1. Introduction
Chronic spinal cord injury (SCI) is associated with increased risk for cardiometabolic disease (CMD), all-cause cardiovascular disease (CVD), and type 2 diabetes mellitus (T2DM) [
1,
2]. Pervasive risk factors for these diseases are commonly observed after SCI and are associated with a sedentary lifestyle, hypercaloric nutrition relative to daily need [
3,
4,
5,
6], a stark loss of lean tissue [
7,
8,
9,
10,
11], increased adiposity [
12,
13], and disrupted regulation of glucose and insulin [
14,
15]. The Consortium for Spinal Cord Medicine Clinical Practice Guideline for Cardiometabolic Disease Identification and Management identified obesity as the most prevalent cardiometabolic component risk [
16], affecting 55–95.7% of the SCI population [
17,
18]. Alarmingly, obesity is known to pose a more substantial health hazard than the other CMD risks [
19,
20,
21].
The current approach for the management of CMD risks after SCI primarily focuses on exercise rehabilitation and, to a lesser extent, nutritional modification. Studies of moderate-to-intense upper-extremity exercise (70–80% of peak capacity) [
22,
23,
24] and circuit resistance training [
25] report reduced individual CMD risks after a short-term intervention. However, the overall benefits typically fail to reach clinically relevant levels or test the sustainability of the intervention. A more reasoned view recognizes the need for a more comprehensive lifestyle intervention for the effective management of cardiometabolic risk factors in individuals with SCI. This requires a multidisciplinary approach incorporating population-appropriate physical activity, ‘heart-healthy’ nutrition at caloric levels that maintain stable body mass, behavioral support to sustain user engagement and long-term compliance, and close monitoring of cardiovascular health indicators [
26].
Therapeutic lifestyle intervention (TLI)—involving a combination of physical activity, healthy nutrition, stress management techniques, and educational interventions—typically results in improved overall health and well-being. To address these needs, the Diabetes Prevention Program (DPP) was a landmark NIH-sponsored, 27-center randomized clinical trial that tested a lock-step lifestyle intervention program for persons who were at risk of developing T2DM. The DPP was found to be effective for promoting body mass reduction and lessening the conversion rates to frank diabetes [
27,
28] and was favorably compared against first-line pharmacotherapy for T2DM [
29]. Importantly, programmatic modification of the DPP and testing of the long-term follow-up have shown remarkable success in sustaining—or even further improving—the benefits obtained during the initial treatment. The existing evidence similarly suggests an important role for ‘weight-lowering and fitness-promoting nutrition and exercise’ lifestyle interventions to improve the health and function of people aging with disability [
30,
31]. Previous studies have emphasized the need for weight management in SCI [
32,
33], and notably, a preliminary case series in which the DPP was refashioned for persons with SCI found that a clinically significant loss of body mass effectively reduced the component risks for CMD and diabetes [
26]. In this way, TLI may be a promising approach for CMD management in SCI that merits further research to fully understand its potential benefits, sustainability, and optimal implementation strategies.
The health state of persons with SCI has recently been shown to impact their caregivers, as they both are living and aging with a disability. As current-day adjuvant providers, caregivers are challenged with assisting daily activities and promoting healthy living for individuals aging with SCI, while they may be similarly experiencing functional and health decline accompanying their own aging [
34]. In general, the caregivers of individuals who are dependent on wheelchairs face greater physical demands, frequently needing to engage in activities such as lifting, pushing, or rolling an impaired body. Additionally, they often assume caregiving responsibilities at an earlier stage compared to caregivers of individuals with other physical disabilities. Unfortunately, healthcare plans and research efforts tend to primarily focus on the person with the disability, neglecting the inclusion of caregivers. As a result, it is not surprising that caregivers of individuals with spinal cord injuries (SCIs) experience high levels of pain, anxiety, and depression [
35]. While there are limited lifestyle intervention programs targeting cardiometabolic health in SCI, there is an even greater scarcity of programs addressing caregiver health. To the best of our knowledge, there is early evidence supporting the benefits to the care-receiver when a caregiver participates in a linked behavioral intervention program [
36]. Consequently, the concept of a coordinated intervention between the caregiver and care-receiver, where both partners can mutually benefit from the intervention, presents an intriguing therapeutic paradigm for consideration.
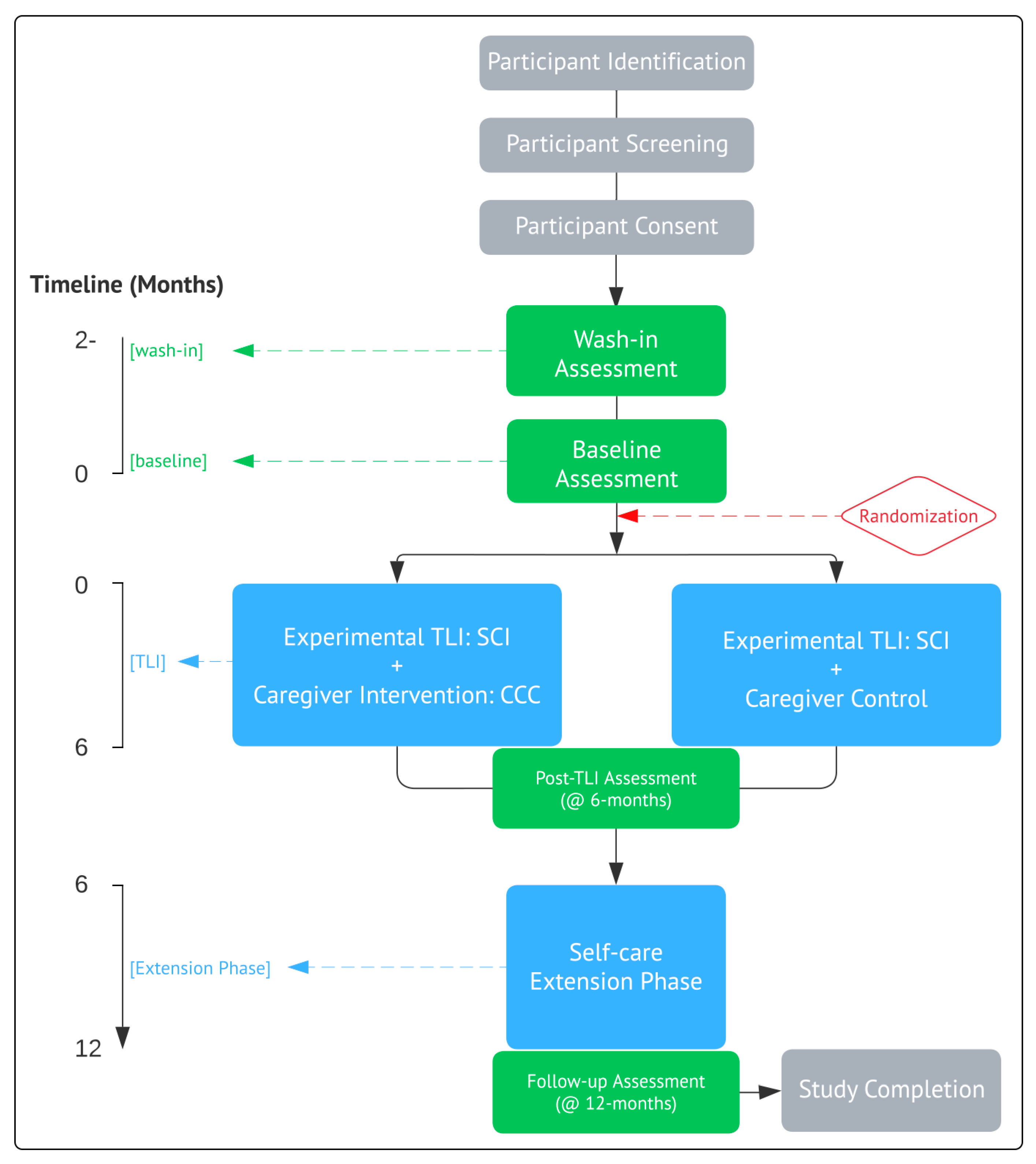
5. Outcome Measures and Assessments
The trial outcome measures and assessments, timepoints, and participant time burdens are summarized in
Table 1.
5.1. Description of Testing for SCI Participants
Anthropometry: Primary Outcome: Body Mass (BM): Participants will be weighed both in and out of their wheelchairs on a calibrated wheelchair scale, and the outcome of mass will be expressed as the average of the two measurements. Height/Waist Circumference/BMI: Height will be measured on a plinth to the nearest 0.25 inch. Waist circumference will be measured using a Gulick tension-regulated tape measure at the level of the umbilicus and recorded to the nearest 0.25 inch. Height [m] and weight [kg] will be used to calculate the body mass index (BMI) in kg/m2.
Cardiorespiratory Endurance: Cardiorespiratory endurance will be assessed as the peak oxygen consumption (VO2peak) on an arm crank ergometer using the open circuit spirometry method. Participants will refrain from exercise for 24 h prior to testing. Ten minutes of quiet rest will follow the preparation and precede the exercise to acquire the baseline values. A peak continuous graded exercise test will determine the VO2sub-peak/peak, HRsub-peak/peak, and ratings of perceived exertion (RPE; categorical ratio (0–10) scale) at subpeak and peak work. The participants will perform work using a calibrated electronically braked arm crank ergometer (Angio, Lode BV, Groningen, The Netherlands), or the equivalent heart rate and oxygen consumption will be recorded continuously from baseline through recovery. HR will be measured with standard electrocardiography, while expired respiratory gases will be collected and analyzed with an online open-circuit metabolic cart (Vmax ENCORE, CareFusion, San Diego, CA, USA) or equivalent. The participants will position their wheelchair at a distance from the arm crank ergometer where their arms can remain slightly flexed at the furthest point from the trunk, and wedges will be placed under the rear wheels to restrict chair movement. Individuals with tetraplegia will have their hands affixed to the arm cranks with hand wraps and athletic tape. Thereafter, the participants will begin cranking on the arm crank ergometer at an initial load of 5 W and will be instructed to maintain a cadence of 55–65 rpm. A digital display will provide real-time revolutions per minute (rpm) feedback.
In one-minute work stages [
42], the resistance will increase at a rate of 5 W for all women and individuals with tetraplegia, or 10 W for individuals with paraplegia. The participants will continue until volitional fatigue, manifesting as either a verbal or nonverbal communication of the desire to stop or an inability to maintain cadence above 55 rpm. Upon cessation, participants will rest quietly for 10 min. The exercise termination will be based on the American College of Sports Medicine Guidelines for Exercise Testing and Training [ACSM, 9th Edition], with peak work defined as volitional exhaustion, the inability to maintain the targeted workload, or when increasing the workload fails to further increase VO
2. The expired gases will be continuously analyzed using an open circuit indirect calorimetry system (Encore
®, with Cardiosoft
® EKG monitoring, VIASYS, Inc., (Conshohocken, PA, USA) or equivalent). EKG rhythm will be continuously monitored to screen ischemia, rhythm disturbances, and signs/symptoms of exertional intolerance.
Perceived exertion (Borg Categorical-Ratio (0–10) Scale) will be obtained at the end of each exercise stage and at test termination.
- 3.
Strength: Strength will be measured as upper-extremity dynamic strength on a Helms Equalizer 7000 multi-station exerciser (Helms Distributing, Polson, MT, or equivalent resistance equipment) using the same maneuvers adopted for training (
Table 2). Subjects will be instructed to perform eight repetitions of each maneuver with each repetition lasting six seconds (3 s concentric, 3 s eccentric). If eight repetitions are completed in a controlled fashion, the weight will be increased and the exercise repeated. Incremental increases in weight (5 kg each for paraplegia, 2.5 kg for tetraplegia) will be added until eight controlled repetitions cannot be completed. The 1-RM will be calculated by the Mayhew regression equation [
43], as we have previously reported [
26]:
where ‘1-RM’ is the calculated one-repetition maximum strength, “Wt” is the resistance used in the last set, in which more than three repetitions but less than eight repetitions were completed, and ‘reps’ equals the number of repetitions completed in the last set of testing. We note that these strength assessments are safe, relevant to daily activities, reliably measurable, and align with the goals of promoting independence and enhancing quality of life in individuals participating in the TLI.
- 4.
Insulin Resistance, CVD Risk, and Inflammatory State: The participants will abstain from caffeine and alcohol consumption for 24 h before testing and will be tested in the post-absorptive state (8 h overnight fast and 48 h after the last exercise bout). Blood will be drawn into citrate and Gel and Lysis Activator (serum) tubes between 8:00– and 10:00 AM. The Biomarker and Immunoassay Laboratory at UM will be used to process the samples. We will use internet freeware (
www.dtu.ox.ac.uk (accessed on 2 August 2016)/homacalculator/download.php) to calculate the second-generation Homeostatic Model Assessment 2 (HOMA2), a paradigm method used for assessing β-cell function and insulin resistance (IR) from basal (fasting) glucose and insulin or C-peptide concentrations. The HOMA2 model has been validated against physiological methods, compares favorably with other models (i.e., euglycemic clamp, minimal model, and ISI0-120), and requires only a single plasma sample assayed for insulin and glucose. The sample will also be assayed using automated methods for hemoglobin A1C. To assess the global CVD risk, we will use the proxy of the TC:HDL ratio (and, secondarily, the LDL:HDL ratio for dyslipidemia risk and the TG:HDL ratio for insulin resistance, respectively), which has predictive power for future CVD risk) that approaches the hazard forecasting of the Framingham regression equation. The assays for TC, TG, and HDL-C will be performed using automated methods and commercially available kits according to manufacturers’ instructions and run procedures [
25]. HDL-C will be assayed after the precipitation of ApoB-containing lipoproteins. LDL-C will be computed using the method of Friedewald [
44,
45].
To test proatherogenic inflammatory stress, we will assess inflammatory biomarkers as surrogates for systemic inflammatory stress profiles as measured by cytokine and antibody production and gene expression in blood serum (inflammasome formation/activation and cytokine (TNF-α, IL-1β, and IL-6) production) using Western blotting and commercially available ELISAs.
- 5.
Dietary Record: To track caloric intake and expenditure and ascertain the potential dietary drift in the caregiver control arm, participants will be instructed to complete and return a sample 4-day dietary record, including two representative weekdays and two weekend days to account for the changes in eating habits. As outlined by the DPP, physical food logs will be provided weekly, where habitual food and drink consumption are recorded, and the logs will be returned to the study personnel. The data will be analyzed at the lead center for total caloric intake and dietary composition, including discriminated macro/micronutrient content, using a nutritional software package (Food Processor II Windows v. 7.6; ESHA Research, Salem, OR, USA). The collected data will provide a comprehensive food intake assessment for scrutiny by both the investigators and study participants undergoing nutritional intervention.
- 6.
(SCI) Function: We will operationalize ‘function’ as the participant-reported outcomes (PRO) of physical function. This refers to an individual’s capacity to carry out activities that require bodily movement. Most importantly, PROs capture their personal assessment of the function. PROs are defined as “a measurement of any aspect of a patient’s health status that comes directly from the patient,” and capture the impact of a disease or condition on the individual. We will administer the following:
SCI Functional Index (SCI-FI): Computer-adapted test (CAT short forms): The SCI-FI is a PRO that captures the activity limitations of persons with SCI [
46,
47]. It has six domains: basic mobility, self-care, fine motor function, ambulation, manual wheelchair, and power wheelchair. We will assess basic mobility and self-care in all participants and fine motor, ambulation, manual wheelchair, and power wheelchair as befits the individual’s injury and mobility characteristics. SCI-FI is patient-centered, accounting for the individual’s perspective on their own functioning, which is important for assessing the effectiveness of the behavioral component of the TLI. SCI-FI is widely recognized and accepted in both research and clinical settings. Its standardized assessment tools and scoring systems make it useful for comparing results across different studies and populations, enhancing the validity and reliability of research findings. Furthermore, SCI-FI is frequently used as an outcome measure in clinical trials and research studies focused on spinal cord injury and is validated for use in community-dwelling individuals with SCI [
48].
Craig Handicap Assessment and Reporting Technique Short Form (CHART-SF): This measures the level of participation in a community setting. The CHART collects information on the degree to which the respondent fulfills the roles typically expected from people without disabilities. There are five dimensions that can be answered in quantifiable behavioral terms (e.g., hours of physical assistance, how much time is someone with you to assist you, how many relatives do you visit, etc.). For each CHART dimension, a scoring rubric allows a score from 0 to 100 points, the latter being the maximum attainable, which corresponds to a role fulfillment equivalent to that of most individuals without disabilities. We will focus our attention on the Physical Independence, Mobility, Occupation, and Social Integration Subscales. Extensive research has shown good levels of reliability and validity [
49,
50,
51,
52].
- 7.
Pain: We consider that pain is multidimensional, and this is reflected in our choice of tests for assessment and classification. We will administer the following:
Multidimensional Pain Inventory-SCI Version (MPI-SCI): This is a psychometric instrument designed to assess pain and a range of psychosocial factors associated with chronic pain [
53]. The answers are given on a numerical rating scale ranging from 0 to 6. The MPI-SCI consists of three sections: (1) pain impact; (2) perceived social support; and (3) activities. The internal consistency, stability, and validity of the MPI-SCI have been demonstrated in the SCI chronic pain population.
The International SCI Pain Basic Data Set (ISCIPBDS): This evaluates the worst, second worst, and third worst pain when a person experiences one or more pains [
54]. The ISCIPBDS includes a pain classification made by a healthcare professional and self-reported information regarding the number of pains and self-reported information regarding the number of pain problems, location, intensity, and temporal pattern. It also assesses the pain interference with activities, mood, and sleep for each specific pain problem.
Neuropathic Pain Symptom Inventory (NPSI): This is sensitive to change and evaluates five common features of neuropathic pain [
55]. The psychometric properties of the NPSI, including its sensitivity to change, provide a useful evaluation in clinical practice and clinical trials. The NPSI shows many similarities among different patient groups with peripheral or central lesions, which supports its utility as a method for pain evaluation in diverse neurotrauma populations.
The International Standards for Neurological Classification of Spinal Cord Injury will be administered before this assessment if one has not been performed within 2 years.
- 8.
QoL, health perceptions, social functioning, and vitality. We will administer the following:
Spinal Cord Independence Measure-III (SCIM-III): This is a 19-item questionnaire assessing domains of 36 self-care, respiration and sphincter management, and mobility. The measure has been shown to have excellent internal consistency with α ranging from 0.77 to 0.85 [
56]. The SCIM III correlates with Functional Independence Measure subscales with r values of 0.78 to 0.80 [
57].
- 9.
Treatment acceptance, life satisfaction, and self-efficacy. We will administer the following:
SCI Exercise Self-Efficacy Scale (ESES): A self-report to measure perceived exercise self-efficacy in individuals with SCI [
58]. The scale requires individuals to indicate their confidence in performing physical activities and exercise. The scale is SCI-specific and measures perceived self-efficacy for various types of physical exercise. The measure has acceptable reliability and validity in SCI populations.
Credibility and Expectancy Questionnaire (CEQ): This is used to measure treatment expectancy. The CEQ demonstrates high internal consistency (
a = 0.79–0.90). The retest reliability is
r = 0.82 for the expectancy factor and
r = 0.75 for the credibility factor. The items are rated based on cognitive appraisal and based on feelings about the therapy [
59].
Satisfaction Questionnaire 9 (LSQ-9): This is used to assess whether the treatment improves life satisfaction. The nine-item version contains a single item assessing overall life satisfaction, along with eight additional items that are domain-specific. Normative data for chronic SCI have been obtained by Post et al. [
60]. The measure has been used extensively in research with persons with SCI and has been shown to have excellent reliability and internal validity.
5.2. Description of Testing for Caregiver Participants
Prior to starting the training program, each subject will complete a basic fitness assessment. The data collected will be used to assess program progress and develop the initial exercise prescription. The testing procedures and order are as follows:
| Stage | Speed (mph) | Incline (%) | Duration (min) |
| 1 | 1.7 | 0 | 3 |
| 2 | 1.7 | 10 | 3 |
| 3 | 2.5 | 12 | 3 |
| 4 | 3.4 | 14 | 3 |
| 5 | 4.2 | 16 | 3 |
- 3.
Heart rate, Blood Pressure, and Rating of Perceived Exertion (RPE; categorical scale 6–20) will be measured 30 s prior to the end of each work stage. The test will be terminated when two consecutive stages elicit heart rates above 60% of the subject’s estimated maximal effort. The test will be terminated if the subject’s heart rate meets 85% of their estimated maximal heart rate. The estimated maximal heart will be calculated by the formula 208—(age × 0.66) (American College of Sports Medicine (ACSM), 2014) [
62].
- 4.
Cardiorespiratory Endurance is estimated by extrapolating the VO2 estimates achieved at the two consecutive submaximal steady-state heart rates to predict the maximum heart rate.
- 5.
Strength: 1-RM strength for chest press and leg press will be estimated using the 7–10 repetition method and regression equation (see below). The resistance will be increased by 10 pounds when the high end of the repetition range can be completed before fatigue.
| Resistance Exercise | Prediction Equation for 7–10 RM Test | r | Adjusted R2 |
| Chest Press | −1.89 + (1.16 × Wt) + (1.68 × reps) | 0.95 | 0.91 |
| Leg Press/Extension | 95.00 + (0.65 × Wt) + (8.52 × reps) | 0.76 | 0.56 |
- 6.
Insulin Resistance, CVD Risk, and Inflammatory State will be measured as described above for the SCI participants.
- 7.
Dietary Record will be kept as described above for the SCI participants.
- 8.
Function and Pain: We will administer the following:
Function: The four sub-domains of the PROMIS Physical Function CAT are
mobility (lower extremity function),
dexterity (upper extremity function),
axial (neck and back function), and the
ability to carry out instrumental ADLs [
63].
West Haven–Yale Multidimensional Pain Inventory (MPI): This is a comprehensive instrument designed to assess a range of self-reported behavioral and psychosocial factors that are associated with chronic pain syndromes [
64]. The MPI comprises three sections:
Section 1 (Pain Impact),
Section 2 (Responses by Significant Others), and
Section 3 (Common Activities). The Pain Severity, Life Interference, Life Control, Affective Distress, Support, Negative Responses from Others, Solicitous Responses (SR) from Others, and Distracting Responses from Others subscales measure cognitive, affective, social, and behavioral responses. The remaining subscales assess the degree of participation in various types of daily activities: Household Activities Away from Home, Social Activities, and Outdoor Activities.
- 9.
QoL, health perceptions, social functioning, and vitality. We will administer the following:
Credibility and Expectancy Questionnaire (CEQ): This is used to measure treatment expectancy. The CEQ demonstrates high internal consistency, (
a = 0.79–0.90). The retest reliability is
r = 0.82 for the expectancy factor and
r = 0.75 for the credibility factor [
59]. The items are rated based on a cognitive appraisal and based on the participants’ feelings about the therapy (e.g., how confident would you be in recommending this treatment to a friend who experiences similar problems?).
PROMIS—Satisfaction Social Satisfaction Short Form: The PROMIS Adult Satisfaction with Social Roles and Activities short form item bank assesses satisfaction with performing usual social roles and activities (e.g., “I am satisfied with my ability to participate in family activities”). We will be using the subset banks Satisfaction with Participation in Social Roles (v1.0) with revised item pools [
65].
7. Trial Safety and Adverse Events
Each trial site will have an assigned medical director to serve as the trial physician and oversee all aspects of participant medical care. The trial will also have an assigned independent medical monitor providing trial medical oversight and project evaluation plans. All testing will be performed at a medical center that is located near an emergency room, and all facilities and trial personnel are trained on the standing policies and procedures for emergencies. This will be designed to reduce the risks and burdens, with the further aim of reducing the potential risks and burdens through strict adherence to best practices [
79].
Any adverse events or serious adverse events that occur during the study will be promptly reported to the Institutional Review Board (IRB) within the required timeframe. In the event of an adverse event, the principal investigator will immediately inform and seek advice from the study physician. The study physician will assess whether the event(s) can be attributed to the study procedures outlined in the protocol. The evaluation of adverse events by the study physician will be conducted using the following criteria [
80]:
- i.
Grade 1 (mild): Individuals experience an awareness of symptoms, which can be easily tolerated. These symptoms are typically transient and do not necessitate any specific treatment. They do not interfere with the individual’s usual or normal daily activities.
- ii.
Grade 2 (moderate): Symptoms may be improved with simple therapeutic measures. While they may interfere with the individual’s normal daily activities, they do not prevent them from participating in these activities.
- iii.
Grade 3: This level of adverse event represents an incapacitating event where the individual experiences an inability to perform their usual activities.
- iv.
Grade 4 (life-threatening/disabling): In this category, the participant is in a critical condition where there is a risk of death, worsening disability, or impairment compared to their pre-existing condition at the time of the event.
For adverse events categorized as grades 1 and 2, the investigators will closely monitor the participant and provide standard medical or therapeutic care as needed. If there are repeated instances of grade 1 and 2 events, the investigators may deem it necessary to notify the Institutional Review Board (IRB) and consider halting the study. In such cases, the investigators will take the appropriate action and inform the IRB accordingly. For grade 3 and 4 events, each occurrence will be individually assessed. If any grade 3 or 4 event takes place, it may prompt the investigators to notify the IRB and consider stopping the study. The investigators will follow the necessary steps and inform the IRB accordingly. In the absence of such circumstances, if the IRB determines that the serious adverse event is related to the protocol, the study will be halted and undergo evaluation to determine if it should continue.
8. Data Management
8.1. Electronic Data Records
All electronic files will be stored on password-protected computer terminals located in the Christine E. Lynn Rehabilitation Center for the Miami Project to Cure Paralysis, the University of Miami Miller School of Medicine, and Craig Hospital. Computer security is provided through data encryption, firewall protection, and data backup on the Miami Project server, accompanied by a DUO Multifactor Authorization. All source data obtained will be entered into a de-identified data bank and stored securely on the network.
8.2. Physical Data Records
Data will be stored in a locked filing cabinet, within a locked room, and behind two levels of access security at the Christine E. Lynn Rehabilitation Center and Craig Hospital. The source data from the laboratory and testing equipment are kept under the same level of access security described above.
8.3. Confidentiality
The identified data (name, date of birth, contact details, next of kin, and any protected health information (PHI) obtained) will be kept by the University of Miami and Craig Hospital. This data will be stored in a password-protected file and on a computer under the same level of access security described above. The deidentified study data, coded with a unique study ID assigned to each participant, will be kept by the University of Miami and Craig Hospital. The electronic records will be kept indefinitely, and the physical records will be kept for a minimum of 3 years after enrollment is closed and destroyed by the shredding services provided by each center. The participants’ samples will be stored in accordance with the Human Tissue Act 2004.
10. Discussion
This study is designed to test the effects and durability of a structured multi-modal intervention on enhancing the health and function of persons with SCI and their caregivers. The expected results from this trial may serve as an important guide for long-term rehabilitation strategies for the cardiometabolic risk factors observed in patients with chronic SCI and the general health and quality of life of their caregivers. The recently published clinical practice guidelines on “Identification and Management of Cardiometabolic Risk after Spinal Cord Injury” [
36] recommend lifestyle intervention as the primary management approach to CMD risk components after SCI. Moreover, the CMD prevention guidelines established by the American College of Cardiology/American Heart Association (ACC/AHA) state that one of the principal components of an “effective high intensity…lifestyle intervention” is the “use of behavioral strategies to facilitate adherence”, and it is asserted that this therapy should provide a “structured behavioral change program”.
Past reviews on CMD risks in SCI suggest the use of clinical pathways incorporating a comprehensive TLI to foster a more effective health-centered culture for those with SCI and their health care providers [
3], and preliminary studies suggest that the TLI described in this trial can effectively reduce the component risks for CMD in SCI [
26]. The results of this trial will have more widespread implications for the management and prevention of CMD in SCI and an effective cross-over of benefits for both the caregiver and care-receiver. Overall, this trial will enhance the validity and applicability of the current recommended guidelines for CMD risks in SCI and the growing health concerns for their caregivers.