Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance
Abstract
1. Introduction
2. Results
2.1. The Maize Genome Contains 46 TCP Family Genes
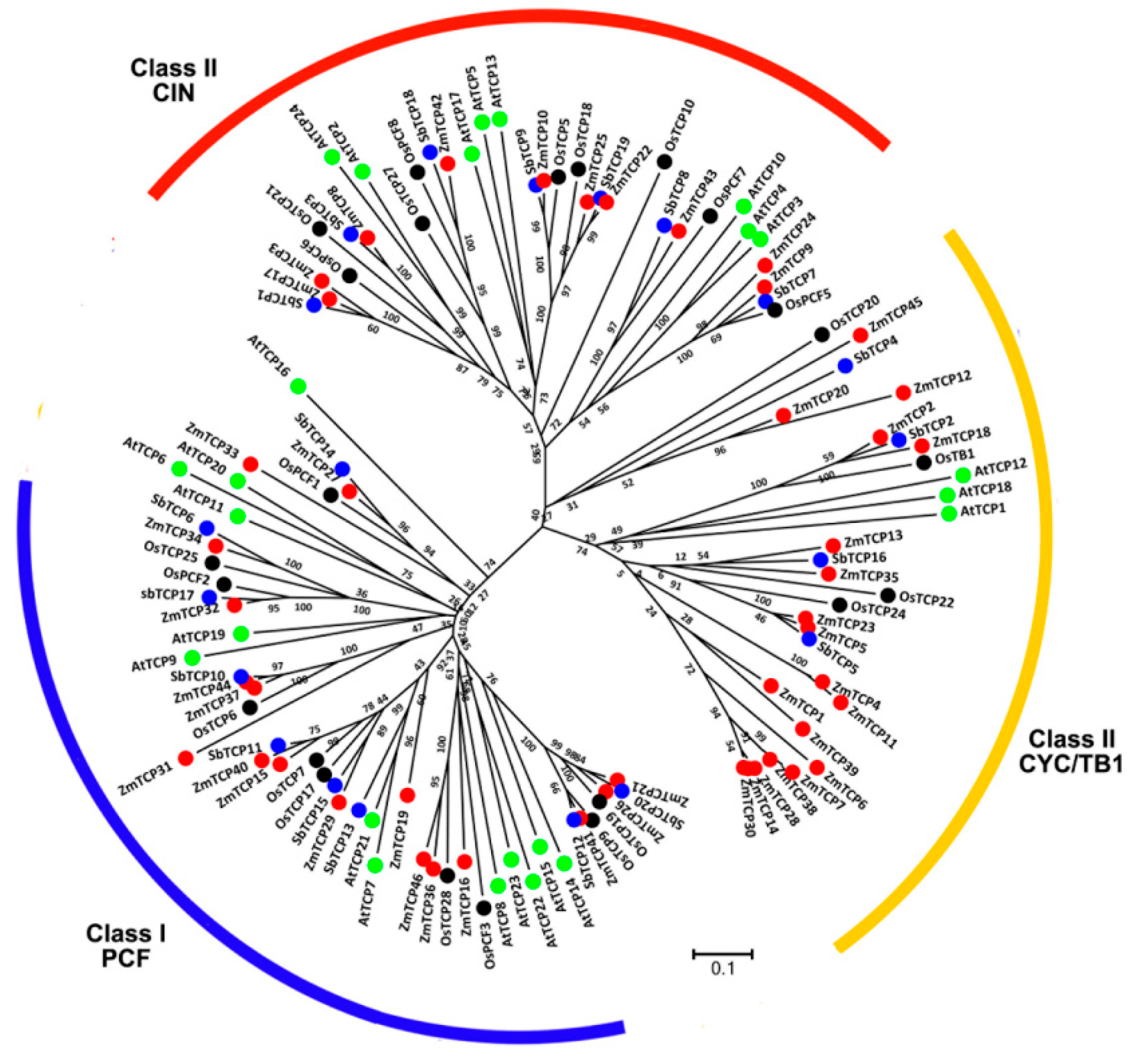
2.2. Maize Contains Roughly Twice as Many TCP Genes as Rice and Sorghum
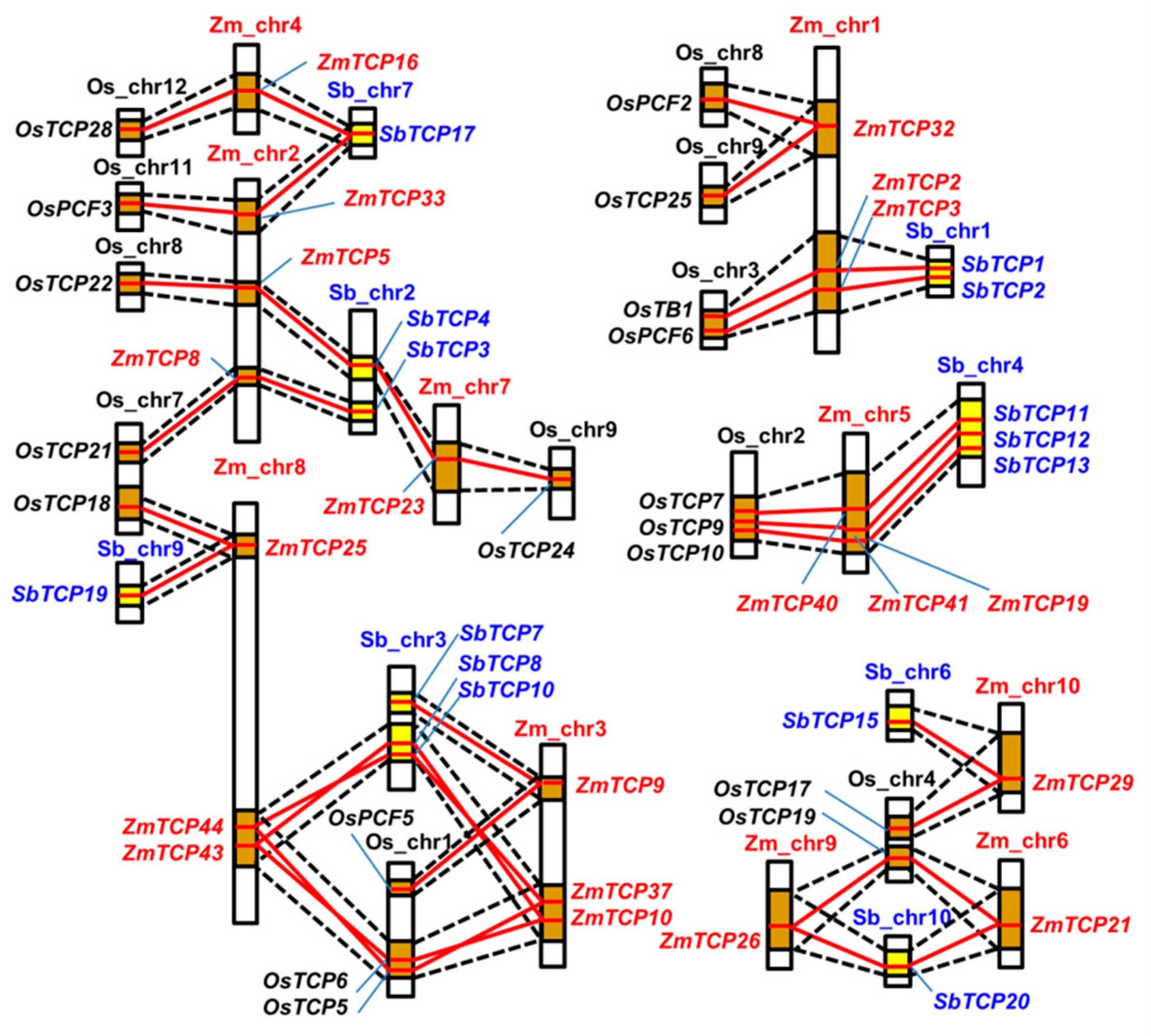
2.3. Many TCP Genes are Found in the Syntenic Segments of Rice, Sorghum, and Maize Genomes
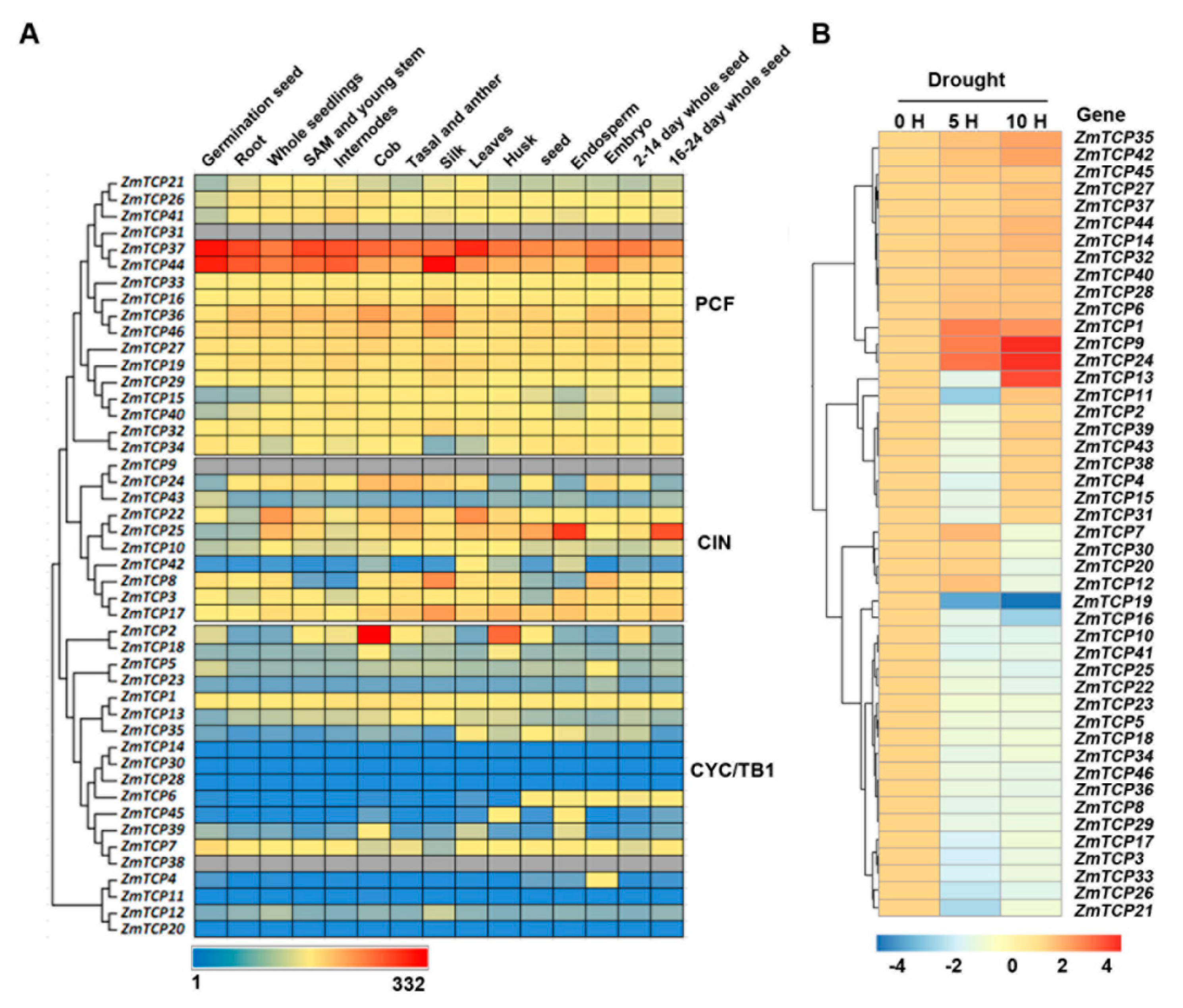
2.4. Expression Profiles of ZmTCP Genes
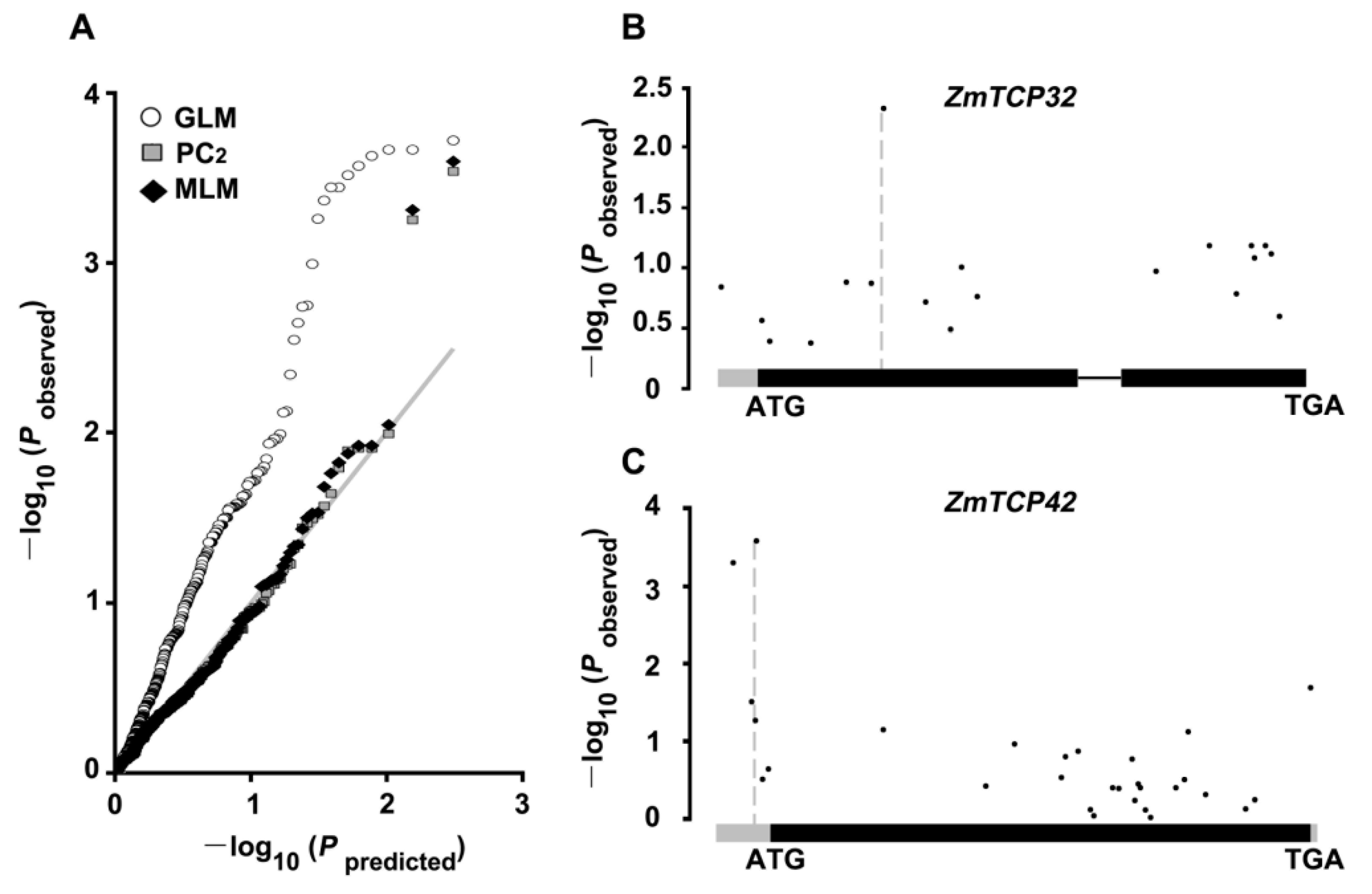
2.5. Association Analysis of Natural Variations in ZmTCP Genes Identified Two ZmTCP Genes Associated with Drought Tolerance in Maize
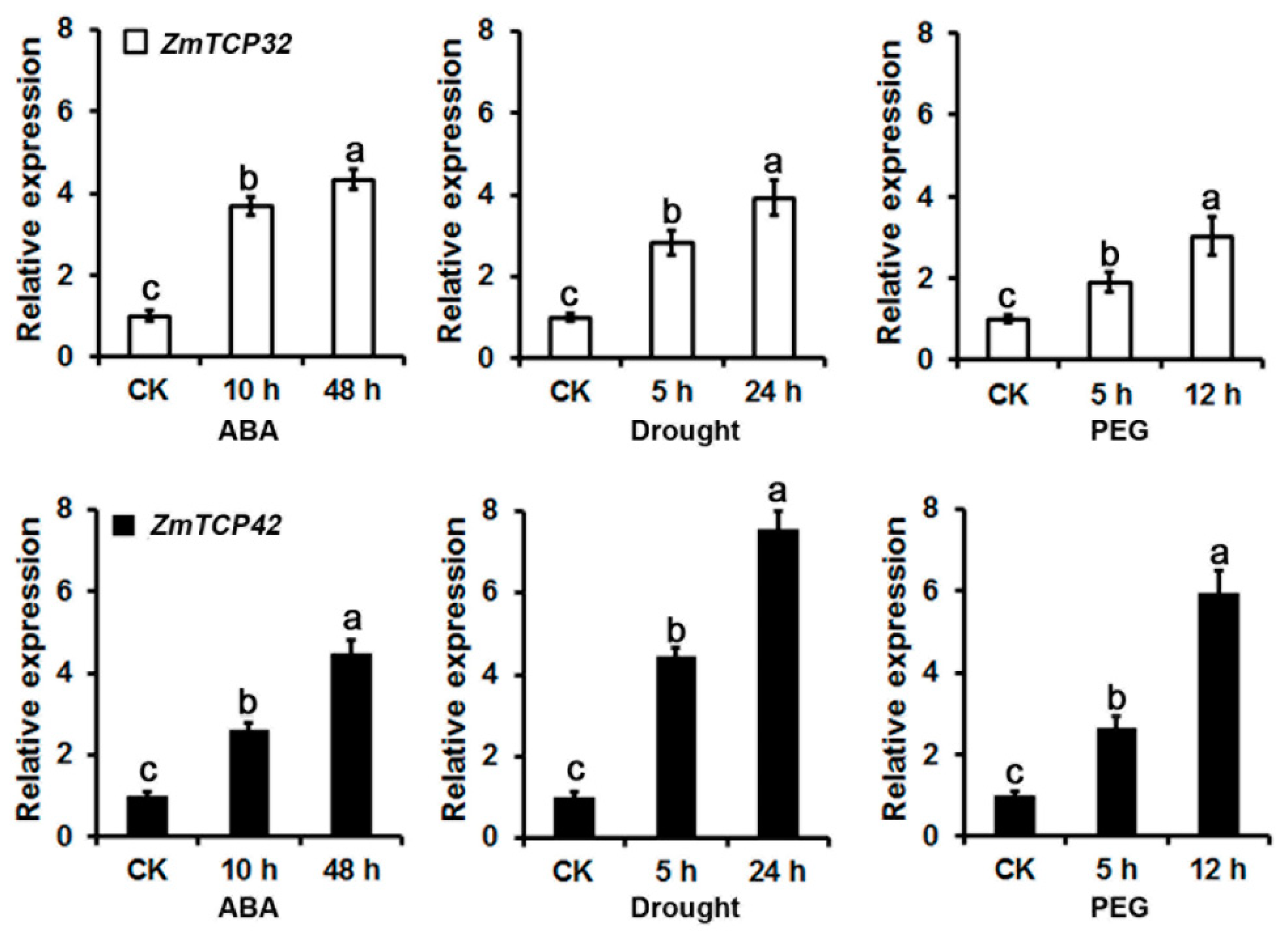
2.6. ZmTCP32 and ZmTCP42 Are Both Induced by ABA Treatment and Drought Stress
2.7. Overexpression of ZmTCP42 Enhances Drought Resistance in Transgenic Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Stress Treatments
4.2. Reverse Transcription PCR and RT-qPCR Analysis
4.3. Generation of Transgenic Plants
4.4. Seed Germination and Drought Phenotype Analysis
4.5. Identification of the TCP Proteins in the Maize Genome
4.6. Gene Structure and Phylogenetic Analysis
4.7. Association Analysis
4.8. Tissue Expression Profile and Microarray Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TCP | the teosinte branched1/cycloidea/proliferating |
| TF | transcription factor |
| CYC/TB1 | cycloidea/teosinte branched 1 |
| PCF | proliferating cell nuclear antigen factor |
| CIN | CINCINNATA of Antirrhinum |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| GLM | general linear model |
| SNP | single nucleotide polymorphism |
| MLM | mixed linear model |
| ABA | abscisic acid |
| PEG | polyethylene glycol |
References
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2007, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Roberts, M.J.; Schlenker, W.; Braun, N.; Little, B.B.; Rejesus, R.M.; Hammer, G.L. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 2014, 344, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF transcription factors: Their structure, function and role in abiotic stress tolerance in plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Martin-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J. 2002, 30, 337–348. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Herve, C. TCP Transcription Factors Predate the Emergence of Land Plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.E.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis Among Eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ma, H.; Wang, J.; Zhang, D. Genome-Wide Comparative Analysis and Expression Pattern of TCP Gene Families in Arabidopsis thaliana and Oryza sativa. J. Integr. Plant Biol. 2007, 49, 885–897. [Google Scholar] [CrossRef]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Q.; Sun, R.; Xie, F.; Jones, D.C.; Zhang, B. Genome-wide identification and expression analysis of TCP transcription factors in Gossypium raimondii. Sci. Rep. 2014, 4, 6645. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, Z.; Li, Y.; Geng, S.; Song, G.; Guan, J.; Jia, M.; Wang, F.; Sun, G.; Feng, N.; et al. Genome-Wide Identification and Expression Profiling of the TCP Family Genes in Spike and Grain Development of Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 1282. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Dhaka, N.; Bakshi, M.; Jung, K.H.; Sharma, M.K.; Sharma, R. Comparative phylogenomic analysis provides insights into TCP gene functions in Sorghum. Sci. Rep. 2016, 6, 38488. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Li, S. The Arabidopsis thaliana TCP transcription factors: A broadening horizon beyond development. Plant Signal. Behav. 2015, 10, e1044192. [Google Scholar]
- Danisman, S. TCP Transcription Factors at the Interface between Environmental Challenges and the Plant’s Growth Responses. Front. Plant Sci. 2016, 7, 1930. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, N.; Bhardwaj, V.; Sharma, M.K.; Sharma, R. Evolving Tale of TCPs: New Paradigms and Old Lacunae. Front. Plant Sci. 2017, 8, 479. [Google Scholar] [CrossRef]
- Nicolas, M.; Cubas, P. The Role of TCP Transcription Factors in Shaping Flower Structure, Leaf Morphology, and Plant Architecture. In Plant Transcription Factors; Elsevier: Amsterdam, The Netherlands, 2016; pp. 249–267. [Google Scholar]
- Resentini, F.; Felipo-Benavent, A.; Colombo, L.; Blázquez, M.A.; Alabadí, D.; Masiero, S. TCP14 and TCP15 Mediate the Promotion of Seed Germination by Gibberellins in Arabidopsis thaliana. Mol. Plant 2015, 8, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Yanai, O.; Shani, E.; Russ, D.; Ori, N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 2011, 68, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of Jasmonate Biosynthesis and Senescence by miR319 Targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. TCP Transcription Factors Regulate the Activities of ASYMMETRIC LEAVES1 and miR164, as Well as the Auxin Response, during Differentiation of Leaves in Arabidopsis. Plant Cell 2010, 22, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Efroni, I.; Han, S.-K.; Kim, H.; Wu, M.-F.; Steiner, E.; Birnbaum, K.; Hong, J.; Eshed, Y.; Wagner, D. Regulation of Leaf Maturation by Chromatin-Mediated Modulation of Cytokinin Responses. Dev. Cell 2013, 24, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Hanhart, C.J.; Hilhorst, H.W.; Karssen, C.M. In Vivo Inhibition of Seed Development and Reserve Protein Accumulation in Recombinants of Abscisic Acid Biosynthesis and Responsiveness Mutants in Arabidopsis thaliana. Plant Physiol. 1989, 90, 463–469. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Tyagi, A.K. OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 2015, 5, 9998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive Expression of a miR319 Gene Alters Plant Development and Enhances Salt and Drought Tolerance in Transgenic Creeping Bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Romero, P.; Barrero-Sicilia, C.; Gomez-Cadenas, A.; Carbonero, P.; Onate-Sanchez, L. Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J. Exp. Bot. 2012, 63, 1937–1949. [Google Scholar] [CrossRef]
- Gonzalez-Grandio, E.; Poza-Carrion, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1 Promotes Axillary Bud Dormancy in Response to Shade in Arabidopsis. Plant Cell 2013, 25, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Grandio, E.; Pajoro, A.; Franco-Zorrilla, J.M.; Tarancon, C.; Immink, R.G.; Cubas, P. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 2017, 114, E245–E254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, Z.; Yang, X.; Wang, W.; Fu, J.; Wang, J.; Han, Y.; Chai, Y.; Guo, T.; Yang, N.; et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef]
- Yan, J.; Kandianis, C.B.; Harjes, C.E.; Bai, L.; Kim, E.-H.; Yang, X.; Skinner, D.J.; Fu, Z.; Mitchell, S.; Li, Q.; et al. Rare genetic variation at Zea mays crtRB1 increases beta-carotene in maize grain. Nat. Genet. 2010, 42, 322. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Li, W.; Ku, L.; Wang, C.; Ye, J.; Li, K.; Yang, N.; Li, Y.; Zhong, T.; et al. CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the postdomestication spread of maize. Proc. Natl. Acad. Sci. USA 2013, 110, 16969–16974. [Google Scholar] [CrossRef]
- Wang, H.; Qin, F. Genome-Wide Association Study Reveals Natural Variations Contributing to Drought Resistance in Crops. Front. Plant Sci. 2017, 8, 1110. [Google Scholar] [CrossRef]
- Chai, W.; Jiang, P.; Huang, G.; Jiang, H.; Li, X. Identification and expression profiling analysis of TCP family genes involved in growth and development in maize. Physiol. Mol. Biol. Plants 2017, 23, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, R.S.; Lin, H.; Childs, K.L.; Hansey, C.N.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. Genome-wide atlas of transcription during maize development. Plant J. 2011, 66, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.D.; Han, L.H.; Tao, M.; Hu, Q.Q.; Wu, W.Y.; Zhang, J.B.; Li, X.B.; Huang, G.Q. Genome-wide identification and characterization of TCP transcription factor genes in upland cotton (Gossypium hirsutum). Sci. Rep. 2017, 7, 10118. [Google Scholar] [CrossRef]
- Ma, X.; Ma, J.; Fan, D.; Li, C.; Jiang, Y.; Luo, K. Genome-wide Identification of TCP Family Transcription Factors from Populus euphratica and Their Involvement in Leaf Shape Regulation. Sci. Rep. 2016, 6, 32795. [Google Scholar] [CrossRef]
- Mondragón-Palomino, M.; Trontin, C. High time for a roll call: Gene duplication and phylogenetic relationships of TCP-like genes in monocots. Ann. Bot. 2011, 107, 1533–1544. [Google Scholar] [CrossRef]
- Sharma, R.; Kapoor, M.; KTyagi, A.; Kapoor, S. Comparative transcript profiling of TCP family genes provide insight into gene functions and diversification in rice and Arabidopsis. J. Plant Mol. Biol. Biotechnol. 2010, 1, 24–38. [Google Scholar]
- Feng, W.; Lindner, H.; Robbins, N.E., 2nd; Dinneny, J.R. Growing Out of Stress: The Role of Cell- and Organ-Scale Growth Control in Plant Water-Stress Responses. Plant Cell 2016, 28, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Gaut, B.S.; Doebley, J. DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl. Acad. Sci. USA 1997, 94, 6809–6814. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Mao, D.; Liu, X.; Ji, C.; Li, X.; Zhao, X.; Cheng, Z.; Chen, C.; Zhu, L. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Environ. 2013, 36, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Xu, S.; Zhang, Z.; Xu, Y.; Zhang, J.; Chong, K. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 2018, 218, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, B.; Qin, F. Arabidopsis RZFP34/CHYR1, a Ubiquitin E3 Ligase, Regulates Stomatal Movement and Drought Tolerance via SnRK2.6-Mediated Phosphorylation. Plant Cell 2015, 27, 3228–3244. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.W.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Yu, L.; Li, Z.; Liu, H.; Han, R. Molecular evolution and gene expression differences within the HD-Zip transcription factor family of Zea mays L. Genetica 2016, 144, 243–257. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Locus ID | Chr | CDS Length (bp) | Protein Length (aa) | Number of Exons | MW | pI | Class |
|---|---|---|---|---|---|---|---|---|
| ZmTCP1 | GRMZM2G166687 | 1 | 723 | 240 | 1 | 26.11 | 5.15 | CYC/TB1 |
| ZmTCP2 | AC233950.1_FG002 | 1 | 1131 | 376 | 1 | 39.86 | 7.8 | CYC/TB1 |
| ZmTCP3 | GRMZM2G115516 | 1 | 1161 | 386 | 2 | 39.36 | 9.33 | CIN |
| ZmTCP4 | AC234521.1_FG006 | 2 | 768 | 255 | 2 | 27.71 | 10.62 | CYC/TB1 |
| ZmTCP5 | GRMZM2G110242 | 2 | 831 | 276 | 1 | 29.9 | 6.26 | CYC/TB1 |
| ZmTCP6 | GRMZM2G088440 | 2 | 549 | 182 | 5 | 21.14 | 8.65 | CYC/TB1 |
| ZmTCP7 | GRMZM2G414114 | 2 | 1104 | 367 | 6 | 42.29 | 8.44 | CYC/TB1 |
| ZmTCP8 | GRMZM2G020805 | 2 | 1323 | 440 | 2 | 46.22 | 9.46 | CIN |
| ZmTCP9 | AC205574.3_FG006 | 3 | 1698 | 565 | 2 | 58.83 | 7.1 | CIN |
| ZmTCP10 | GRMZM2G166946 | 3 | 852 | 283 | 2 | 30.48 | 8.98 | CIN |
| ZmTCP11 | GRMZM2G055024 | 4 | 1263 | 420 | 3 | 45.5 | 10.82 | CYC/TB1 |
| ZmTCP12 | GRMZM2G062711 | 4 | 564 | 187 | 1 | 21.58 | 12.05 | CIN |
| ZmTCP13 | GRMZM2G060319 | 4 | 825 | 274 | 1 | 29.35 | 5.96 | CYC/TB1 |
| ZmTCP14 | GRMZM2G135461 | 4 | 402 | 132 | 3 | 15.09 | 7.32 | CYC/TB1 |
| ZmTCP15 | GRMZM2G078077 | 4 | 657 | 218 | 1 | 22.97 | 10.02 | PCF |
| ZmTCP16 | GRMZM2G003944 | 4 | 1164 | 387 | 1 | 40.76 | 9.33 | PCF |
| ZmTCP17 | GRMZM2G089361 | 5 | 1146 | 381 | 1 | 39.05 | 9.58 | CIN |
| ZmTCP18 | AC190734.2_FG003 | 5 | 1080 | 359 | 1 | 38.89 | 7.01 | CYC/TB1 |
| ZmTCP19 | GRMZM2G445944 | 5 | 624 | 207 | 1 | 21.12 | 10.26 | PCF |
| ZmTCP20 | GRMZM2G031905 | 6 | 435 | 144 | 3 | 16.01 | 9.89 | CIN |
| ZmTCP21 | GRMZM2G142751 | 6 | 1140 | 379 | 1 | 39.09 | 8.02 | PCF |
| ZmTCP22 | GRMZM2G120151 | 6 | 897 | 298 | 2 | 30.9 | 8.07 | CIN |
| ZmTCP23 | GRMZM2G064628 | 7 | 843 | 280 | 1 | 30.23 | 6.33 | CYC/TB1 |
| ZmTCP24 | GRMZM2G015037 | 8 | 1974 | 657 | 4 | 69.55 | 6.61 | CIN |
| ZmTCP25 | GRMZM2G035944 | 8 | 828 | 275 | 2 | 29.59 | 9.02 | CIN |
| ZmTCP26 | GRMZM2G113888 | 9 | 1209 | 402 | 1 | 40.66 | 8.81 | PCF |
| ZmTCP27 | GRMZM2G096610 | 10 | 531 | 176 | 1 | 18.33 | 7.43 | PCF |
| ZmTCP28 | GRMZM2G458087 | 10 | 495 | 164 | 3 | 18.83 | 8.37 | CYC/TB1 |
| ZmTCP29 | GRMZM2G465091 | 10 | 624 | 207 | 1 | 21.01 | 10.01 | PCF |
| ZmTCP30 | GRMZM2G454571 | 1 | 399 | 132 | 3 | 15.11 | 7.27 | CYC/TB1 |
| ZmTCP31 | AC213524.3_FG003 | 1 | 831 | 276 | 2 | 29.56 | 9.83 | PCF |
| ZmTCP32 | GRMZM2G107031 | 1 | 972 | 323 | 2 | 34.02 | 5.98 | PCF |
| ZmTCP33 | GRMZM2G416524 | 2 | 894 | 297 | 3 | 32.37 | 10.43 | PCF |
| ZmTCP34 | AC199782.5_FG003 | 2 | 1032 | 343 | 1 | 35.43 | 5.13 | PCF |
| ZmTCP35 | GRMZM5G824514 | 3 | 297 | 98 | 1 | 11.76 | 12.23 | CYC/TB1 |
| ZmTCP36 | GRMZM2G089638 | 3 | 1251 | 416 | 1 | 42.91 | 5.79 | PCF |
| ZmTCP37 | GRMZM2G092214 | 3 | 975 | 324 | 2 | 34.35 | 6.7 | PCF |
| ZmTCP38 | GRMZM2G359599 | 4 | 522 | 173 | 4 | 19.58 | 8.6 | CYC/TB1 |
| ZmTCP39 | GRMZM2G170232 | 4 | 396 | 131 | 4 | 14.38 | 8.87 | CYC/TB1 |
| ZmTCP40 | GRMZM2G178603 | 5 | 663 | 220 | 1 | 22.76 | 10.02 | PCF |
| ZmTCP41 | GRMZM2G077755 | 5 | 1200 | 400 | 1 | 40.49 | 9.2 | PCF |
| ZmTCP42 | GRMZM2G180568 | 7 | 972 | 323 | 1 | 33.71 | 8.11 | CIN |
| ZmTCP43 | GRMZM2G148022 | 8 | 2337 | 778 | 15 | 84.78 | 7.35 | CIN |
| ZmTCP44 | GRMZM2G034638 | 8 | 948 | 315 | 2 | 33.08 | 6.57 | PCF |
| ZmTCP45 | GRMZM2G424261 | 10 | 489 | 162 | 3 | 17.27 | 5.91 | CIN |
| ZmTCP46 | GRMZM2G093895 | 10 | 1218 | 405 | 1 | 41.09 | 5.95 | PCF |
| Locus ID | Gene Name | Polymorphic Name | GLM | PCA | PCA + K | |
|---|---|---|---|---|---|---|
| p ≤ 0.01 | p ≤ 0.01 | p ≤ 0.01 | p ≤ 0.001 | |||
| GRMZM2G166687 | ZmTCP1 | - | - | - | - | - |
| AC233950.1_FG002 | ZmTCP2 | 3 | 2 | 0 | 0 | 0 |
| GRMZM2G115516 | ZmTCP3 | 33 | 0 | 0 | 0 | 0 |
| AC234521.1_FG006 | ZmTCP4 | - | - | - | - | - |
| GRMZM2G110242 | ZmTCP5 | 1 | 0 | 0 | 0 | 0 |
| GRMZM2G088440 | ZmTCP6 | - | - | - | - | - |
| GRMZM2G414114 | ZmTCP7 | - | - | - | - | - |
| GRMZM2G020805 | ZmTCP8 | 21 | 0 | 0 | 0 | 0 |
| AC205574.3_FG006 | ZmTCP9 | 19 | 0 | 0 | 0 | 0 |
| GRMZM2G166946 | ZmTCP10 | 1 | 0 | 0 | 0 | 0 |
| GRMZM2G055024 | ZmTCP11 | - | - | - | - | - |
| GRMZM2G062711 | ZmTCP12 | - | - | - | - | - |
| GRMZM2G060319 | ZmTCP13 | 1 | 0 | 0 | 0 | 0 |
| GRMZM2G135461 | ZmTCP14 | - | - | - | - | - |
| GRMZM2G078077 | ZmTCP15 | 5 | 0 | 0 | 0 | 0 |
| GRMZM2G003944 | ZmTCP16 | - | - | - | - | - |
| GRMZM2G089361 | ZmTCP17 | 28 | 1 | 0 | 0 | 0 |
| AC190734.2_FG003 | ZmTCP18 | - | - | - | - | - |
| GRMZM2G445944 | ZmTCP19 | 7 | 0 | 0 | 0 | 0 |
| GRMZM2G031905 | ZmTCP20 | - | - | - | - | - |
| GRMZM2G142751 | ZmTCP21 | 2 | 0 | 0 | 0 | 0 |
| GRMZM2G120151 | ZmTCP22 | 2 | 0 | 0 | 0 | 0 |
| GRMZM2G064628 | ZmTCP23 | - | - | - | - | - |
| GRMZM2G015037 | ZmTCP24 | 2 | 0 | 0 | 0 | 0 |
| GRMZM2G035944 | ZmTCP25 | 27 | 1 | 0 | 0 | 0 |
| GRMZM2G113888 | ZmTCP26 | 4 | 0 | 0 | 0 | 0 |
| GRMZM2G096610 | ZmTCP27 | 12 | 0 | 0 | 0 | 0 |
| GRMZM2G458087 | ZmTCP28 | - | - | - | - | - |
| GRMZM2G465091 | ZmTCP29 | 1 | 0 | 0 | 0 | 0 |
| GRMZM2G454571 | ZmTCP30 | - | - | - | - | - |
| AC213524.3_FG003 | ZmTCP31 | - | - | - | - | - |
| GRMZM2G107031 | ZmTCP32 | 19 | 0 | 1 | 1 | 0 |
| GRMZM2G416524 | ZmTCP33 | - | - | - | - | - |
| AC199782.5_FG003 | ZmTCP34 | 12 | 0 | 0 | 0 | 0 |
| GRMZM5G824514 | ZmTCP35 | - | - | - | - | - |
| GRMZM2G089638 | ZmTCP36 | 2 | 0 | 0 | 0 | 0 |
| GRMZM2G092214 | ZmTCP37 | 34 | 3 | 0 | 0 | 0 |
| GRMZM2G359599 | ZmTCP38 | - | - | - | - | - |
| GRMZM2G170232 | ZmTCP39 | - | - | - | - | - |
| GRMZM2G178603 | ZmTCP40 | 2 | 1 | 0 | 0 | 0 |
| GRMZM2G077755 | ZmTCP41 | - | - | - | - | - |
| GRMZM2G180568 | ZmTCP42 | 29 | 2 | 2 | 2 | 2 |
| GRMZM2G148022 | ZmTCP43 | 1 | 0 | 0 | 0 | 0 |
| GRMZM2G034638 | ZmTCP44 | 22 | 6 | 0 | 0 | 0 |
| GRMZM2G424261 | ZmTCP45 | - | - | - | - | - |
| GRMZM2G093895 | ZmTCP46 | 27 | 2 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. https://doi.org/10.3390/ijms20112762
Ding S, Cai Z, Du H, Wang H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. International Journal of Molecular Sciences. 2019; 20(11):2762. https://doi.org/10.3390/ijms20112762
Chicago/Turabian StyleDing, Shuangcheng, Zhenzhen Cai, Hewei Du, and Hongwei Wang. 2019. "Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance" International Journal of Molecular Sciences 20, no. 11: 2762. https://doi.org/10.3390/ijms20112762
APA StyleDing, S., Cai, Z., Du, H., & Wang, H. (2019). Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. International Journal of Molecular Sciences, 20(11), 2762. https://doi.org/10.3390/ijms20112762





