Characteristics of the Passive Muscle Stiffness of the Vastus Lateralis: A Feasibility Study to Assess Muscle Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
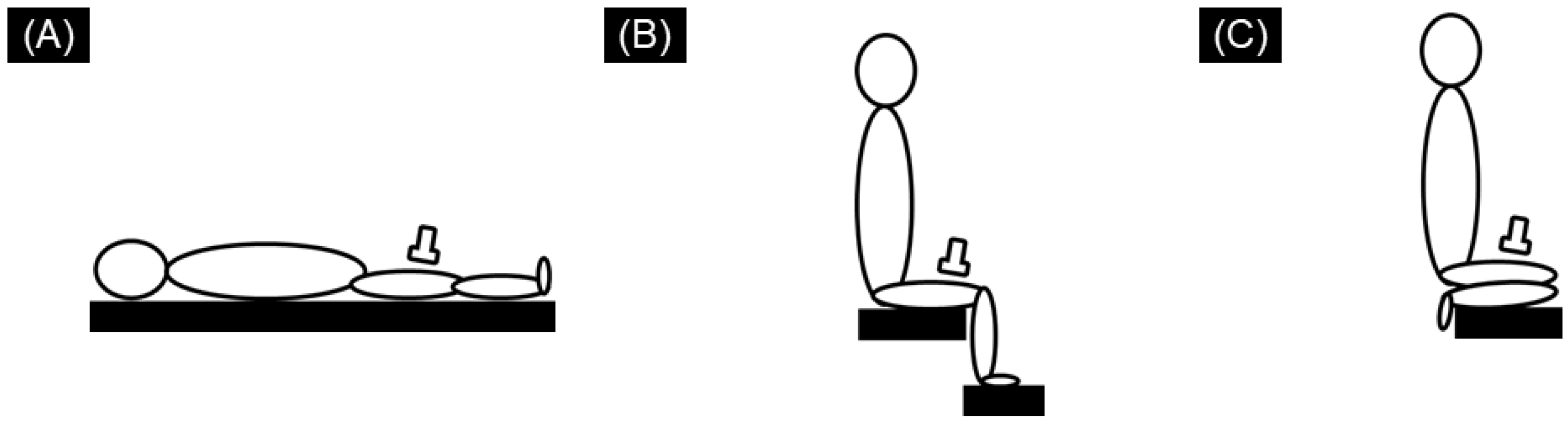
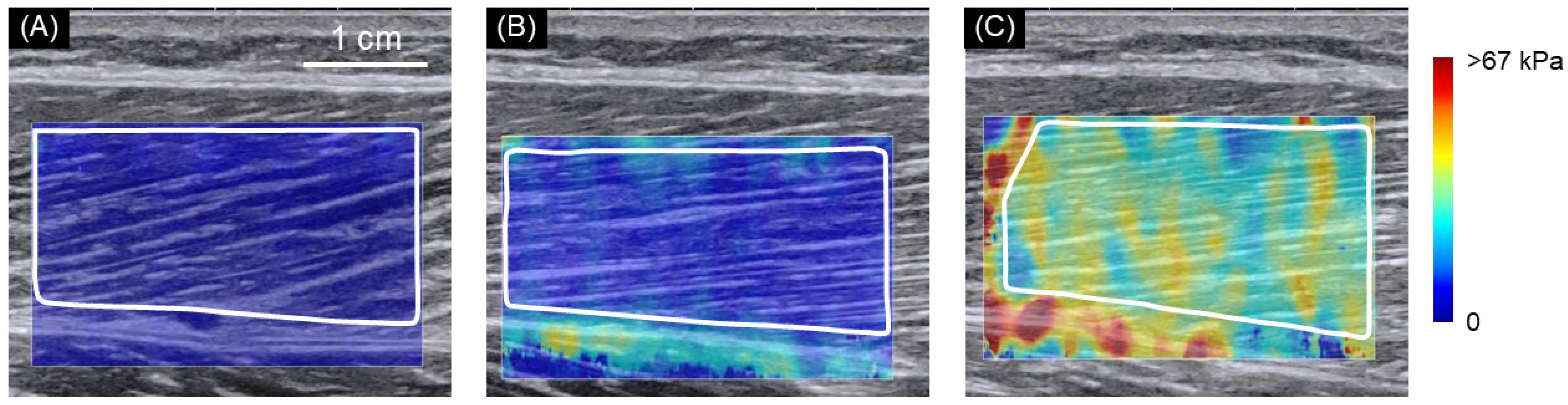
2.2. SWE Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wood, L.K.; Kayupov, E.; Gumucio, J.P.; Mendias, C.L.; Claflin, D.R.; Brooks, S.V. Intrinsic Stiffness of Extracellular Matrix Increases with Age in Skeletal Muscles of Mice. J. Appl. Physiol. 2014, 117, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Mahdy, M.A.A. Skeletal Muscle Fibrosis: An Overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the Skeletal Muscle Extracellular Matrix Drives a Stem Cell Fibrogenic Conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purslow, P.P. Strain-Induced Reorientation of an Intramuscular Connective Tissue Network: Implications for Passive Muscle Elasticity. J. Biomech. 1989, 22, 21–31. [Google Scholar] [CrossRef]
- Meyer, G.A.; Lieber, R.L. Elucidation of Extracellular Matrix Mechanics from Muscle Fibers and Fiber Bundles. J. Biomech. 2011, 44, 771–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieber, R.L.; Runesson, E.; Einarsson, F.; Fridén, J. Inferior Mechanical Properties of Spastic Muscle Bundles Due to Hypertrophic but Compromised Extracellular Matrix Material. Muscle Nerve 2003, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gajdosik, R.L. Passive Extensibility of Skeletal Muscle: Review of the Literature with Clinical Implications. Clin. Biomech. 2001, 16, 87–101. [Google Scholar] [CrossRef]
- Martins-Bach, A.B.; Bachasson, D.; Araujo, E.C.A.; Soustelle, L.; Loureiro de Sousa, P.; Fromes, Y.; Carlier, P.G. Non-Invasive Assessment of Skeletal Muscle Fibrosis in Mice Using Nuclear Magnetic Resonance Imaging and Ultrasound Shear Wave Elastography. Sci. Rep. 2021, 11, 284. [Google Scholar] [CrossRef]
- Akagi, R.; Yamashita, Y.; Ueyasu, Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound. Med. Biol. 2015, 41, 2906–2912. [Google Scholar] [CrossRef]
- Yoshida, K.; Itoigawa, Y.; Maruyama, Y.; Saita, Y.; Takazawa, Y.; Ikeda, H.; Kaneko, K.; Sakai, T.; Okuwaki, T. Application of Shear Wave Elastography for the Gastrocnemius Medial Head to Tennis Leg. Clin. Anat. 2017, 30, 114–119. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Li, T.-J.; Zheng, Y.-P. Shear Modulus Estimation on Vastus Intermedius of Elderly and Young Females over the Entire Range of Isometric Contraction. PLoS ONE 2014, 9, e101769. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.-K.; Sheng, S.-Y.; Liang, S.-M.; Lu, H.; Chen, R.-Y.; Pan, M.; Wen, Z.-B. Quantitative Evaluation of Passive Muscle Stiffness by Shear Wave Elastography in Healthy Individuals of Different Ages. Eur. Radiol. 2021, 31, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Rosant, C.; Nagel, M.-D.; Pérot, C. Aging Affects Passive Stiffness and Spindle Function of the Rat Soleus Muscle. Exp. Gerontol. 2007, 42, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Hirata, K.; Miyamoto-Mikami, E.; Yasuda, O.; Kanehisa, H. Associations of Passive Muscle Stiffness, Muscle Stretch Tolerance, and Muscle Slack Angle with Range of Motion: Individual and Sex Differences. Sci. Rep. 2018, 8, 8274. [Google Scholar] [CrossRef]
- Hansen, M.; Kongsgaard, M.; Holm, L.; Skovgaard, D.; Magnusson, S.P.; Qvortrup, K.; Larsen, J.O.; Aagaard, P.; Dahl, M.; Serup, A.; et al. Effect of Estrogen on Tendon Collagen Synthesis, Tendon Structural Characteristics, and Biomechanical Properties in Postmenopausal Women. J. Appl. Physiol. 2009, 106, 1385–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.-N. Shear Wave Elastography of Passive Skeletal Muscle Stiffness: Influences of Sex and Age throughout Adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; O’Dell, M.; He, W.; Du, L.-J.; Li, P.-C.; Gao, J. Ultrasound Shear Wave Elastography in the Assessment of Passive Biceps Brachii Muscle Stiffness: Influences of Sex and Elbow Position. Clin. Imaging 2017, 45, 26–29. [Google Scholar] [CrossRef]
- Brooks, S.V.; Faulkner, J.A. Skeletal Muscle Weakness in Old Age: Underlying Mechanisms. Med. Sci. Sports Exerc. 1994, 26, 432–439. [Google Scholar] [CrossRef]
- Miyamoto, N.; Hirata, K.; Inoue, K.; Hashimoto, T. Muscle Stiffness of the Vastus Lateralis in Sprinters and Long-Distance Runners. Med. Sci. Sports Exerc. 2019, 51, 2080–2087. [Google Scholar] [CrossRef]
- Csapo, R.; Malis, V.; Sinha, U.; Du, J.; Sinha, S. Age-Associated Differences in Triceps Surae Muscle Composition and Strength—An MRI-Based Cross-Sectional Comparison of Contractile, Adipose and Connective Tissue. BMC Musculoskelet. Disord. 2014, 15, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Sato, K.; Hasegawa, N.; Kurihara, T.; Matsutani, K.; Sanada, K.; Hamaoka, T.; Fujita, S.; Iemitsu, M. Serum C1q as a Novel Biomarker of Sarcopenia in Older Adults. FASEB J. 2015, 29, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Barry, B.K.; Carson, R.G. The Consequences of Resistance Training for Movement Control in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 730–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18-88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Loftin, M.; Fukunaga, T. Prevalence of Site-Specific Thigh Sarcopenia in Japanese Men and Women. Age 2014, 36, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age Group (years) | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | |
|---|---|---|---|---|---|---|
| Female | N | 9 | 9 | 9 | 7 | 9 |
| Age (years) | 34.78 ± 3.01 | 45.11 ± 2.23 | 54.67 ± 2.49 | 65.29 ± 3.10 | 71.78 ± 2.20 | |
| Height (cm) | 158.71 ± 4.69 | 155.88 ± 4.07 | 155.41 ± 4.58 | 154.43 ± 2.71 | 151.94 ± 2.75 | |
| Weight (kg) | 51.22 ± 7.24 | 51.73 ± 4.23 | 53.80 ± 6.16 | 46.20 ± 2.64 | 51.27 ± 5.73 | |
| BMI | 20.30 ± 2.47 | 21.31 ± 1.85 | 22.30 ± 2.60 | 19.36 ± 0.77 | 22.19 ± 2.23 | |
| Number of menopause | 0 | 0 | 8 | 7 | 9 | |
| Male | N | 9 | 9 | 9 | 9 | 7 |
| Age (years) | 34.44 ± 2.79 | 43.89 ± 2.88 | 53.44 ± 2.71 | 65.56 ± 1.64 | 73.14 ± 2.53 | |
| Height (cm) | 175.40 ± 4.70 | 171.57 ± 4.36 | 169.97 ± 4.85 | 169.49 ± 6.28 | 165.31 ± 3.72 | |
| Weight (kg) | 68.10 ± 5.84 | 70.32 ± 9.00 | 69.66 ± 7.05 | 69.67 ± 9.57 | 63.51 ± 10.50 | |
| BMI | 22.19 ± 2.33 | 23.83 ± 2.29 | 24.09 ± 2.02 | 24.37 ± 4.04 | 23.12 ± 2.96 |
| Age Group (years) | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | Two-Way ANOVA p-Value (Partial η2) | |||
|---|---|---|---|---|---|---|---|---|---|
| (Age) | (Sex) | (Age X Sex) | |||||||
| Muscle shear modulus (kPa) | |||||||||
| The knee fully extended | |||||||||
| Female | 4.16 ± 0.97 | 4.31 ± 1.41 | 4.66 ± 2.27 | 4.90 ± 1.15 | 4.28 ± 0.67 | 0.413 (0.050) | 0.408 (0.009) | 0.645 (0.032) | |
| Male | 3.92 ± 0.83 | 4.01 ± 0.46 | 3.93 ± 0.54 | 4.58 ± 1.25 | 4.80 ± 0.97 | ||||
| The knee flexed to 90° | |||||||||
| Female | 8.63 ± 1.82 | 7.40 ± 1.23 | 10.69 ± 6.12 | 8.42 ± 2.11 | 10.71 ± 6.96 | 0.128 (0.089) | 0.272 (0.016) | 0.876 (0.016) | |
| Male | 7.93 ± 0.97 | 7.16 ± 0.83 | 9.15 ± 2.17 | 8.75 ± 2.24 | 8.81 ± 1.83 | ||||
| The knee fully flexed | |||||||||
| Female | 43.02 ± 9.44 | 41.03 ± 10.23 | 45.66 ± 16.74 | 46.26 ± 12.76 | 51.68 ± 14.77 | 0.006 (0.170) | 0.001 (0.145) | 0.497 (0.043) | |
| Male | 56.72 ± 14.93 | 46.38 ± 10.44 | 53.60 ± 10.46 | 51.52 ± 13.67 | 70.79 ± 16.59 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, A.; Yamagishi, M.; Otsuka, Y.; Izumo, T.; Rogi, T.; Shibata, H.; Fukuda, M.; Arimitsu, T.; Yamada, Y.; Miyamoto, N.; et al. Characteristics of the Passive Muscle Stiffness of the Vastus Lateralis: A Feasibility Study to Assess Muscle Fibrosis. Int. J. Environ. Res. Public Health 2021, 18, 8947. https://doi.org/10.3390/ijerph18178947
Maeda A, Yamagishi M, Otsuka Y, Izumo T, Rogi T, Shibata H, Fukuda M, Arimitsu T, Yamada Y, Miyamoto N, et al. Characteristics of the Passive Muscle Stiffness of the Vastus Lateralis: A Feasibility Study to Assess Muscle Fibrosis. International Journal of Environmental Research and Public Health. 2021; 18(17):8947. https://doi.org/10.3390/ijerph18178947
Chicago/Turabian StyleMaeda, Akifumi, Maito Yamagishi, Yuta Otsuka, Takayuki Izumo, Tomohiro Rogi, Hiroshi Shibata, Masahiro Fukuda, Takuma Arimitsu, Yosuke Yamada, Naokazu Miyamoto, and et al. 2021. "Characteristics of the Passive Muscle Stiffness of the Vastus Lateralis: A Feasibility Study to Assess Muscle Fibrosis" International Journal of Environmental Research and Public Health 18, no. 17: 8947. https://doi.org/10.3390/ijerph18178947
APA StyleMaeda, A., Yamagishi, M., Otsuka, Y., Izumo, T., Rogi, T., Shibata, H., Fukuda, M., Arimitsu, T., Yamada, Y., Miyamoto, N., & Hashimoto, T. (2021). Characteristics of the Passive Muscle Stiffness of the Vastus Lateralis: A Feasibility Study to Assess Muscle Fibrosis. International Journal of Environmental Research and Public Health, 18(17), 8947. https://doi.org/10.3390/ijerph18178947






