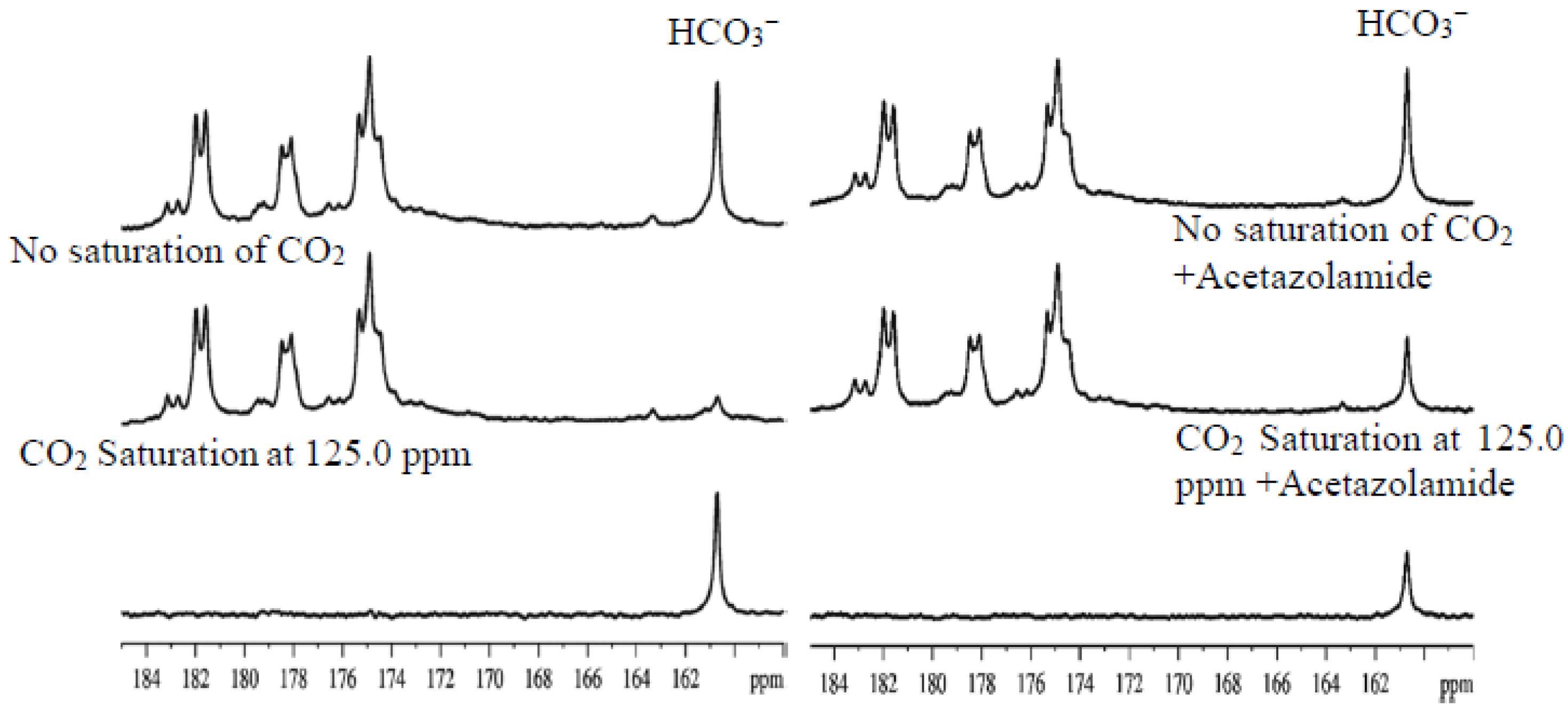
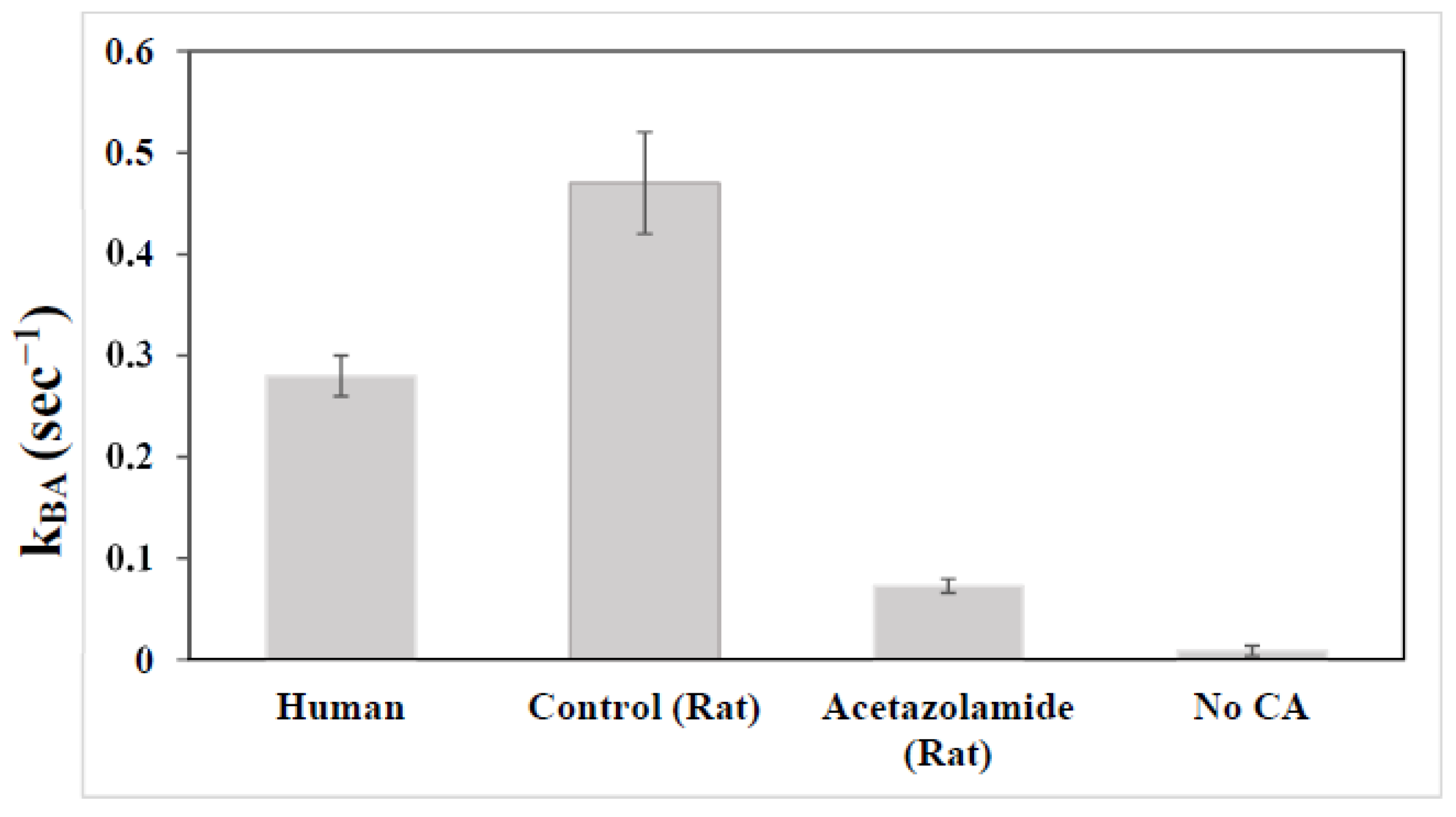
2.4. Characterization of Carbonic Anhydrase Reaction in the Human Brain
3. Future Applications of in Vivo MRS of Carbonic Anhydrase Reaction
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Carbonic anhydrase |
| CAI | Carbonic anhydrases inhibitor |
| MRS | Magnetic resonance spectroscopy |
References
- Maren, T.H. Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol. Rev. 1967, 47, 595–781. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Andring, J.T.; McKenna, R. Crystallography and its impact on carbonic anhydrase research. Int. J. Med. Chem. 2018, 2018, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Aspatwar, A.; Tolvanen, M.E.E.; Ortutay, C.; Parkkila, S. Carbonic anhydrase related proteins: Molecular biology and evolution. Subcell Biochem. 2014, 75, 135–156. [Google Scholar] [PubMed]
- Aspatwar, A.; Tolvanen, M.E.E.; Parkkila, S. An update on carbonic anhydrase-related proteins VIII, X and XI. J. Enzyme Inhib. Med. Chem. 2013, 28, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Tashian, R.E.; Hewett-Emmett, D.; Carter, N.; Bergenhem, N.C.H. Carbonic anhydrase (CA)-related proteins (CA-RPs) and transmembrane proteins with CA or CA-RP domains. In The carbonic Anhydrases: New Horizons; Chegwidden, W.R., Carter, N.D., Edwards, Y.H., Eds.; Birkhäuser: Basel, Switzerland, 2000; pp. 105–120. [Google Scholar]
- Aspatwar, A.; Tolvanen, M.E.; Ortutay, C.; Parkkila, S. Carbonic anhydrase related protein VIII and its role in neurodegeneration and cancer. Curr. Pharm. Des. 2010, 16, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, A.M.; Hynninen, P.; Mecklin, J.P.; Kuopio, T.; Kokko, A.; Aaltonen, L.; Parkkila, A.K.; Pastorekova, S.; Pastorek, J.; Waheed, A.; et al. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.H.; Chia, S.K.; Wykoff, C.C.; Han, C.; Leek, R.D.; Sly, W.S.; Gatter, K.C.; Ratcliffe, P.; Harris, A.L. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br. J. Cancer 2003, 88, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Mazure, N.M.; Hofman, V.; Ammadi, R.E.; Ortholan, C.; Bonnetaud, C.; Havet, K.; Venissac, N.; Mograbi, B.; Mouroux, J.; et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br. J. Cancer. 2010, 102, 1627–1635. [Google Scholar] [CrossRef]
- Karjalainen, S.L.; Haapasalo, H.K.; Aspatwar, A.; Barker, H.; Parkkila, S.; Haapasalo, J.A. Carbonic anhydrase related protein expression in astrocytomas and oligodendroglial tumors. BMC Cancer 2018, 18, 584–593. [Google Scholar] [CrossRef]
- Haapasalo, J.A.; Nordfors, K.M.; Hilvo, M.; Rantala, I.J.; Soini, Y.; Parkkila, A.-K.; Pastoreková, S.; Pastorek, J.; Parkkila, S.M.; Haapasalo, H.K. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin. Cancer Res. 2006, 12, 473–477. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007, 26, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase: Catalytic and inhibition mechanisms, distribution and physiological roles. In Carbonic Anhydrase: Its Inhibitors and Activators; Supuran, C.T., Scozzafava, A., Conway, J., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–23. [Google Scholar]
- Semenza, G.L. Hypoxia and cancer. Cancer Metastasis Rev. 2007, 26, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Trastour, C.; Benizri, E.; Ettore, F.; Ramaioli, A.; Chamorey, E.; Pouysségur, J.; Berra, E. HIF-1α and CA IX staining in invasive breast carcinomas: Prognosis and treatment outcome. Int. J. Cancer. 2007, 120, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; Supuran, C.T.; De Simone, G. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert. Opin. Ther. Pat. 2013, 23, 737–749. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ganesan, R.; Reynolds, G.; Gross, L.; Stevens, A.; Pastorek, J.; Murray, P.G.; Perunovic, B.; Anwar, M.S.; Billingham, L.; et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br. J. Cancer 2007, 96, 104–109. [Google Scholar] [CrossRef]
- Potter, C.P.; Harris, A.L. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br. J. Cancer 2003, 89, 2–7. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018). Expert. Opin. Ther. Pat. 2018, 28, 729–740. [Google Scholar] [CrossRef]
- Pastorekova, S.; Parkkila, S.; Pastorek, J.; Supuran, C.T. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J. Enzyme Inhib. Med. Chem. 2004, 19, 199–229. [Google Scholar] [CrossRef]
- Proescholdt, M.A.; Mayer, C.; Kubitza, M.; Schubert, T.; Liao, S.Y.; Stanbridge, E.J.; Ivanov, S.; Oldfield, E.H.; Brawanski, A.; Merrill, M.J. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005, 7, 465–475. [Google Scholar] [CrossRef]
- Järvelä, S.; Parkkila, S.; Bragge, H.; Kähkönen, M.; Parkkila, A.K.; Soini, Y.; Pastorekova, S.; Pastorek, J.; Haapasalo, H. Carbonic anhydrase IX in oligodendroglial brain tumors. BMC Cancer 2008, 8, 1. [Google Scholar] [CrossRef]
- Vuotikka, P.; Uusimaa, P.; Niemelä, M.; Väänänen, K.; Vuori, J.; Peuhkurinen, K. Serum myoglobin/carbonic anhydrase III ratio as a marker of reperfusion after miocardial infarction. Int. J. Cardiol. 2003, 91, 137–144. [Google Scholar] [CrossRef]
- Beuerle, J.R.; Azzazy, H.M.; Styba, G.; Duh, S.H.; Christenson, R.H. Characteristics of myoglobin, carbonic anhydrase III and the myoglobin/carbonic anhydrase III ratio in trauma, exercise, and myocardial infarction patients. Clin. Chim. Acta 2000, 294, 115–128. [Google Scholar] [CrossRef]
- Sapirstein, V.S.; Strocchi, P.; Gilbert, J.M. Properties and function of brain carbonic anhydrase. Ann. N. Y. Acad. Sci. 1984, 429, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, M.S.; Parkkila, A.K.; Parkkila, S.; Waheed, A.; Sly, W.S. Mitochondrial carbonic anhydrase in the nervous system: Expression in neuronal and glial cells. J. Neurochem. 2000, 75, 2212–2220. [Google Scholar] [CrossRef]
- Ruusuvuori, E.; Huebner, A.K.; Kirilkin, I.; Yukin, A.Y.; Blaesse, P.; Helmy, M.; Kang, H.J.; El Muayed, M.; Hennings, J.C.; Voipio, J.; et al. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013, 32, 2275–2286. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Agnati, L.F.; Tinner, B.; Staines, W.A.; Väänänen, K.; Fuxe, K. On the cellular localization and distribution of carbonic anhydrase II immunoreactivity in the rat brain. Brain. Res. 1995, 676, 10–24. [Google Scholar] [CrossRef]
- Giacobini, E. Localization of carbonic anhydrase in the nervous system. Science 1961, 134, 1524–1525. [Google Scholar] [CrossRef]
- Deitmer, J.W. Strategies for metabolic exchange between glial cells and neurons. Respir. Physiol. 2001, 129, 71–81. [Google Scholar] [CrossRef]
- Deitmer, J.W. Glial strategy for metabolic shuttling and neuronal function. Bioessays 2000, 22, 747–752. [Google Scholar] [CrossRef]
- Cammer, W.B.; Brion, L.P. Carbonic anhydrase in the nervous system. In The Carbonic Anhydrases: New Horizons; Chegwidden, W.R., Carter, N.D., Edwards, Y.H., Eds.; Birkhäuser: Basel, Switzerland, 2000; pp. 475–489. [Google Scholar]
- Sun, M.K.; Alkon, D.L. Carbonic anhydrase gating of attention: Memory therapy and enhancement. Trends Pharmacol. Sci. 2002, 23, 83–89. [Google Scholar] [CrossRef]
- Gavernet, L. Carbonic Anhydrase and Epilepsy. In Antiepileptic Drug Discovery; Talevi, A., Rocha, L., Eds.; Humana Press: New York, NY, USA, 2016; pp. 37–51. [Google Scholar]
- Shen, J.; Xu, S. Theoretical analysis of carbon-13 magnetization transfer for in vivo exchange between a-ketoglutarate and glutamate. NMR Biomed. 2006, 19, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Shen, J. In vivo carbon-13 magnetization transfer effect. Detection of aspartate aminotransferase reaction. Magn. Reson. Med. 2005, 54, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, J.; Shen, J. In vivo 13C saturation transfer effect of the lactate dehydrogenase reaction. Magn. Reson. Med. 2007, 57, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, J. Relayed 13C magnetization transfer: Detection of malate dehydrogenase reaction in vivo. J. Magn. Reson. 2007, 184, 344–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Singh, S.; Shen, J. 13C saturation transfer effect of carbon dioxide-bicarbonate exchange catalyzed by carbonic anhydrase in vivo. Magn. Reson. Med. 2008, 59, 492–498. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Shen, J. Inverse Polarization Transfer for Detecting in Vivo 13C Magnetization Transfer Effect of Specific Enzyme Reactions in 1H Spectra. Magn. Reson. Imaging 2008, 26, 413–419. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Yang, J.; Shen, J. Novel strategy for cerebral 13C MRS using very low RF power for proton decoupling. Magn. Reson. Med. 2007, 57, 265–271. [Google Scholar] [CrossRef]
- Li, S.; An, L.; Duan, Q.; Araneta, M.F.; Johnson, C.S.; Shen, J. Determining the rate of carbonic anhydrase reaction in the human brain. Sci. Rep. 2018, 8, 2328. [Google Scholar] [CrossRef]
- Johnston-Wilson, N.L.; Sims, C.D.; Hofmann, J.P.; Anderson, L.; Shore, A.D.; Torrey, E.F.; Yolken, R.H. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol. Psychiatry 2000, 5, 142–149. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med. Chem. 2011, 3, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.; Ossipov, M.H.; Kaila, K.; Price, T.J. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain 2010, 148, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Reiss, W.G.; Oles, K.S. Acetazolamide in the treatment of seizures. Ann. Pharmacother. 1996, 30, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Carta, F.; Cozzi, A.; Ghelardini, C.; Supuran, C.T. Carbonic anhydrase inhibition for the management of cerebral ischemia: In vivo evaluation of sulfonamide and coumarin inhibitors. Enzyme Inhib. Med. Chem. 2016, 31, 894–899. [Google Scholar] [CrossRef]
- Cowen, M.A.; Gree, M.; Bertoll, D.N.; Abbot, K. A treatment for tardive dyskinesia and some other extrapyramidal symptoms. J. Clin. Psychopharmacol. 1997, 17, 190–193. [Google Scholar] [CrossRef]
- Uitti, R.J. Medical treatment of essential tremor and Parkinson’s disease. Geriatrics 1998, 53, 46–48, 53–57. [Google Scholar]
- Bernhard, W.N.; Schalik, L.M.; Delaney, P.A.; Bernhard, T.M.; Barnas, G.M. Acetazolamide plus low-dose dexamethasone is better than acetazolamide alone to ameliorate symptoms of acute mountain sickness. Aviat. Space. Environ. Med. 1998, 69, 883–886. [Google Scholar]
- Makoto, H.; Masaaki, I.; Haruo, S.; Hiroshi, I.; Koji, M. A case of schizophrenia with favorable effect of acetazolamide. Kyushu Neuropsychiatry 1996, 42, 189–193. [Google Scholar]
- Ahmed, S.E.; Khan, A.H. Acetazolamide: Treatment of psychogenic polydipsia. Cureus. 2017, 9, e1553. [Google Scholar] [CrossRef]
- Hayes, S.G. Acetazolamide in bipolar affective disorders. Ann. Clin. Psychiatry. 1994, 6, 91–98. [Google Scholar] [CrossRef]
- Brandt, C.; Grunze, H.; Normann, C.; Walden, J. Acetazolamide in the treatment of acute mania. A case report. Neuropsychobiology 1998, 38, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Di Cesare Mannelli, L.; Pinard, M.; Ghelardini, C.; Scozzafava, A.; McKenna, R.; Supurana, C.T. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg. Med. Chem. 2015, 23, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert. Rev. Neurother. 2015, 15, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert. Opin. Ther. Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef]
- Shank, R.P.; Gardocki, J.F.; Streeter, A.J.; Maryanoff, B.E. An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 2000, 41, S3–S9. [Google Scholar] [CrossRef]
- Kudin, A.P.; Debska-Vielhaber, G.; Vielhaber, S.; Elger, C.E.; Kunz, W.S. The mechanism of neuroprotection by topiramate in an animal model of epilepsy. Epilepsia 2004, 45, 1478–1487. [Google Scholar] [CrossRef]
- Bermejo, P.E.; Dorado, R. Zonisamide for migraine prophylaxis in patient’s refractory to topiramate. Clin. Neuropharmacol. 2009, 32, 103–106. [Google Scholar] [CrossRef]
- Lai, E.C.; Chang, C.H.; Kao Yang, Y.H.; Lin, S.J.; Lin, C.Y. Effectiveness of sulpiride in adult patients with schizophrenia. Schizophr. Bull. 2013, 3, 673–683. [Google Scholar] [CrossRef]
- Kaila, K.; Voipio, J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 1987, 330, 163–165. [Google Scholar] [CrossRef]
- Marini, S.; De Berardis, D.; Vellante, F.; Santacroce, R.; Orsolini, L.; Valchera, A.; Girinelli, G.; Carano, A.; Fornaro, M.; Gambi, F.; et al. Celecoxib Adjunctive Treatment to Antipsychotics in Schizophrenia: A Review of Randomized Clinical Add-On Trials. Mediators Inflamm. 2016, 2016, 3476240. [Google Scholar] [CrossRef]
- Bavaresco, D.V.; Colonetti, T.; Grande, A.J.; Colom, F.; Valvassori, S.S.; Quevedo, J.; da Rosa, M.I. Efficacy of Celecoxib Adjunct Treatment on Bipolar Disorder: Systematic Review and Meta-Analysis. CNS Neurol. Disord. Drug. Targets. 2019, 18, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Shalbafan, M.; Malekpour, F.; Donboli, S.; Shirazi, E.; Moridian, M. The role of celecoxib in treatment of psychiatric disorders: A review article. J. Neurol. Psychol. 2018, 6, 4. [Google Scholar]
- Dodgson, S.J.; Shank, R.P.; Maryanoff, B.E. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 2000, 41, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Alger, J.R.; Shulman, R.G. NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q. Rev. Biophys. 1984, 17, 83–124. [Google Scholar] [CrossRef] [PubMed]
- Kuchel, P.W. Spin-exchange NMR spectroscopy in studies of the kinetics of enzymes and membrane transport. NMR Biomed. 1990, 3, 102–119. [Google Scholar] [CrossRef]
- Rudin, M.; Sauter, A. Measurement of Reaction Rates In Vivo Using Magnetization Transfer Techniques. In In-Vivo Magnetic Resonance Spectroscopy II: Localization and Spectral Editing; Rudin, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 257–293. [Google Scholar]
- Ames, A. CNS energy metabolism as related to function. Brain. Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef]
- Weyne, J.; Demeester, G.; Leusen, I. Bicarbonate and chloride shifts in rat brain during acute and prolonged respiratory acid-base changes. Arch. Int. Physiol. Biochim. 1968, 76, 415–433. [Google Scholar] [CrossRef]
- Torrey, H.C. Bloch Equations with Diffusion Terms. Phys. Rev. 1956, 104, 563–565. [Google Scholar] [CrossRef]
- Zhou, J.; van Zijl, P.C.M. Chemical exchange saturation transfer imaging and spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 48, 109–136. [Google Scholar] [CrossRef]
- Baguet, E.; Roby, C. Off-resonance irradiation effect in steady-state NMR saturation transfer. J. Magn. Reson. 1997, 128, 149–160. [Google Scholar] [CrossRef]
- Kingsley, P.B.; Monahan, W.G. Corrections for off-resonance effects and incomplete saturation in conventional (two-site) saturation-transfer kinetic measurements. Magn. Reson. Med. 2000, 43, 810–819. [Google Scholar] [CrossRef]
- Brown, T.R. Saturation transfer in living systems. Philos Trans. R. Soc. Lond. B. Biol. Sci. 1980, 289, 441–444. [Google Scholar] [PubMed]
- Led, J.J.; Gesmar, H. The applicability of the magnetization-transfer NMR technique to determine chemical exchange rates in extreme cases. The importance of complementary experiments. J. Magn. Reson. 1982, 49, 444–463. [Google Scholar] [CrossRef]
- Shporer, M.; Forster, R.E.; Civan, M.M. Kinetics of CO2 exchange in human erythrocytes analyzed by 13C-NMR. Am. J. Physiol. 1984, 246, C231–C234. [Google Scholar] [CrossRef]
- Ruusuvuori, E.; Kaila, K. Carbonic anhydrases and brain pH in the control of neuronal excitability. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Frost, S.C., McKenna, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 271–290. [Google Scholar]
- Xiong, Z.Q.; Stringer, J.L. Regulation of extracellular pH in the developing hippocampus. Dev. Brain Res. 2000, 122, 113–117. [Google Scholar] [CrossRef]
- Aram, J.A.; Lodge, D. Epileptiform activity induced by alkalosis in rat neocortical slices: Block by antagonists of N-methyl-D-aspartate. Neurosci. Lett. 1987, 83, 345–350. [Google Scholar] [CrossRef]
- White, H.S. Woodbury, D.M.; Chen, C.F.; Kemp, J.W.; Chow, S.Y.; Yen-Chow, Y.C. Role of glial cation and anion transport mechanisms in etiology and arrest of seizures. Adv. Neurol. 1986, 44, 695–712. [Google Scholar]
- Thiry, A.; Dogné, J.M.; Supuran, C.T.; Masereel, B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr. Top. Med. Chem. 2007, 7, 855–864. [Google Scholar] [CrossRef]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: A patent review. Expert. Opin. Ther. Pat. 2013, 23, 717–724. [Google Scholar] [CrossRef]
- Hamidi, S.; Avoli, M. Carbonic anhydrase inhibition by acetazolamide reduces in vitro epileptiform synchronization. Neuropharmacology 2015, 95, 377–387. [Google Scholar] [CrossRef]
- Petroff, O.A.; Rothman, D.L.; Behar, K.L.; Mattson, R.H. Low brain GABA level is associated with poor seizure control. Ann. Neurol. 1996, 40, 908–991. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.; Edden, R.A. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.M.; Degnan, A.J. GABA-Based Evaluation of Neurologic Conditions: MR Spectroscopy. AJNR. Am. J. Neuroradiol. 2013, 34, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.L.; Petroff, O.A.; Behar, K.L.; Mattson, R.H. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc. Nat. Acad. Sci USA 1993, 90, 5662–5666. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005, 562, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.C.; McKenna, R. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease and Industrial Applications; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Inoue, H.; Hazama, H.; Hamazoe, K.; Ichikawa, M.; Omura, F.; Fukuma, E.; Inoue, K.; Umezawa, Y. Antipsychotic and prophylactic effects of acetazolamide (Diamox) on atypical psychosis. Folia Psychiatr. Neurol. Jpn. 1984, 38, 425–436. [Google Scholar] [CrossRef]
- Karunakaran, K.B.; Chaparala, S.; Ganapathiraju, M.K. Potentially repurposable drugs for schizophrenia identified from its interactome. Sci. Rep. 2019, 9, 12682. [Google Scholar] [CrossRef]
- Kakunje, A.; Prabhu, A.; Es, S.P.; Karkal, R.; Pookoth, R.K.; Rekha, P.D. Acetazolamide for antipsychotic-associated weight gain in schizophrenia. J. Clin. Psychopharmacol. 2018, 38, 652–653. [Google Scholar] [CrossRef]
- Erzengin, M.; Bilen, C.; Ergun, A.; Gencer, N. Antipsychotic agents screened as human carbonic anhydrase I and II inhibitors. Arch. Physiol. Biochem. 2014, 120, 29–33. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase activators. Future Med. Chem. 2018, 10, 561–573. [Google Scholar] [CrossRef]
- Sacks, W.; Esser, A.H.; Sacks, S. Inhibition of pyruvate dehydrogenase complex (PDHC) by antipsychotic drugs. Biol Psychiatry 1991, 29, 176–182. [Google Scholar] [CrossRef]
- Sacks, W.; Esser, A.H.; Feitel, B.; Abbott, K. Acetazolamide and thiamine: An ancillary therapy for chronic mental illness. Psychiatry Res. 1989, 28, 279–288. [Google Scholar] [CrossRef]
- Mechtcheriakov, S.; Oehl, M.A.; Hausmann, A.; Fleischhacker, W.W.; Boesch, S.; Schocke, M.; Donnemiller, E. Schizophrenia and episodic ataxia type 2. J. Neurol. Neurosurg. Psychiatry 2003, 74, 688–689. [Google Scholar] [CrossRef] [PubMed][Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomar, J.S.; Shen, J. Characterization of Carbonic Anhydrase In Vivo Using Magnetic Resonance Spectroscopy. Int. J. Mol. Sci. 2020, 21, 2442. https://doi.org/10.3390/ijms21072442
Tomar JS, Shen J. Characterization of Carbonic Anhydrase In Vivo Using Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences. 2020; 21(7):2442. https://doi.org/10.3390/ijms21072442
Chicago/Turabian StyleTomar, Jyoti Singh, and Jun Shen. 2020. "Characterization of Carbonic Anhydrase In Vivo Using Magnetic Resonance Spectroscopy" International Journal of Molecular Sciences 21, no. 7: 2442. https://doi.org/10.3390/ijms21072442
APA StyleTomar, J. S., & Shen, J. (2020). Characterization of Carbonic Anhydrase In Vivo Using Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences, 21(7), 2442. https://doi.org/10.3390/ijms21072442