Positive Childhood Experiences, Cognition, and Biomarkers of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cognitive Assessment Measures
2.3. Positive Childhood Experience Measures
2.4. MRI Imaging Measures
2.5. Amyloid PET Imaging Measures
2.6. Covariates
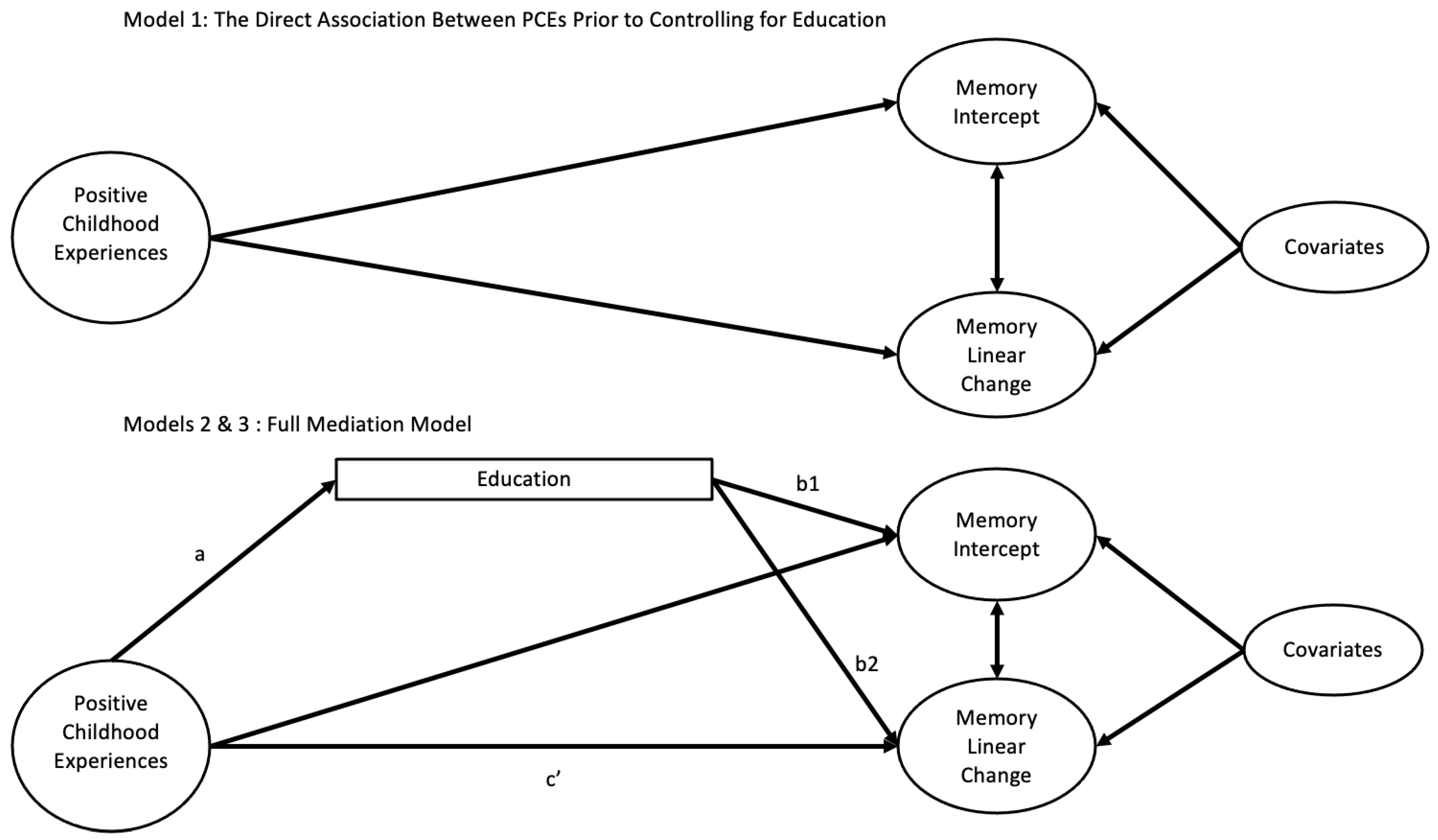
2.7. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Direct Effects of PCEs and Education on Memory
3.3. Indirect Effects of Education Through PCEs
3.4. The Association Between PCEs and Education on Hippocampal Volume and Amyloid Burden
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gold, A.L.; Meza, E.; Ackley, S.F.; Mungas, D.; Whitmer, R.; Mayeda, E.R.; Miles, S.; Eng, C.W.; Gilsanz, P.; Glymour, M.M. Are adverse childhood experiences associated with late-life cognitive performance across racial/ethnic groups: Results from the Kaiser Healthy Aging and Diverse Life Experiences study baseline. BMJ Open 2021, 11, e042125. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Jaussent, I.; Stewart, R.; Dupuy, A.M.; Courtet, P.; Malafosse, A.; Ancelin, M.L. Adverse childhood environment and late-life cognitive functioning. Int. J. Geriatr. Psychiatry 2011, 26, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Schickedanz, H.B.; Jennings, L.A.; Schickedanz, A. The Association Between Adverse Childhood Experiences and Positive Dementia Screen in American Older Adults. J. Gen. Intern. Med. 2022, 37, 2398–2404. [Google Scholar] [CrossRef]
- Tani, Y.; Fujiwara, T.; Kondo, K. Association Between Adverse Childhood Experiences and Dementia in Older Japanese Adults. JAMA Netw. Open 2020, 3, e1920740. [Google Scholar] [CrossRef]
- Briggs, E.C.; Amaya-Jackson, L.; Putnam, K.T.; Putnam, F.W. All adverse childhood experiences are not equal: The contribution of synergy to adverse childhood experience scores. Am. Psychol. 2021, 76, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Dekhtyar, S.; Wang, H.X.; Scott, K.; Goodman, A.; Koupil, I.; Herlitz, A. A Life-Course Study of Cognitive Reserve in Dementia--From Childhood to Old Age. Am. J. Geriatr. Psychiatry 2015, 23, 885–896. [Google Scholar] [CrossRef]
- Greenfield, E.A.; Moorman, S.M. Childhood Socioeconomic Status and Later Life Cognition: Evidence from the Wisconsin Longitudinal Study. J. Aging Health 2019, 31, 1589–1615. [Google Scholar] [CrossRef]
- Sege, R.D.; Harper Browne, C. Responding to ACEs with HOPE: Health Outcomes from Positive Experiences. Acad. Pediatr. 2017, 17, S79–S85. [Google Scholar] [CrossRef]
- Rees, C. Childhood attachment. Br. J. Gen. Pract. 2007, 57, 920–922. [Google Scholar] [CrossRef]
- Baglivio, M.T.; Wolff, K.T. Positive childhood experiences (PCE): Cumulative resiliency in the face of adverse childhood experiences. Youth Violence Juv. Justice 2021, 19, 139–162. [Google Scholar]
- Kalmakis, K.A.; Chandler, G.E. Adverse childhood experiences: Towards a clear conceptual meaning. J. Adv. Nurs. 2014, 70, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Schafer, M. Are Positive Childhood Experiences Linked to Better Cognitive Functioning in Later Life?: Examining the Role of Life Course Pathways. J. Aging Health 2021, 33, 217–226. [Google Scholar] [CrossRef]
- Crandall, A.; Miller, J.R.; Cheung, A.; Novilla, L.K.; Glade, R.; Novilla, M.L.B.; Magnussoa, B.M.; Leavitt, B.L.; Barnes, M.D.; Hanson, C.L. ACEs and counter-ACEs: How positive and negative childhood experiences influence adult health. Child Abus. Negl. 2019, 96, 104089. [Google Scholar] [CrossRef] [PubMed]
- Cunha, O.; Sousa, M.; Pereira, B.; Pinheiro, M.; Machado, A.B.; Caridade, S.; Almeida, T.C. Positive Childhood Experiences and Adult Outcomes: A Systematic Review. Trauma Violence Abuse 2024. [Google Scholar] [CrossRef]
- Bethell, C.; Jones, J.; Gombojav, N.; Linkenbach, J.; Sege, R. Positive Childhood Experiences and Adult Mental and Relational Health in a Statewide Sample: Associations Across Adverse Childhood Experiences Levels. JAMA Pediatr. 2019, 173, e193007. [Google Scholar] [CrossRef]
- Slopen, N.; Chen, Y.; Guida, J.L.; Albert, M.A.; Williams, D.R. Positive childhood experiences and ideal cardiovascular health in midlife: Associations and mediators. Prev. Med. 2017, 97, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, K.; Penman-Aguilar, A.; Chang, M.H.; Moonesinghe, R.; Castellanos, T.; Rodriguez-Lainz, A.; Schieber, R. Vital signs: Leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States—2009–2013. MMWR Morb. Mortal. Wkly Rep. 2015, 64, 469–478. [Google Scholar]
- Maness, S.B.; Merrell, L.; Thompson, E.L.; Griner, S.B.; Kline, N.; Wheldon, C. Social Determinants of Health and Health Disparities: COVID-19 Exposures and Mortality Among African American People in the United States. Public Health Rep. 2021, 136, 18–22. [Google Scholar] [CrossRef]
- Vega, W.A.; Rodriguez, M.A.; Gruskin, E. Health disparities in the Latino population. Epidemiol. Rev. 2009, 31, 99–112. [Google Scholar] [CrossRef]
- Gilman, S.E.; Kawachi, I.; Fitzmaurice, G.M.; Buka, L. Socio-economic status, family disruption and residential stability in childhood: Relation to onset, recurrence and remission of major depression. Psychol. Med. 2003, 33, 1341–1355. [Google Scholar] [CrossRef]
- Larson, K.; Halfon, N. Parental divorce and adult longevity. Int. J. Public Health 2013, 58, 89–97. [Google Scholar] [CrossRef]
- Meadows, S.O.; Brown, J.S.; Elder, G.H. Depressive Symptoms, Stress, and Support: Gendered Trajectories from Adolescence to Young Adulthood. J. Youth Adolesc. 2006, 35, 89–99. [Google Scholar] [CrossRef]
- Crouch, E.; Radcliff, E.; Merrell, M.A.; Hung, P.; Bennett, K.J. Positive Childhood Experiences Promote School Success. Matern. Child Health J. 2021, 25, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Staff, R.T.; Murray, A.D.; Ahearn, T.S.; Mustafa, N.; Fox, H.C.; Whalley, L.J. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann. Neurol. 2012, 71, 653–660. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 2013, 170, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Woon, F.L.; Hedges, D.W. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus 2008, 18, 729–736. [Google Scholar] [CrossRef]
- Corlier, F.W.; Shaw, C.; Hayes-Larson, E.; Mungas, D.; Farias, S.T.; Glymour, M.M.; Whitmer, R.A.; Mayeda, E.R. Association Between Cognitive Test Performance and Subjective Cognitive Decline in a Diverse Cohort of Older Adults: Findings from the KHANDLE Study. Alzheimer Dis. Assoc. Disord. 2020, 34, 198–205. [Google Scholar] [CrossRef]
- George, K.M.; Gilsanz, P.; Peterson, R.L.; Barnes, L.L.; DeCarlis, C.; Mayeda, E.R.; Mungas, D.M.; Whitmer, R.A. Impact of Cardiovascular Risk Factors in Adolescence, Young Adulthood, and Midlife on Late-Life Cognition: Study of Healthy Aging in African. J. Gerontol. Ser. A 2021, 76, 1692–1698. [Google Scholar] [CrossRef]
- Mungas, D.; Reed, B.R.; Marshall, S.C.; González, H.M. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 2000, 14, 209–223. [Google Scholar] [CrossRef]
- George, K.M.; Maillard, P.; Gilsanz, P.; Fletcher, E.; Peterson, R.L.; Fong, J.; Mayeda, E.R.; Mungas, D.M.; Barnes, L.L.; Glymour, M.M.; et al. Association of Early Adulthood Hypertension and Blood Pressure Change with Late-Life Neuroimaging Biomarkers. JAMA Netw. Open. 2023, 6, e236431. [Google Scholar] [CrossRef]
- Mungas, D.; Reed, B.R.; Crane, P.K.; Haan, M.N.; González, H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychol. Assess. 2004, 16, 347–359. [Google Scholar] [CrossRef]
- Arnold, S.E.; Hyman, B.T.; Flory, J.; Damasio, A.R.; Van Hoesen, G.W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1991, 1, 103–116. [Google Scholar]
- Aljabar, P.; Heckemann, R.A.; Hammers, A.; Hajnal, J.V.; Rueckert, D. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. Neuroimage 2009, 46, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; DeCarli, C.; Fan, A.P.; Knaack, A. Convolutional Neural Net Learning Can Achieve Production-Level Brain Segmentation in Structural Magnetic Resonance Imaging. Front. Neurosci. 2021, 15, 683426. [Google Scholar] [CrossRef]
- Landau, S.M.; Breault, C.; Joshi, A.D.; Pontecorvo, M.; Mathis, C.A.; Jagust, W.; Mintun, M.A. Amyloid-β imaging with Pittsburgh compound B and florbetapir: Comparing radiotracers and quantification methods. J. Nucl. Med. 2013, 54, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Landau, S.M.; Mintun, M.A.; Joshi, A.D.; Koeppe, R.A.; Petersen, R.C.; Aisen, P.S.; Weiner, M.W.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging Initiative. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012, 72, 578–586. [Google Scholar] [CrossRef]
- Mormino, E.C.; Kluth, J.T.; Madison, C.M.; Rabinovici, G.D.; Baker, S.L.; Miller, B.L.; Koeppe, R.A.; Mathis, C.A.; Weiner, M.W.; Jagust, W.J.; et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009, 132, 1310–1323. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. Available online: http://www.jstatsoft.org/v48/i02/ (accessed on 10 October 2024).
- Lane, R.D.; Reiman, E.M.; Ahern, G.L.; Schwartz, G.E.; Davidson, R.J. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry 1997, 154, 926–933. [Google Scholar] [CrossRef]
- Wang, L.; Paul, N.; Stanton, S.J.; Greeson, J.M.; Smoski, M.J. Loss of sustained activity in the ventromedial prefrontal cortex in response to repeated stress in individuals with early-life emotional abuse: Implications for depression vulnerability. Front. Psychol. 2013, 4, 320. [Google Scholar] [CrossRef]
- Kelly, M.E.; Duff, H.; Kelly, S.; McHugh Power, J.E.; Brennan, S.; Lawlor, B.A.; Loughrey, D.G. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Syst. Rev. 2017, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Soederberg Miller, L.M.; Lachman, M.E. Cognitive performance and the role of control beliefs in midlife. Aging Neuropsychol. Cogn. 2000, 7, 69–85. [Google Scholar] [CrossRef]
- Seblova, D.; Berggren, R.; Lövdén, M. Education and age-related decline in cognitive performance: Systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2020, 58, 101005. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Peterson, R.L.; George, K.M.; Barnes, L.L.; Gilsanz, P.; Mayeda, E.R.; Glymour, M.; Mungas, D.M.; Whitmer, R.A. Association of Timing of School Desegregation in the United States with Late-Life Cognition in the Study of Healthy Aging in African Americans (STAR) Cohort. JAMA Netw. Open 2021, 4, e2129052. [Google Scholar] [CrossRef]
- Calem, M.; Bromis, K.; McGuire, P.; Morgan, C.; Kempton, M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017, 14, 471–479. [Google Scholar] [CrossRef]
- Förster, K.; Danzer, L.; Redlich, R.; Opel, N.; Grotgerd, D.; Leehr, E.J.; Dohm, K.; Enneking, V.; Meinert, S.; Goltermann, J.; et al. Social support and hippocampal volume are negatively associated in adults with previous experience of childhood maltreatment. J. Psychiatry Neurosci. 2021, 46, E328–E336. [Google Scholar] [CrossRef]
- Davis, A.C.; Voelkel, J.L.; Remmers, C.L.; Adams, J.L.; McGlynn, E.A. Comparing Kaiser Permanente Members to the General Population: Implications for Generalizability of Research. Perm J. 2023, 27, 87–98. [Google Scholar] [CrossRef]
- Liebenberg, L.; Ungar, M.; LeBlanc, J.C. The CYRM-12: A brief measure of resilience. Can. J. Public Health 2013, 104, e131–e135. [Google Scholar] [CrossRef]
- Leyhe, T.; Müller, S.; Milian, M.; Eschweiler, G.W.; Saur, R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 2009, 47, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research; Fed Regist: Washington, WA, USA, 1994; pp. 1408–1413.
- Piccolo, L.R.; Roby, E.; Canfield, C.F.; Seery, A.M.; Weisleder, A.; Cates, C.B.; Tutasig, L.; Matalon, M.; Custode, A.; Rodriguez, L.; et al. Supporting responsive parenting in real-world implementation: Minimal effective dose of the Video Interaction Project. Pediatr. Res. 2024, 95, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Roby, E.; Canfield, C.F.; Seery, A.M.; Dreyer, B.; Mendelsohn, A.L. Promotion of Positive Childhood Experiences and Early Relational Health in Pediatric Primary Care: Accumulating Evidence. Acad. Pediatr. 2024, 24, 201–203. [Google Scholar] [CrossRef] [PubMed]
| KHANDLE | STAR | MRI Sample | PET Sample | Total | |||
|---|---|---|---|---|---|---|---|
| Mean Age at First Assessment (SD) | 76 (6.7) | 69 (8.8) | 72 (8.0) | 75 (5.8) | 74 (8.2) | ||
| % Female | 59 | 69 | 60 | 53 | 62 | ||
| Race, N (%) | |||||||
| Black | 436 (26) | 743 (99) | 298 (53) | 50 (18) | 1179 (48) | ||
| White | 498 (30) | 0 | 93 (17) | 84 (30) | 498 (20) | ||
| Latinx | 343 (20) | 6 (1) | 93 (17) | 80 (28) | 349 (14) | ||
| Asian | 406 (24) | 0 | 76 (14) | 67 (24) | 406 (17) | ||
| Education, N (%) | |||||||
| Grade School | 50 (3) | 2 (0) | 3 (1) | 3 (1) | 52 (2) | ||
| Some High School | 63 (4) | 17 (2) | 14 (3) | 8 (3) | 80 (3) | ||
| Tech/Trade School | 75 (4) | 31 (4) | 17 (3) | 8 (3) | 106 (4) | ||
| High School Graduate | 167 (10) | 114 (15) | 59 (11) | 31 (13) | 281 (12) | ||
| Some College | 576 (33) | 332 (44) | 207 (27) | 88 (38) | 838 (34) | ||
| College Graduate | 424 (24) | 130 (17) | 141 (25) | 32 (14) | 554 (23) | ||
| Graduate School | 387 (22) | 133 (18) | 119 (22) | 61 (26) | 520 (21) | ||
| Total N | 1683 | 749 | 560 | 283 | 2432 | ||
| Ethnocultural Group Means | |||||||
| Black | White | Latinx | Asian | ||||
| Mean Age (SD) | 71 (8.7) | 77 (7.2) | 76 (6.5) | 76 (6.6) | |||
| % Female | 68 | 58 | 59 | 53 | |||
| Education (SD) | 5.2 (1.3) | 5.5 (1.4) | 4.7 (1.7) | 5.8 (1.2) | |||
| Childhood Finances | 0.726 (0.604) | 0.892 (0.555) | 0.665 (0.627) | 0.762 (0.633) | |||
| ACE | 1.81 (1.40) | 1.45 (1.36) | 1.85(1.41) | 0.980 (1.14) | |||
| PCEs (SD) | 4.1 (0.9) | 4.1 (0.9) | 3.4 (1.2) | 3.5 (1.0) | |||
| Education (SD) | 5.2 (1.3) | 5.5 (1.4) | 4.7 (1.7) | 5.8 (1.2) | |||
| Verbal Episodic Memory (SD) | 0.02 (0.9) | 0.06 (0.9) | −0.17 (0.9) | 0.09 (0.9) | |||
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| b Weight (95% CI) | Std. Beta | SE | p Value | b Weight (95% CI) | Std. Beta | SE | p Value | |
| Age | −0.357 (−0.390, −0.324) | −0.485 | 0.017 | 0.000 | −0.334 (−0.368, −0.304) | −0.459 | 0.017 | 0.000 |
| Gender | 0.465 (0.406, 0.527) | 0.309 | 0.032 | 0.000 | 0.487 (0.421, 0.545) | 0.327 | 0.032 | 0.000 |
| Childhood Finances | −0.001 (−0.044, 0.045) | −0.001 | 0.022 | 0.970 | −0.013 (−0.055, 0.029) | −0.014 | 0.022 | 0.549 |
| ACE | 0.003 (−0.020, 0.027) | 0.006 | 0.012 | 0.797 | 0.005 (−0.018, 0.028) | 0.009 | 0.012 | 0.673 |
| White | 0.336 (0.251, 0.431) | 0.186 | 0.045 | 0.000 | 0.269 (0.186, 0.354) | 0.151 | 0.042 | 0.000 |
| Latinx | 0.096 (−0.003, 0.188) | 0.046 | 0.047 | 0.040 | 0.117 (0.026, 0.209) | 0.057 | 0.046 | 0.010 |
| Asian | 0.393 (0.298, 0.499) | 0.201 | 0.051 | 0.000 | 0.296 (0.195, 0.402) | 0.153 | 0.050 | 0.000 |
| PCEs | 0.033 (0.003, 0.064) | 0.048 | 0.016 | 0.035 | 0.011 (−0.019, 0.040) | 0.016 | 0.015 | 0.460 |
| Education | -- | -- | -- | -- | 0.185 (0.151, 0.218) | 0.234 | 0.017 | 0.000 |
| R Squared | 0.330 | 0.364 | ||||||
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| b Weight (95% CI) | Std. Beta | SE | p Value | b Weight (95% CI) | Std. Beta | SE | p Value | |
| Age | −0.014 (−0.034, 0.007) | −0.083 | 0.010 | 0.149 | −0.016 (−0.036, 0.004) | −0.096 | 0.010 | 0.108 |
| Gender | 0.024 (−0.012, 0.059) | 0.068 | 0.018 | 0.202 | 0.024 (−0.011, 0.063) | 0.069 | 0.018 | 0.188 |
| Childhood Finances | −0.021 (−0.044, 0.002) | −0.099 | 0.012 | 0.085 | −0.021 (−0.043, 0.002) | −0.098 | 0.012 | 0.074 |
| ACE | −0.005 (−0.018, 0.008) | −0.041 | 0.007 | 0.464 | −0.005 (−0.017, 0.008) | −0.041 | 0.006 | 0.445 |
| White | 0.053 (0.004, 0.102) | 0.127 | 0.024 | 0.028 | 0.057 (0.011, 0.106) | 0.137 | 0.024 | 0.019 |
| Latinx | 0.092 (0.037, 0.144) | 0.190 | 0.028 | 0.001 | 0.091 (0.038, 0.143) | 0.190 | 0.028 | 0.001 |
| Asian | −0.021 (−0.072, 0.032) | −0.046 | 0.027 | 0.445 | −0.016 (−0.070, 0.038) | −0.036 | 0.027 | 0.554 |
| PCEs | 0.019 (0.000, 0.036) | 0.116 | 0.009 | 0.039 | 0.020 (0.001, 0.038) | 0.121 | 0.009 | 0.037 |
| Education | -- | -- | -- | -- | −0.004 (−0.026, 0.016) | −0.021 | 0.011 | 0.730 |
| R Squared | 0.066 | 0.067 | ||||||
| b Weight (95% CI) | Std. Beta | SE | p Value | ||
|---|---|---|---|---|---|
| Black | Age | −0.313 (−0.353, −0.278) | −0.489 | 0.018 | 0.000 |
| Gender | 0.461 (0.388, 0.540) | 0.315 | 0.039 | 0.000 | |
| Childhood Finances | −0.020 (−0.071, 0.030) | −0.024 | 0.026 | 0.428 | |
| ACE | 0.002 (−0.025, 0.030) | 0.003 | 0.014 | 0.911 | |
| PCEs | 0.019 (−0.020, 0.055) | 0.026 | 0.019 | 0.322 | |
| Education | 0.160 (0.117, 0.209) | 0.200 | 0.023 | 0.000 | |
| R Squared | 0.390 | ||||
| White | Age | −0.418 (−0.482, −0.351) | −0.464 | 0.034 | 0.000 |
| Gender | 0.567 (0.445, 0.688) | 0.365 | 0.061 | 0.000 | |
| Childhood Finances | −0.022 (−0.124, 0.070) | −0.021 | 0.048 | 0.641 | |
| ACE | 0.018 (−0.027, 0.067) | 0.032 | 0.024 | 0.446 | |
| PCEs | 0.021 (−0.038, 0.083) | 0.028 | 0.031 | 0.496 | |
| Education | 0.246 (0.180, 0.313) | 0.298 | 0.033 | 0.000 | |
| R Squared | 0.451 | ||||
| Latinx | Age | −0.401 (−0.500, −0.305) | −0.438 | 0.051 | 0.000 |
| Gender | 0.478 (0.337, 0.621) | 0.339 | 0.072 | 0.000 | |
| Childhood Finances | −0.135 (−0.223, −0.039) | −0.159 | 0.048 | 0.005 | |
| ACE | −0.024 (−0.073, 0.031) | −0.049 | 0.027 | 0.366 | |
| PCEs | 0.045 (−0.018, 0.104) | 0.074 | 0.032 | 0.158 | |
| Education | 0.124 (0.051, 0.194) | 0.184 | 0.037 | 0.001 | |
| R Squared | 0.371 | ||||
| Asian | Age | −0.375 (−0.473, −0.278) | −0.372 | 0.050 | 0.000 |
| Gender | 0.586 (0.449, 0.741) | 0.374 | 0.074 | 0.000 | |
| Childhood Finances | 0.035 (−0.054, 0.124) | 0.037 | 0.046 | 0.446 | |
| ACE | −0.002 (−0.070, 0.062) | −0.003 | 0.034 | 0.949 | |
| PCEs | 0.053 (−0.020, 0.127) | 0.071 | 0.038 | 0.155 | |
| Education | 0.195 (0.100, 0.290) | 0.201 | 0.048 | 0.000 | |
| R Squared | 0.342 | ||||
| Association Between PCE and Education (a Path) | ||||
| b Weight (95% CI) | Std. Beta | SE | p Value | |
| Asian | 0.182 (0.099, 0.260) | 0.236 | 0.042 | 0.000 |
| White | 0.168 (0.079, 0.256) | 0.184 | 0.045 | 0.000 |
| Latinx | 0.245 (0.150, 0.331) | 0.272 | 0.046 | 0.000 |
| Black | 0.077 (0.023, 0.130) | 0.087 | 0.027 | 0.005 |
| Combined Sample | 0.127 (0.089, 0.163) | 0.146 | 0.019 | 0.000 |
| Total Indirect Effect of Education Through PCEs | ||||
| Black | 0.012 (0.004, 0.023) | 0.017 | 0.005 | 0.011 |
| White | 0.041 (0.019, 0.066) | 0.055 | 0.012 | 0.001 |
| Latinx | 0.030 (0.011, 0.051) | 0.050 | 0.010 | 0.003 |
| Asian | 0.035 (0.015, 0.059) | 0.047 | 0.012 | 0.002 |
| Combined Sample Intercept | 0.023 (0.015, 0.032) | 0.034 | 0.004 | 0.000 |
| Combined Sample Linear Slope | 0.000 (−0.003, 0.002) | −0.003 | 0.001 | 0.734 |
| Total Effect of Education | ||||
| Black | 0.031 (−0.008, 0.069) | 0.044 | 0.019 | 0.111 |
| White | 0.062 (−0.001, 0.126) | 0.083 | 0.033 | 0.062 |
| Latinx | 0.075 (0.010, 0.135) | 0.124 | 0.032 | 0.019 |
| Asian | 0.089 (0.017, 0.163) | 0.119 | 0.037 | 0.017 |
| Combined Sample Intercept | 0.035 (0.004, 0.064) | 0.050 | 0.016 | 0.027 |
| Combined Sample Linear Slope | 0.019 (0.001, 0.038) | 0.118 | 0.009 | 0.041 |
| b Weight (95% CI) | Std. Beta | SE | p Value | ||
|---|---|---|---|---|---|
| Black | Age at MRI | −0.032 (−0.044, −0.020) | −0.293 | 0.006 | 0.000 |
| Gender | −0.562 (−0.777, −0.348) | −0.286 | 0.109 | 0.000 | |
| Childhood Finances | −0.098 (−0.233, 0.036) | −0.084 | 0.068 | 0.152 | |
| ACE | −0.048 (−0.123, 0.027) | −0.072 | 0.038 | 0.207 | |
| PCEs | −0.097 (−0.212, 0.018) | −0.096 | 0.058 | 0.099 | |
| Education | 0.105 (−0.024, 0.233) | 0.089 | 0.065 | 0.111 | |
| R Squared | 0.1535 | ||||
| White | Age at MRI | −0.049 (−0.084, −0.014) | −0.267 | 0.018 | 0.007 |
| Gender | −0.998 (−1.426, −0.570) | −0.441 | 0.215 | 0.000 | |
| Childhood Finances | −0.025 (−0.365, 0.314) | −0.015 | 0.171 | 0.882 | |
| ACE | 0.102 (−0.069, 0.273) | 0.117 | 0.086 | 0.239 | |
| PCEs | 0.142 (−0.070, 0.354) | 0.125 | 0.107 | 0.188 | |
| Education | 0.013 (−0.248, 0.274) | 0.010 | 0.131 | 0.921 | |
| R Squared | 0.2472 | ||||
| Latinx | Age at MRI | −0.062 (−0.100, −0.024) | −0.348 | 0.019 | 0.002 |
| Gender | −0.517 (−0.943, −0.092) | −0.252 | 0.214 | 0.018 | |
| Childhood Finances | −0.101 (−0.377, 0.176) | −0.081 | 0.139 | 0.470 | |
| ACE | 0.013 (−0.135, 0.161) | 0.019 | 0.074 | 0.861 | |
| PCEs | 0.167 (−0.041, 0.375) | 0.182 | 0.104 | 0.114 | |
| Education | 0.053 (−0.215, 0.322) | 0.045 | 0.135 | 0.693 | |
| R Squared | 0.1671 | ||||
| Asian | Age at MRI | −0.061 (−0.091, −0.030) | −0.385 | 0.015 | 0.000 |
| Gender | −0.668 (−1.012, −0.324) | −0.381 | 0.172 | 0.000 | |
| Childhood Finances | −0.022 (−0.223, 0.180) | −0.021 | 0.101 | 0.831 | |
| ACE | 0.062 (−0.068, 0.193) | 0.095 | 0.065 | 0.344 | |
| PCEs | 0.033 (−0.134, 0.200) | 0.041 | 0.083 | 0.692 | |
| Education | 0.276 (0.042, 0.509) | 0.241 | 0.117 | 0.021 | |
| R Squared | 0.3799 | ||||
| Combined Sample | Age at MRI | −0.038 (−0.048, −0.027) | −0.311 | 0.005 | 0.000 |
| Gender | −0.656 (−0.812, −0.500) | −0.324 | 0.079 | 0.000 | |
| Childhood Finances | −0.083 (−0.184, 0.019) | −0.066 | 0.052 | 0.109 | |
| ACE | 0.000 (−0.057, 0.056) | 0.000 | 0.029 | 0.991 | |
| White | 0.617 (0.385, 0.849) | 0.231 | 0.118 | 0.000 | |
| Latinx | 0.550 (0.316, 0.784) | 0.202 | 0.119 | 0.000 | |
| Asian | 0.509 (0.258, 0.760) | 0.176 | 0.128 | 0.000 | |
| PCEs | 0.022 (−0.058, 0.102) | 0.023 | 0.041 | 0.586 | |
| Education | 0.102 (0.006, 0.197) | 0.084 | 0.049 | 0.037 | |
| R Squared | 0.2231 | ||||
| b Weight (95% CI) | Std. Beta | SE | p Value | ||
|---|---|---|---|---|---|
| Black | Age at PET | −0.020 (−0.063, 0.023) | −0.156 | 0.021 | 0.349 |
| Gender | 0.285 (−0.196, 0.766) | 0.192 | 0.238 | 0.237 | |
| Childhood Finances | −0.097 (−0.406, 0.211) | −0.108 | 0.152 | 0.528 | |
| ACE | 0.010 (−0.141, 0.162) | 0.022 | 0.075 | 0.889 | |
| PCEs | −0.136 (−0.384, 0.112) | −0.176 | 0.123 | 0.274 | |
| Education | −0.066 (−0.347, 0.214) | −0.079 | 0.138 | 0.634 | |
| R Squared | 0.034 | ||||
| White | Age at PET | −0.002 (−0.051, 0.048) | −0.008 | 0.025 | 0.949 |
| Gender | −0.087 (−0.671, 0.498) | −0.034 | 0.293 | 0.769 | |
| Childhood Finances | −0.131 (−0.587, 0.325) | −0.066 | 0.229 | 0.569 | |
| ACE | −0.271 (−0.505, −0.038) | −0.268 | 0.117 | 0.023 | |
| PCEs | −0.006 (−0.300, 0.289) | −0.004 | 0.148 | 0.970 | |
| Education | −0.342 (−0.713, 0.029) | −0.218 | 0.186 | 0.070 | |
| R Squared | 0.025 | ||||
| Latinx | Age at PET | 0.006 (−0.033, 0.045) | 0.042 | 0.019 | 0.750 |
| Gender | −0.081 (−0.518, 0.355) | −0.047 | 0.219 | 0.711 | |
| Childhood Finances | 0.153 (−0.124, 0.429) | 0.145 | 0.139 | 0.275 | |
| ACE | −0.050 (−0.205, 0.105) | −0.084 | 0.078 | 0.523 | |
| PCEs | −0.014 (−0.216, 0.189) | −0.018 | 0.101 | 0.894 | |
| Education | −0.170 (−0.450, 0.110) | −0.171 | 0.140 | 0.231 | |
| R Squared | 0.037 | ||||
| Asian | Age at PET | −0.004 (−0.043, 0.034) | −0.030 | 0.019 | 0.819 |
| Gender | 0.206 (−0.220, 0.633) | 0.129 | 0.213 | 0.337 | |
| Childhood Finances | −0.054 (−0.297, 0.190) | −0.058 | 0.121 | 0.660 | |
| ACE | −0.100 (−0.268, 0.067) | −0.160 | 0.084 | 0.236 | |
| PCEs | −0.082 (−0.284, 0.120) | −0.112 | 0.101 | 0.420 | |
| Education | 0.244 (−0.044, 0.532) | 0.232 | 0.144 | 0.095 | |
| R Squared | 0.028 | ||||
| Combined Sample | Age at PET | 0.001 (−0.020, 0.023) | 0.008 | 0.011 | 0.900 |
| Gender | 0.043 (−0.202, 0.288) | 0.021 | 0.124 | 0.730 | |
| Childhood Finances | −0.012 (−0.173, 0.149) | −0.010 | 0.082 | 0.883 | |
| ACE | −0.087 (−0.177, 0.004) | −0.122 | 0.046 | 0.061 | |
| White | 0.341 (−0.036, 0.719) | 0.157 | 0.192 | 0.076 | |
| Latinx | −0.063 (−0.443, 0.316) | −0.028 | 0.193 | 0.743 | |
| Asian | −0.220 (−0.618, 0.177) | −0.095 | 0.202 | 0.277 | |
| PCEs | −0.015 (−0.134, 0.103) | −0.017 | 0.060 | 0.801 | |
| Education | −0.106 (−0.260, 0.047) | −0.090 | 0.078 | 0.172 | |
| R Squared | 0.029 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owens, J.H.; Windon, C.C.; Mungas, D.; Whitmer, R.A.; Gilsanz, P.; Manly, J.J.; Glymour, M.M. Positive Childhood Experiences, Cognition, and Biomarkers of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2025, 22, 525. https://doi.org/10.3390/ijerph22040525
Owens JH, Windon CC, Mungas D, Whitmer RA, Gilsanz P, Manly JJ, Glymour MM. Positive Childhood Experiences, Cognition, and Biomarkers of Alzheimer’s Disease. International Journal of Environmental Research and Public Health. 2025; 22(4):525. https://doi.org/10.3390/ijerph22040525
Chicago/Turabian StyleOwens, Joshua H., Charles C. Windon, Dan Mungas, Rachel A. Whitmer, Paola Gilsanz, Jennifer J. Manly, and M. Maria Glymour. 2025. "Positive Childhood Experiences, Cognition, and Biomarkers of Alzheimer’s Disease" International Journal of Environmental Research and Public Health 22, no. 4: 525. https://doi.org/10.3390/ijerph22040525
APA StyleOwens, J. H., Windon, C. C., Mungas, D., Whitmer, R. A., Gilsanz, P., Manly, J. J., & Glymour, M. M. (2025). Positive Childhood Experiences, Cognition, and Biomarkers of Alzheimer’s Disease. International Journal of Environmental Research and Public Health, 22(4), 525. https://doi.org/10.3390/ijerph22040525







