Effects of a Multifaceted Intervention Program on the Eating Ability of Nursing Home Residents
Abstract
:1. Introduction
2. Methods
2.1. Design
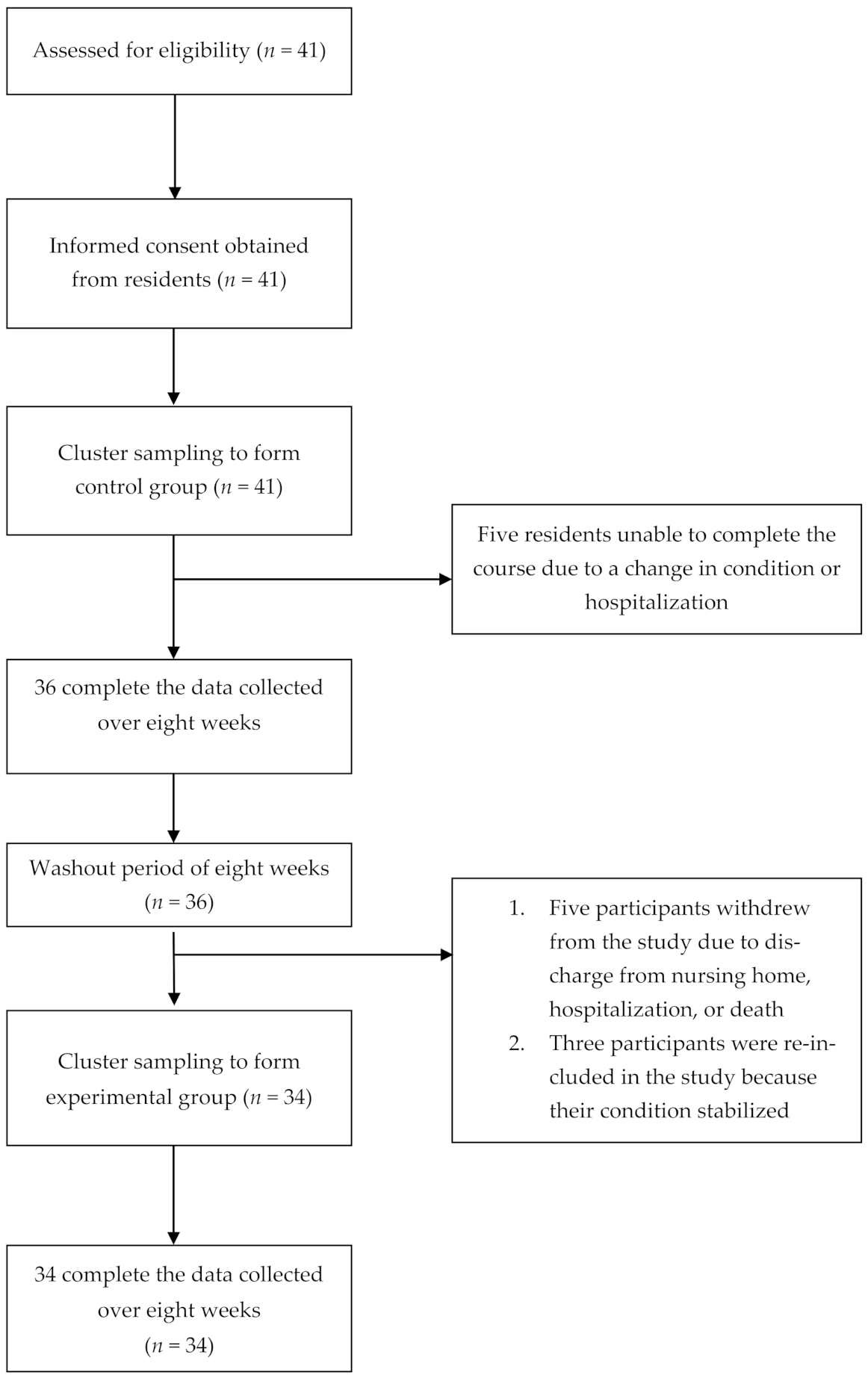
2.2. Sample and Setting
2.3. Multifaceted Intervention Program
2.3.1. Interactive Oral Activities
2.3.2. Tongue Strength Training
2.3.3. Oral Cleaning Procedure
2.4. Measures
2.4.1. Measurement of the Maximum Isometric Pressure of the Tongue
2.4.2. Body Weight
2.4.3. Amount of Food Consumed
2.4.4. Mealtime Duration
2.4.5. Oral Health
2.4.6. Gugging Swallowing Screen (GUSS) (Indirect Test)
2.5. Data Collection
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
3.1. Demographics and Baseline Data
3.2. Effects of Multifaceted Intervention Program on Outcome Variables
3.3. Lasting Effects of the Multifaceted Intervention Program
3.4. Subjective Feelings of the Participants of the Multifaceted Intervention Program
4. Discussion
5. Limitations
6. Conclusions and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Chang, E.; Brownhill, S.; Bidewell, J.; Johnson, A.; Ratnayake, S. Focus on feeding! Evaluation of a frame work for maximizing mealtime in aged care facilities. Int. J. Nurs. Stud. 2015, 21, 269–277. [Google Scholar]
- Aghdassi, E.; McArther, M.; Liu, B.; McGeer, A.; Simor, A.; Allard, J.P. Dietary intake of elderly living in To ronto long-term care facilities: Comparison to the dietary reference intake. Rejuvenation Res. 2007, 10, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.; Whear, R.; Thompson-Coon, J.; Ukoumunne, O.; Rogers, M.; Betje, A. Effectiveness of mealtime interventions on nutritional outcomes for the elderly living in residential care: A systematic review and meta-analysis. Ageing Res. Rev. 2013, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Yamashita, Y. Oral health and swallowing problems. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laguna, L.; Chen, J. The eating capability: Constituents and assessments. Food Qual. Prefer. 2016, 48, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Ney, D.; Weiss, J.; Kind, A.; Robbins, J.A. Senescent swallowing: Impact, strategies and interventions. Nutr. Clin. Pract. 2009, 24, 395–413. [Google Scholar] [CrossRef] [Green Version]
- Baijens, L.W.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O.; et al. European Society for Swallowing Disorders–European Union Geriatric Medicine Society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef] [Green Version]
- Ortega, O.; Cabré, M.; Clavé, P. Oropharyngeal dysphagia: Aetiology and effects of ageing. J. Gastroenterol. Hepatol. 2014, 3, 1049–1054. [Google Scholar]
- Di Pede, C.; Mantovani, M.E.; Del Felice, A.; Masiero, S. Dysphagia in the elderly: Focus on rehabilitation strategies. Aging Clin. Exp. Res. 2016, 28, 607–617. [Google Scholar] [CrossRef]
- Imaizumi, M.; Suzuki, T.; Ikeda, M.; Matsuzuka, T.; Goto, A.; Omori, K. Implementing a flexible endoscopic evaluation of swallowing at elderly care facilities to reveal characteristics of elderly subjects who screened positive for a swallowing disorder. Auris Nasus Larynx 2020, 47, 602–608. [Google Scholar] [CrossRef]
- Trawitzki, L.V.V.; Borges, C.G.P.; Giglio, L.D.; Silva, J.B. Tongue strength of healthy young adults. J. Oral Rehabil. 2011, 38, 482–486. [Google Scholar] [CrossRef]
- Szynkiewicz, S.H.; Nobriga, C.V.; O’Donoghue, C.R.; Becerra, B.J.; LaForge, G. Motor imagery practice and increased tongue strength: A case series feasibility report. J. Speech Lang. Hear. Res. 2019, 62, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Peladeau-Pigeon, M.; Steele, C.M. Age-related variability in tongue pressure patterns for maximum isometric and saliva swallowing tasks. J. Speech Lang. Hear. Res. 2017, 60, 3177–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Chung, S.Y.; Lin, C.T.; Hwu, Y.J. Effect of tongue-to-palate resistance training on tongue strength in healthy adults. Auris Nasus Larynx 2021, 48, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Bailey, G.L.; Polacco, R.E.C.; Hori, S.F.; Molfenter, S.M.; Oshalla, M.; Yeates, E.M. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int. J. Speech Lang. Pathol. 2013, 15, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, L.; Vanderwegen, J.; Guns, C.; Elen, R.; De Bodt, M.; Van Nuffelen, G. Tongue-strengthening exercises in healthy older adults: Does exercise load matter? A randomized controlled trial. Dysphagia 2019, 34, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.A.W.T. Basic oral care for patients with dysphagia: A special needs dentistry perspective. JCPSLP 2018, 20, 142–149. [Google Scholar]
- VanRavenhorst-Bell, H.A.; Coufal, K.L.; Patterson, J.A.; Mefferd, A.S. A comparative study: Tongue muscle performance in weightlifters and runners. Physiol. Rep. 2018, 6, e13923. [Google Scholar] [CrossRef]
- Toniazzo, M.P.; Amorim, P.S.; Muniz, F.W.M.G.; Weidlich, P. Relationship of nutritional status and oral health in elderly: Systematic review with meta-analysis. Clin. Nutr. 2018, 37, 824–830. [Google Scholar] [CrossRef]
- Liu, W.M.; Chiang, C.K.; Hwu, Y.J. Oral care for clients in long-term care. J. Nurs. Res. 2019, 66, 21–26. [Google Scholar]
- Sarkar, A.; Andablo-Reyes, E.; Bryant, M.; Dowson, D.; Neville, A. Lubrication of soft oral surfaces. Curr. Opin. Colloid Interface Sci. 2019, 39, 61–75. [Google Scholar] [CrossRef]
- Agrawal, D.; Kern, M.; Edeani, F.; Balasubramania, G.; Hyngstrom, A.; Sanvanson, P.; Shaker, R. Swallow strength training exercise for elderly: A health maintenance need. Neurogastroenterol. Motil. 2018, 30, e13382. [Google Scholar] [CrossRef]
- Wu, S.J.; Shieh, S.H.; Lai, Y.J.; Shin, Y.T.; Hwu, Y.J. Effects of an eating ability program for community-dwelling older adults. J. Am. Med. Dir. Assoc. 2020, 21, 1336–1340. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Lauretani, F.; Pelá., G.; Meschi, T.; Maggio, M. The risk of dysphagia is associated with malnutrition and poor functional outcomes in a large population of outpatient older individuals. Clin. Nutr. 2019, 38, 2684–2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.W.H.; Greenwood, C.E.; van Reekum, R.; Binns, M.A. A randomized, crossover trial of high-carbohydrate foods in nursing home residents with Alzheimer’s disease: Associations among intervention response, body mass index, and behavioral and cognitive function. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health and Welfare (Online). Guidelines for Eating, Dysphagia Care and Guidance Protocol. Taipei, Taiwan, 2019. Available online: http://nhplatform.mohw.gov.tw/dl-1023-141080a9990a4d5bbaeeb7aab39bd36c7.html (accessed on 29 June 2021).
- McKenna, V.S.; Zhang, B.; Haines, M.B.; Kelchner, L.N. A systematic review of isometric lingual strength-training programs in adults with and without dysphagia. Am. J. Speech Lang. Pathol. 2017, 26, 524–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.Y.; Liu, Y.C.; Li, C.L.; Yang, Y.Y.; Chiu, Y.C. Validation of the Chinese version in oral health assessment tool (OHAT) for clinical non-dentist professionals. Chang. Gung Nurs. 2015, 26, 400–409. [Google Scholar]
- Trapl, M.; Enderle, P.; Nowotny, M.; Teuschl, Y.; Matz, K.; Dachenhausen, A.; Brainin, M. Dysphagia bedside screening for acute-stroke patients: The Gugging Swallowing Screen. Stroke 2007, 38, 2948–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, S.F.; Keeler, E.; Zhuo, X.; Hickey, K.A.; Sato, H.W.; Schnelle, J.F. Prevention of unintentional weight loss in nursing home residents: A controlled trial of feeding assistance. J. Am. Geriatr. Soc. 2008, 56, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.P.; Ngu, B.H. Undertaking experiments in social sciences: Sequential, multiple times series designs for consideration. Educ. Psychol. Rev. 2017, 29, 847–867. [Google Scholar] [CrossRef]
- Molfenter, S.M.; Lenell, C.; Lazarus, C.L. Volumetric changes to the pharynx in healthy aging: Consequence for pharyngeal swallow mechanics and function. Dysphagia 2019, 34, 129–137. [Google Scholar] [CrossRef]
- De Almeida Mello, J.; Tran, T.D.; Krausch-Hofmann, S.; Meehan, B.; van Hout, H.; Turcotte, L.; van der Roest, H.G.; Garms-Homolová, V.; Jónsson, P.; Onder, G.; et al. Cross-country validation of the association between oral health and general health in community-dwelling older adults. J. Am. Med. Dir. Assoc. 2019, 20, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S. Ethical considerations in community oral health. J. Dent. Educ. 2015, 79, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Spanemberg, J.C.; Cardoso, J.A.; Slob, E.M.G.B.; López-López, J. Quality of life related to oral health and its impact in adults. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 234–239. [Google Scholar] [CrossRef] [PubMed]
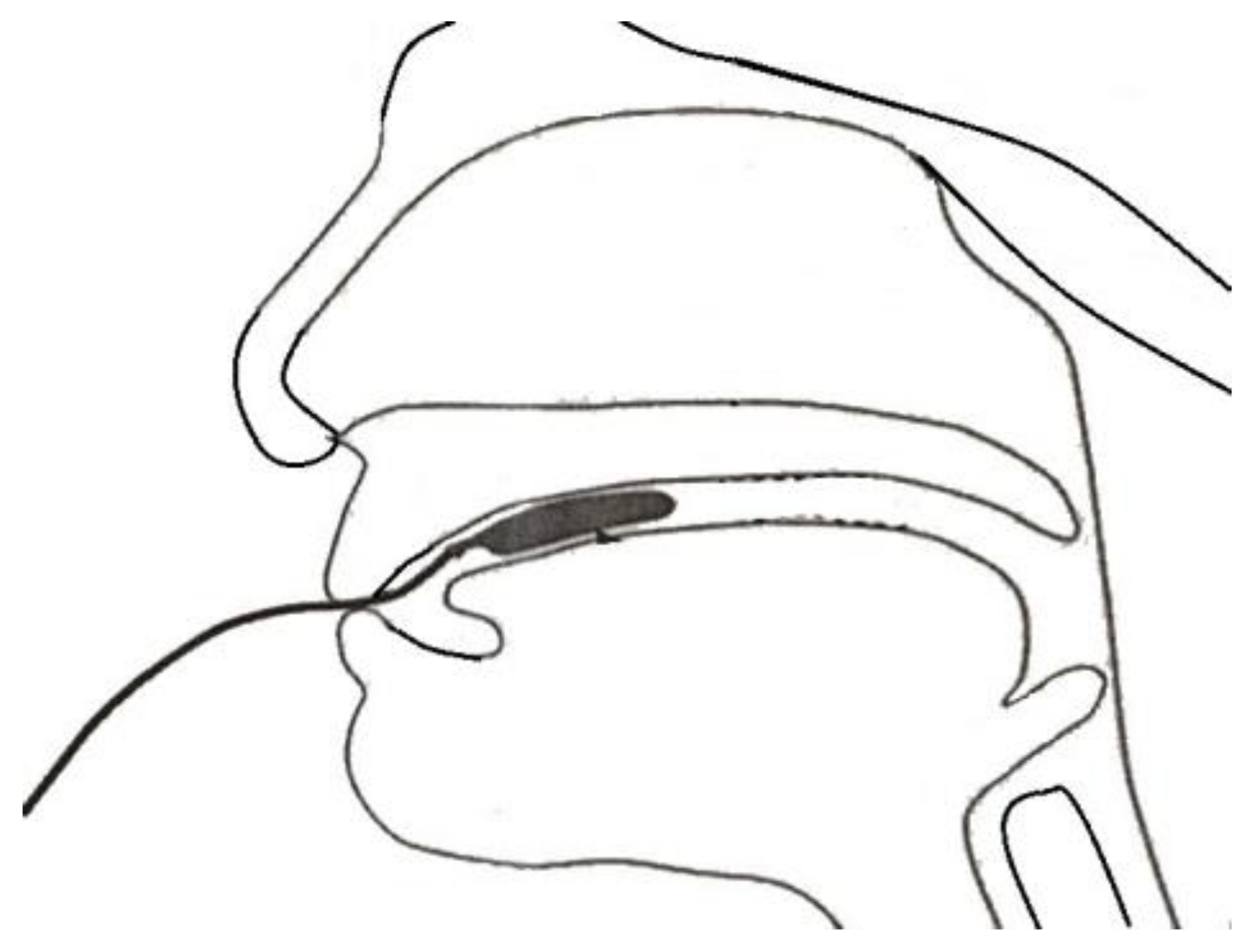
| The Training Protocol. | |
|---|---|
| 1 | Ask the participant to sit in a chair with a backrest and relax. |
| 2 | Instruct them to place the tip of their tongue behind their upper front teeth and feel the edge of the teeth. Tell them that this is where their tongue must go when they swallow, so that the food and liquid are sent back to the pharynx. |
| 3 | Instruct the participant to try and place the tip of their tongue behind their upper teeth, and then push it up forcefully. Explain that during the oral phase of swallowing, the tongue moves upward and forward. |
| 4 | Put the pressure bulb in the participant’s mouth and lie it flat on the tongue. The seal of the pressure bulb should be behind the front teeth. Once the bulb is in position, mark the connecting tube near the lips with an oil-based pen. |
| 5 | Hold the connecting tube with your thumb and index finger and ask the participant to lift their tongue and push the pressure bulb up. Count to six (two seconds). Rest for 5–10 s and repeat the above actions a total of 30 times. |
| 6 | Instruct the participant to do the tongue-to-palate pressure bulb exercise 30 min before meals, and then rest for a while before eating. They should perform this exercise three times a day for eight weeks. |
| 7 | If the connecting tube shifts, ask the participant to open their mouth, adjust the position of the pressure bulb, and then perform the tongue-to-palate pressure bulb exercise. |
| 8 | Verbally encourage the participant, telling them that they have done a good job. |
| 9 | Adjust the frequency and timing according to the participant’s condition. |
| 10 | Record the number and duration of the tongue-to-palate exercises the participant performs each day in a datasheet. |
| Variable | Total (n = 41) n (%)/Mean (SD) | Phase 1 (n = 36) n (%)/Mean (SD) | Phase 3 (n = 34) n (%)/Mean (SD) | t/χ2 | p |
|---|---|---|---|---|---|
| Gender | 0.364 | 0.433 | |||
| Male | 15 (36.6) | 12 (33.3) | 8 (23.5) | ||
| Female | 26 (63.4) | 24 (66.7) | 26 (76.5) | ||
| Diet | 8.10 | 0.088 | |||
| 7 (17.1) | 6 (16.7) | 15 (44.1) | ||
| 6 (14.6) | 6 (16.7) | 2 (5.9) | ||
| 12 (29.3) | 11 (30.6) | 5 (14.7) | ||
| 13 (31.7) | 12 (33.3) | 1 (32.4) | ||
| 3 (7.3) | 1 (2.8) | 1 (2.9) | ||
| Mealtime context | 0.00 a | 0.967 | |||
| 38 (92.7) | 35 (97.2) | 33 (97.1) | ||
| 2 (4.9) | 1 (2.8) | 1 (2.9) | ||
| 1 (2.4) | ||||
| Age | 80.14 (10.16) | 79.61 (10.62) | 79.91 (9.51) | 0.124 | 0.901 |
| Body mass index | 21.42 (3.22) | 21.80 (3.03) | 21.44 (3.11) | −0.487 | 0.628 |
| Calf circumference | 28.56 (3.80) | 28.97 (3.54) | 28.79 (3.58) | −0.209 | 0.835 |
| Cognition status (SPMSQ) | 3.73 (2.89) | 3.56 (2.61) | 3.05 (2.25) | −0.849 | 0.399 |
| Barthel index | 30.26 (21.67) | 31.69 (21.47) | 33.08 (21.81) | 0.269 | 0.789 |
| IADL | 23.31 (6.74) | 23.05 (6.74) | 23.85 (6.72) | 0.495 | 0.622 |
| Number of medications | 8.22 (2.93) | 8.16 (3.12) | 8.47 (3.84) | 0.364 | 0.717 |
| Comorbidities | 2.10 (0.80) | 2.11 (0.82) | 2.12 (0.84) | 0.033 | 0.974 |
| Nutrition status (MNA score) | 18.20 (4.72) | 19.05 (4.04) | 19.23 (4.40) | 0.178 | 0.859 |
| Variable | Total (n = 41) Mean (SD) | Phase 1 (n = 36) Mean (SD) | Phase 3 (n = 34) Mean (SD) | t | p |
|---|---|---|---|---|---|
| Tongue strength | 9.83 (4.35) | 10.31 (4.27) | 21.14 (7.53) | 7.46 | 0.000 |
| Body weight (kg) | 52.34 (9.95) | 53.24 (9.91) | 52.35 (10.45) | 7.35 | 0.000 |
| Food consumption (g) | 427.07 (241.12) | 417.97 (246.17) | 437.73 (246.17) | 0.34 | 0.738 |
| Mealtime duration (min) | 20.46 (7.39) | 20.11 (7.29) | 19.26 (4.42) | −0.58 | 0.562 |
| Dysphagia severity (GUSS score) | 4.17 (0.99) | 4.19 (0.88) | 4.23 (1.04) | 0.18 | 0.860 |
| Oral health (OHAT score) | 3.83 (1.94) | 3.72 (1.87) | 3.38 (2.29) | −0.68 | 0.499 |
| Variable | Baseline | Biweekly Measurements | Adjusted Biweekly Measurements | F | p | |||
|---|---|---|---|---|---|---|---|---|
| Phase 3 Mean ± SD | Phase 1 Mean ± SD | Phase 3 Mean ± SD | Phase 1 Mean ± SD | Phase 3 Mean ± SD | Phase 1 Mean ± SD | |||
| Tongue strength | 21.14 ± 7.53 | 10.31 ± 4.27 | ||||||
| Week 2 | 27.73 ± 12.84 | 14.91 ± 6.11 | 22.94 | 19.71 | 5.25 | 0.000 | ||
| Week 4 | 31.14 ± 12.25 | 20.05 ± 9.43 | 26.89 | 24.31 | 3.99 | 0.000 | ||
| Week 6 | 33.17 ± 14.66 | 17.94 ± 7.30 | 29.16 | 21.95 | 3.48 | 0.001 | ||
| Week 8 | 36.38 ± 12.20 | 20.50 ± 7.62 | 32.67 | 24.20 | 3.69 | 0.000 | ||
| Body weight | 52.35 ± 10.45 | 53.24 ± 9.91 | ||||||
| Week 2 | 52.07 ± 10.53 | 53.50 ± 9.62 | 52.51 | 53.06 | 57.51 | 0.000 | ||
| Week 4 | 52.75 ± 10.74 | 53.33 ± 10.44 | 53.21 | 52.88 | 41.09 | 0.000 | ||
| Week 6 | 52.68 ± 10.98 | 52.87 ± 10.36 | 53.14 | 52.41 | 40.51 | 0.000 | ||
| Week 8 | 52.92 ± 11.05 | 53.04 ± 10.43 | 53.38 | 52.59 | 37.22 | 0.000 | ||
| Food consumption (g) | 437.73 ± 246.17 | 417.97 ± 246.17 | ||||||
| Week 2 | 483.58 ± 257.95 | 385.18 ± 237.53 | 477.19 | 391.58 | 6.89 | 0.000 | ||
| Week 4 | 593.64 ± 265.76 | 440.49 ± 134.64 | 590.87 | 443.27 | 2.87 | 0.005 | ||
| Week 6 | 509.23 ± 261.63 | 419.47 ± 240.59 | 503.61 | 425.10 | 5.51 | 0.000 | ||
| Week 8 | 628.94 ± 305.25 | 462.54 ± 279.01 | 623.52 | 467.95 | 4.27 | 0.000 | ||
| Mealtime duration (min) | 19.26 ± 4.42 | 20.11 ± 7.29 | ||||||
| Week 2 | 20.17 ± 5.63 | 23.58 ± 8.18 | 20.35 | 23.40 | 3.11 | 0.003 | ||
| Week 4 | 20.32 ± 5.77 | 24.41 ± 6.21 | 20.53 | 24.20 | 4.83 | 0.000 | ||
| Week 6 | 20.23 ± 5.76 | 20.11 ± 5.52 | 20.40 | 19.93 | 3.99 | 0.000 | ||
| Week 8 | 19.32 ± 5.62 | 19.88 ± 4.99 | 19.46 | 19.74 | 3.44 | 0.001 | ||
| Oral health (OHAT score) | 3.38 ± 2.29 | 3.72 ± 1.87 | ||||||
| Week 2 | 2.64 ± 1.84 | 3.75 ± 1.87 | 2.78 | 3.60 | 21.73 | 0.000 | ||
| Week 4 | 2.38 ± 1.45 | 3.69 ± 1.87 | 2.50 | 3.57 | 16.13 | 0.000 | ||
| Week 6 | 2.29 ± 1.16 | 3.63 ± 1.89 | 2.40 | 3.52 | 14.20 | 0.000 | ||
| Week 8 | 2.29 ± 1.16 | 3.86 ± 2.05 | 2.40 | 3.74 | 12.54 | 0.000 | ||
| Dysphagia severity (GUSS score) | 4.23 ± 1.04 | 4.19 ± 0.88 | ||||||
| Week 2 | 4.47 ± 1.07 | 4.16 ± 0.97 | 4.45 | 4.18 | 11.54 | 0.000 | ||
| Week 4 | 4.82 ± 0.62 | 4.17 ± 0.97 | 4.81 | 4.17 | 8.62 | 0.000 | ||
| Week 6 | 4.79 ± 0.72 | 4.16 ± 0.97 | 4.78 | 4.18 | 7.92 | 0.000 | ||
| Week 8 | 3.94 ± 0.23 | 3.47 ± 0.77 | 3.94 | 3.47 | 3.83 | 0.000 | ||
| Variable | Sphericity Test (p) | Mean Squares | Degrees of Freedom | F | p | LSD Test a |
|---|---|---|---|---|---|---|
| 1. Tongue strength | 0.000 | |||||
| Phase | 15,171.76 | 1 | 41.93 | 0.000 | ||
| Week (0, 2, 4, 6, 8) | 2433.45 | 2.82 | 53.89 | 0.000 | 8 > 4 > 2 > 0, 8 > 6 > 2 > 0 | |
| Time × phase | 133.18 | 2.82 | 2.95 | 0.037 | ||
| 2. Body weight | 0.000 | |||||
| Phase | 35.81 | 1 | 0.07 | 0.798 | ||
| Week (0, 2, 4, 6, 8) | 1.90 | 2.29 | 0.65 | 0.544 | ||
| Time × phase | 8.77 | 2.29 | 3.00 | 0.046 | ||
| 3. Food consumption (g) | 0.000 | |||||
| Phase | 973,060.26 | 1 | 5.41 | 0.023 | ||
| Week (0, 2, 4, 6, 8) | 187,418.02 | 4 | 5.60 | 0.000 | 8 > 0, 8 > 2 | |
| Time × phase | 59,576.60 | 4 | 1.78 | 0.133 | ||
| 4. Mealtime duration (min) | 0.000 | |||||
| Phase | 270.06 | 1 | 2.63 | 0.109 | ||
| Week (0, 2, 4, 6, 8) | 145.52 | 3.21 | 5.82 | 0.001 | 2 > 8, 4 > 0, 4 > 8 | |
| Time × phase | 76.18 | 3.21 | 3.04 | 0.027 | ||
| 5. Oral health (OHAT score) | 0.000 | |||||
| Phase | 112.30 | 1 | 7.56 | 0.008 | ||
| Week (0, 2, 4, 6, 8) | 8.24 | 1.82 | 12.64 | 0.000 | 0 > 2, 0 > 4, 0 > 6, 0 > 8 | |
| Time × phase | 8.61 | 1.82 | 13.21 | 0.000 | ||
| 6. Dysphagia severity (GUSS score) | 0.000 | |||||
| Phase | 15.39 | 1 | 5.54 | 0.022 | ||
| Week (0, 2, 4, 6, 8) | 11.24 | 2.58 | 30.08 | 0.000 | 4 > 0 > 8, 6 > 0 > 8, 2 > 8 | |
| Time × phase | 1.76 | 2.58 | 4.70 | 0.005 |
| Theme | Statement |
|---|---|
| 1. To improve swallowing function | “In the past, when I had meals, I often coughed and had no confidence. I was also afraid of being looked down upon by others. The thought of having a meal frightened me. When I eat now, I can keep my food moving smoothly without it getting stuck, which leaves me feeling comfortable and vigorous.” “I like chicken very much, but I used to eat only boiled chicken, which didn’t taste good. Now I can even eat chicken thighs, and I don’t bite my tongue. I feel very happy.” When I saw other people’s plates, I felt embarrassed. Now I can eat rice and non-pureed meat and vegetables that are cut into pieces. I feel that I have dignity with respect to eating.” |
| 2. To keep the oral cavity clean | “I have followed the attendant’s reminder to brush my teeth and rinse my mouth after meals. I feel refreshed, and I don’t always need to clear my throat and cough.” “I brush my teeth and rinse my mouth after all three meals. The discomfort in my oral cavity has reduced a lot, and I don’t have to go to the dentist as often.” “Ever since I have known that dental plaques form within three minutes of a meal, and that the plaques are full of bacteria that can induce systemic diseases, I am serious about brushing my teeth for at least three minutes and within three minutes after meals. Now I implement oral hygiene very carefully every day. I feel very busy every day.” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-L.; Chiu, C.-H.; Hwu, Y.-J.; Kuo, S.-C. Effects of a Multifaceted Intervention Program on the Eating Ability of Nursing Home Residents. Int. J. Environ. Res. Public Health 2021, 18, 8951. https://doi.org/10.3390/ijerph18178951
Chen M-L, Chiu C-H, Hwu Y-J, Kuo S-C. Effects of a Multifaceted Intervention Program on the Eating Ability of Nursing Home Residents. International Journal of Environmental Research and Public Health. 2021; 18(17):8951. https://doi.org/10.3390/ijerph18178951
Chicago/Turabian StyleChen, Mei-Ling, Chia-Hui Chiu, Yueh-Juen Hwu, and Shu-Chen Kuo. 2021. "Effects of a Multifaceted Intervention Program on the Eating Ability of Nursing Home Residents" International Journal of Environmental Research and Public Health 18, no. 17: 8951. https://doi.org/10.3390/ijerph18178951






