Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol
2.2. Eligibility Criteria
- −
- Types of participants: young humans (children or adolescents) and rodents.
- −
- Types of interventions: prenatal, perinatal, or postnatal environmental exposure to pesticides.
- −
- Types of Comparators: studies comparing the prenatal or postnatal environmental exposure to pesticides, with either a control group, a non-exposed group, or between groups with different levels of exposure (i.e., low, medium, high).
- −
- Types of outcome measures: obesity, overweight, or metabolic syndrome, measured through body weight, height, body mass index, and/or waist circumference. Furthermore, biological outcomes such as temperature, organ weight, biochemical measurements, and brain histomorphological alterations were assessed. Additionally, behavioral measures related to cognitive impairment and emotional disturbances were also assessed.
- −
- Types of study design: experimental studies for literature with animal models, and cohort, cross-sectional in the case of human studies. In this way, rodent models have shown the highest face validity to reproduce the human metabolic syndrome induced by high-carbohydrate and high-fat diets [31]. In addition, many current studies have addressed the induction of metabolic syndrome in rodents [32]. Thus, a recent study refers to the main criteria and reference values used to reproduce this disease in animal models [33].
2.3. Information Sources
2.4. Study Selection and Data Collection Process
2.5. Risk of Bias in Individual Studies
2.6. Summary Measures and Analysis
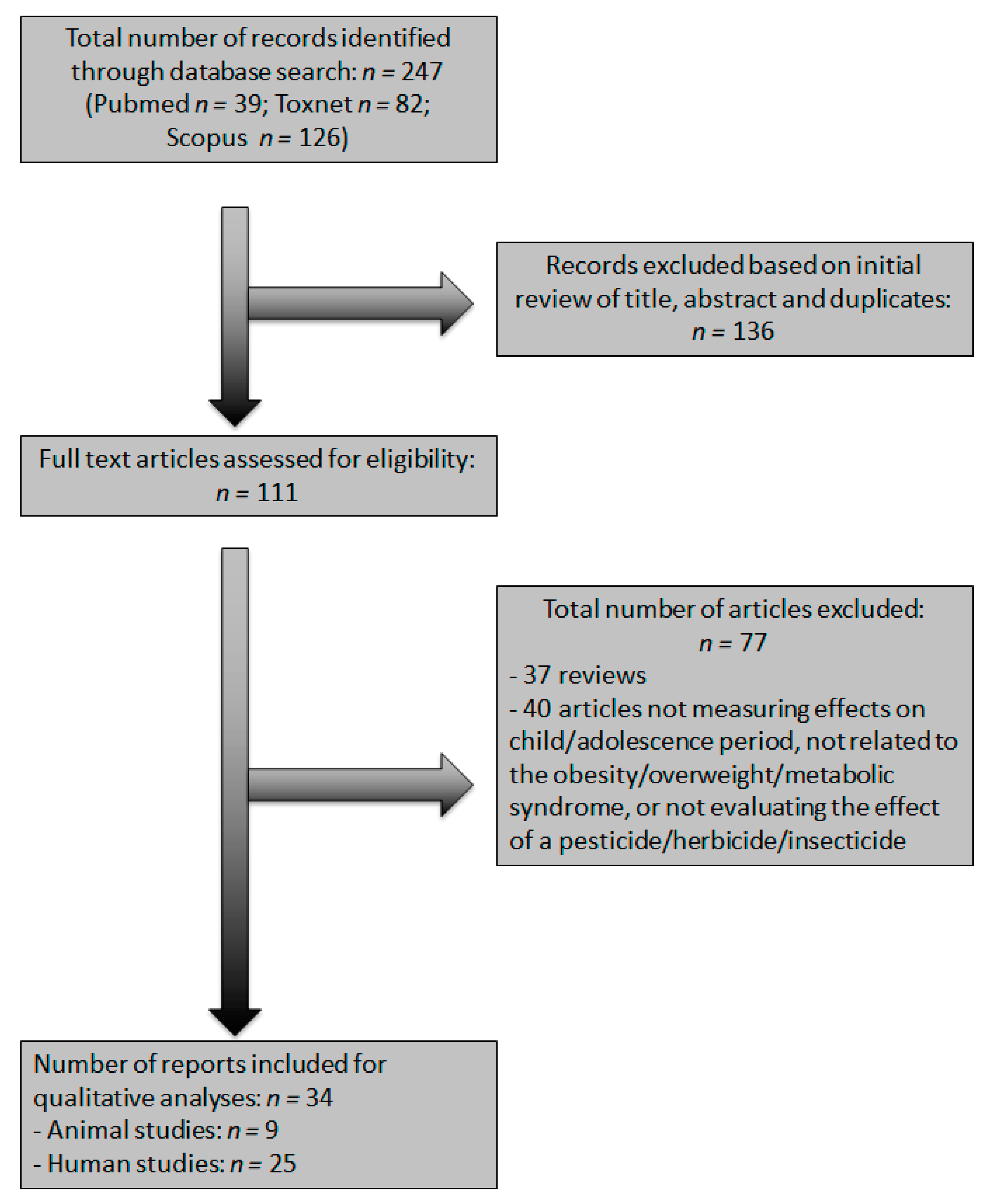
3. Results
3.1. Study Selection
3.2. Clinical Studies Characteristics
3.2.1. Outcomes and Exposure Statement
Organochlorines Pesticides: p,p′-DDE/p,p′-DDD and p,p′-DDT
Organochlorines Pesticides: HCB, β-HCH, Chlordecone
Pyrethroids, Diclorophenols (DCPs), Organophosphatades, and Mixed Pesticides
3.3. Animal Studies
3.3.1. Outcomes and Exposure Statement
Obesity
Physiological, Biochemical, Metabolic, and Behavioral Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef]
- Pinos, H.; Carrillo, B.; Díaz, F.; Chowen, J.A.; Collado, P. Differential vulnerability to adverse nutritional conditions in male and female rats: Modulatory role of estradiol during development. Front. Neuroendocr. 2018, 48, 13–22. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Ren, X.-M.; Kuo, Y.; Blumberg, B. Agrochemicals and obesity. Mol. Cell. Endocrinol. 2020, 515, 110926. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Fossati, S.; Maitre, L.; Márquez, S.; Roumeliotaki, T.; Agier, L.; Andrusaityte, S.; Cadiou, S.; Casas, M.; De Castro, M.; et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ. Health Perspect. 2020, 128, 067009. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Rashid, H.; Alqahtani, S.S.; Alshahrani, S. Diet: A Source of Endocrine Disruptors. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.A. Human and Wildlife Exposures to EDCs; Bergman, A., Heindel, J.J., Jobling, S., Kidd, K.A., Zoeller, R.T., Eds.; State of the Science of Endocrine Disrupting Chemicals–2012; World Health Organization; UNEP: Cham, Switzerland, 2012; pp. 189–250. [Google Scholar]
- Mansouri, A.; Cregut, M.; Abbes, C.; Durand, M.-J.; Landoulsi, A.; Thouand, G. The Environmental Issues of DDT Pollution and Bioremediation: A Multidisciplinary Review. Appl. Biochem. Biotechnol. 2016, 181, 309–339. [Google Scholar] [CrossRef]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A. Persistent organic pollutants (POPs): A global issue, a global challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Guo, G.L. Understanding Environmental Contaminants’ Direct Effects on Non-alcoholic Fatty Liver Disease Progression. Curr. Environ. Health Rep. 2019, 6, 95–104. [Google Scholar] [CrossRef]
- Xiao, X.; Clark, J.M.; Park, Y. Potential contribution of insecticide exposure and development of obesity and type 2 diabetes. Food Chem. Toxicol. 2017, 105, 456–474. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Kim, J.; Yoon, K.S.; Clark, J.; Lee, J.; Park, Y. Imidacloprid, a Neonicotinoid Insecticide, Potentiates Adipogenesis in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2013, 61, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Saal, F.S.V.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health 2015, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kong, A.P.S.; Cai, Z.; Chung, A.C. Persistent Organic Pollutants as Risk Factors for Obesity and Diabetes. Curr. Diabetes Rep. 2017, 17, 132. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.; Cardoso, R.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- Mustieles, V.; Fernández, M.F.; Martin-Olmedo, P.; Alzaga, B.G.; Fontalba-Navas, A.; Hauser, R.; Olea, N.; Arrebola, J.P. Human adipose tissue levels of persistent organic pollutants and metabolic syndrome components: Combining a cross-sectional with a 10-year longitudinal study using a multi-pollutant approach. Environ. Int. 2017, 104, 48–57. [Google Scholar] [CrossRef]
- Grün, F.; Blumberg, B. Environmental Obesogens: Organotins and Endocrine Disruption via Nuclear Receptor Signaling. Endocrinology 2006, 147, s50–s55. [Google Scholar] [CrossRef]
- Evangelou, E.; Ntritsos, G.; Chondrogiorgi, M.; Kavvoura, F.K.; Hernández, A.F.; Ntzani, E.E.; Tzoulaki, I. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ. Int. 2016, 91, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kadawathagedara, M.; De Lauzon-Guillain, B.; Botton, J. Environmental contaminants and child’s growth. J. Dev. Orig. Health Dis. 2018, 9, 632–641. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef]
- Mesnage, R.; Biserni, M.; Wozniak, E.; Xenakis, T.; Mein, C.A.; Antoniou, M.N. Comparison of transcriptome responses to glyphosate, isoxaflutole, quizalofop-p-ethyl and mesotrione in the HepaRG cell line. Toxicol. Rep. 2018, 5, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xiao, X.; Kim, Y.; Kim, D.; Yoon, K.; Clark, J.M.; Park, Y. Imidacloprid Promotes High Fat Diet-Induced Adiposity and Insulin Resistance in Male C57BL/6J Mice. J. Agric. Food Chem. 2016, 64, 9293–9306. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, X.; Yao, X.; Xi, F.; He, Y.; Xu, Y.; Ma, L.; Chen, X.; Zhao, C.; Du, R.; et al. Bifenthrin Induces Fat Deposition by Improving Fatty Acid Uptake and Inhibiting Lipolysis in Mice. J. Agric. Food Chem. 2019, 67, 14048–14055. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Hsieh, T.-H.; Yue, Y.; Sun, Q.; Clark, J.M.; Park, Y. Deltamethrin increases the fat accumulation in 3T3-L1 adipocytes and Caenorhabditis elegans. Food Chem. Toxicol. 2017, 101, 149–156. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef]
- Fuchs, T.; Loureiro, M.D.P.; Macedo, L.E.; Nocca, D.; Nedelcu, M.; Costa-Casagrande, T.A. Modelos animais na síndrome metabólica. Rev. Colégio Bras. Cir. 2018, 45, 45. [Google Scholar] [CrossRef]
- Virgen-Carrillo, C.A.; Moreno, A.G.M.; Rodríguez-Gudiño, J.J.; Pineda-Lozano, J.E. Feeding pattern, biochemical, anthropometric and histological effects of prolonged ad libitum access to sucrose, honey and glucose-fructose solutions in Wistar rats. Nutr. Res. Pract. 2021, 15, 187–202. [Google Scholar] [CrossRef]
- Wells, G.S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugvell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 9 September 2020).
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Güil-Oumrait, N.; Valvi, D.; Garcia-Esteban, R.; Guxens, M.; Sunyer, J.; Torrent, M.; Casas, M.; Vrijheid, M. Prenatal exposure to persistent organic pollutants and markers of obesity and cardiometabolic risk in Spanish adolescents. Environ. Int. 2021, 151, 106469. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Lee, Y.A.; Lee, Y.J.; Shin, C.H.; Lim, Y.-H.; Hong, Y.-C. The relationship of urinary 3-phenoxybenzoic acid concentrations in utero and during childhood with adiposity in 4-year-old children. Environ. Res. 2019, 172, 446–453. [Google Scholar] [CrossRef]
- Valvi, D.; Mendez, M.A.; Garcia-Esteban, R.; Ballester, F.; Ibarluzea, J.; Goñi-Irigoyen, F.; Grimalt, J.O.; Llop, S.; Marina, L.S.; Vizcaino, E.; et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity 2013, 22, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Wesselink, A.; Harley, K.G.; Bradman, A.; Kogut, K.; Eskenazi, B. Prenatal exposure to dichlorodiphenyltrichloroethane and obesity at 9 years of age in the CHAMACOS study cohort. Am. J. Epidemiol. 2014, 179, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Cupul-Uicab, L.A.; Klebanoff, M.A.; Brock, J.W.; Longnecker, M.P. Prenatal Exposure to Persistent Organochlorines and Childhood Obesity in the U.S. Collaborative Perinatal Project. Environ. Health Perspect. 2013, 121, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Schall, R.A.; Harley, K.G.; Bradman, A.; Barr, D.; Eskenazi, B. In Utero DDT and DDE Exposure and Obesity Status of 7-Year-Old Mexican-American Children in the CHAMACOS Cohort. Environ. Health Perspect. 2013, 121, 631–636. [Google Scholar] [CrossRef]
- Valvi, D.; Mendez, M.A.; Martinez, D.; Grimalt, J.O.; Torrent, M.; Sunyer, J.; Vrijheid, M. Prenatal Concentrations of Polychlorinated Biphenyls, DDE, and DDT and Overweight in Children: A Prospective Birth Cohort Study. Environ. Health Perspect. 2012, 120, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cupul-Uicab, L.A.; Hernández-Ávila, M.; Terrazas-Medina, E.A.; Pennell, M.L.; Longnecker, M. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ. Res. 2010, 110, 595–603. [Google Scholar] [CrossRef]
- Smink, A.; Ribas-Fito, N.; Garcia, R.; Torrent, M.; Mendez, M.A.; O Grimalt, J.; Sunyer, J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. Int. J. Paediatr. 2008, 97, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fang, J.; Sun, X.; Zhang, W.; Li, J.; Chen, X.; Yu, L.; Xia, W.; Xu, S.; Cai, Z.; et al. Prenatal exposure to organochlorine pesticides and infant growth: A longitudinal study. Environ. Int. 2021, 148, 106374. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadi, M.; Georgiou, V.; Chalkiadaki, G.; Rantakokko, P.; Kiviranta, H.; Karachaliou, M.; Fthenou, E.; Venihaki, M.; Sarri, K.; Vassilaki, M.; et al. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother–Child Cohort (Crete, Greece). Environ. Health Perspect. 2015, 123, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Delvaux, I.; Van Cauwenberghe, J.; Hond, E.D.; Schoeters, G.; Govarts, E.; Nelen, V.; Baeyens, W.; Van Larebeke, N.; Sioen, I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ. Res. 2014, 132, 24–32. [Google Scholar] [CrossRef]
- Warner, M.; Ye, M.; Harley, K.; Kogut, K.; Bradman, A.; Eskenazi, B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ. Res. 2017, 159, 606–612. [Google Scholar] [CrossRef]
- Burns, J.S.; Williams, P.L.; Sergeyev, O.; Korrick, S.A.; Lee, M.M.; Revich, B.; Altshul, L.; Del Prato, J.T.; Humblet, O.; Patterson, D.G.; et al. Serum Concentrations of Organochlorine Pesticides and Growth among Russian Boys. Environ. Health Perspect. 2012, 120, 303–308. [Google Scholar] [CrossRef]
- Mendez, M.A.; Garcia-Esteban, R.; Guxens, M.; Vrijheid, M.; Kogevinas, M.; Goñi-Irigoyen, F.; Fochs, S.; Sunyer, J. Prenatal Organochlorine Compound Exposure, Rapid Weight Gain, and Overweight in Infancy. Environ. Health Perspect. 2011, 119, 272–278. [Google Scholar] [CrossRef]
- Xu, C.; Yin, S.; Tang, M.; Liu, K.; Yang, F.; Liu, W. Environmental exposure to DDT and its metabolites in cord serum: Distribution, enantiomeric patterns, and effects on infant birth outcomes. Sci. Total Environ. 2017, 580, 491–498. [Google Scholar] [CrossRef]
- Debost-Legrand, A.; Warembourg, C.; Massart, C.; Chevrier, C.; Bonvallot, N.; Monfort, C.; Rouget, F.; Bonnet, F.; Cordier, S. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ. Res. 2016, 146, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hervé, D.; Costet, N.; Kadhel, P.; Rouget, F.; Monfort, C.; Thomé, J.-P.; Multigner, L.; Cordier, S. Prenatal exposure to chlordecone, gestational weight gain, and birth weight in a Guadeloupean birth cohort. Environ. Res. 2016, 151, 436–444. [Google Scholar] [CrossRef]
- Agay-Shay, K.; Martinez, D.; Valvi, D.; Garcia-Esteban, R.; Basagaña, X.; Robinson, O.; Casas, M.; Sunyer, J.; Vrijheid, M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ. Health Perspect. 2015, 123, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Tang-Péronard, J.L.; Jensen, T.K.; Andersen, H.R.; Ried-Larsen, M.; Grøntved, A.; Andersen, L.B.; Timmermann, C.A.; Nielsen, F.; Heitmann, B.L. Associations between Exposure to Persistent Organic Pollutants in Childhood and Overweight up to 12 Years Later in a Low Exposed Danish Population. Obes. Facts 2015, 8, 282–292. [Google Scholar] [CrossRef]
- Høyer, B.; Ramlau-Hansen, C.H.; Henriksen, T.B.; Pedersen, H.S.; Goralczyk, K.; Zviezdai, V.; Jönsson, B.; Heederik, D.; Lenters, V.; Vermeulen, R.; et al. Body mass index in young school-age children in relation to organochlorine compounds in early life: A prospective study. Int. J. Obes. 2014, 38, 919–925. [Google Scholar] [CrossRef]
- Tang-Péronard, J.L.; Heitmann, B.L.; Andersen, H.R.; Steuerwald, U.; Grandjean, P.; Weihe, P.; Jensen, T.K. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: A prospective cohort study of 656 children from the Faroe Islands. Am. J. Clin. Nutr. 2014, 99, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.S.; Williams, P.L.; Korrick, S.A.; Hauser, R.; Sergeyev, O.; Revich, B.; Lam, T.; Lee, M.M. Association Between Chlorinated Pesticides in the Serum of Prepubertal Russian Boys and Longitudinal Biomarkers of Metabolic Function. Am. J. Epidemiol. 2014, 180, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Parastar, S.; Ebrahimpour, K.; Hashemi, M.; Maracy, M.R.; Ebrahimi, A.; Poursafa, P.; Kelishadi, R. Association of urinary concentrations of four chlorophenol pesticides with cardiometabolic risk factors and obesity in children and adolescents. Environ. Sci. Pollut. Res. Int. 2017, 25, 4516–4523. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rodríguez, R.; Luzardo, O.P.; Almeida-González, M.; Boada, L.D.; Zumbado, M.; Dacal, A.C.A.; Rial-Berriel, C.; Henríquez-Hernández, L.A. Association between prenatal exposure to multiple persistent organic pollutants (POPs) and growth indicators in newborns. Environ. Res. 2019, 171, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Tang, J.; Ren, X.; Li, C. Glyphosate exposure induces inflammatory responses in the small intestine and alters gut microbial composition in rats. Environ. Pollut. 2020, 261, 114129. [Google Scholar] [CrossRef]
- Lassiter, T.L.; Ryde, I.T.; MacKillop, E.A.; Brown, K.K.; Levin, E.D.; Seidler, F.J.; Slotkin, T.A. Exposure of Neonatal Rats to Parathion Elicits Sex-Selective Reprogramming of Metabolism and Alters the Response to a High-Fat Diet in Adulthood. Environ. Health Perspect. 2008, 116, 1456–1462. [Google Scholar] [CrossRef][Green Version]
- Lassiter, T.L.; Ryde, I.T.; Levin, E.D.; Seidler, F.J.; Slotkin, T.A. Neonatal exposure to parathion alters lipid metabolism in adulthood: Interactions with dietary fat intake and implications for neurodevelopmental deficits. Brain Res. Bull. 2010, 81, 85–91. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.; Karey, E.; Moshier, E.; Lindtner, C.; La Frano, M.; Newman, J.; Buettner, C. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring. PLoS ONE 2014, 9, e103337. [Google Scholar] [CrossRef] [PubMed]
- Ndonwi, E.N.; Atogho-Tiedeu, B.; Lontchi-Yimagou, E.; Shinkafi, T.S.; Nanfa, D.; Balti, E.V.; Indusmita, R.; Mahmood, A.; Katte, J.-C.; Mbanya, A.; et al. Gestational Exposure to Pesticides Induces Oxidative Stress and Lipid Peroxidation in Offspring that Persist at Adult Age in an Animal Model. Toxicol. Res. 2019, 35, 241–248. [Google Scholar] [CrossRef]
- Lassiter, T.L.; Brimijoin, S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol. Teratol. 2008, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Q.; Zhang, Y.-Z.; Yuan, L.; Li, Y.-F.; Li, J. Neurobehavioral evaluation of adolescent male rats following repeated exposure to chlorpyrifos. Neurosci. Lett. 2014, 570, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, D.; Meng, Z.; Yan, S.; Teng, M.; Jia, M.; Li, R.; Tian, S.; Weiss, C.; Zhou, Z.; et al. Effects of incremental endosulfan sulfate exposure and high fat diet on lipid metabolism, glucose homeostasis and gut microbiota in mice. Environ. Pollut. 2021, 268, 115697. [Google Scholar] [CrossRef]
- Wang, D.; Li, B.; Wu, Y.; Li, B. The Effects of Maternal Atrazine Exposure and Swimming Training on Spatial Learning Memory and Hippocampal Morphology in Offspring Male Rats via PSD95/NR2B Signaling Pathway. Cell. Mol. Neurobiol. 2019, 39, 1003–1015. [Google Scholar] [CrossRef]
- André, S.M.; Markowski, V.P. Learning deficits expressed as delayed extinction of a conditioned running response following perinatal exposure to vinclozolin. Neurotoxicol. Teratol. 2006, 28, 482–488. [Google Scholar] [CrossRef]
- Heggeseth, B.; Harley, K.; Warner, M.; Jewell, N.; Eskenazi, B. Detecting Associations between Early-Life DDT Exposures and Childhood Growth Patterns: A Novel Statistical Approach. PLoS ONE 2015, 10, e0131443. [Google Scholar] [CrossRef]
- Jackson, E.; Shoemaker, R.; Larian, N.; Cassis, L. Adipose Tissue as a Site of Toxin Accumulation. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2017; Volume 7, pp. 1085–1135. [Google Scholar]
- Camacho, W.J.M.; Díaz, J.M.M.; Ortiz, S.P.; Ortiz, J.E.P.; Camacho, M.A.M.; Calderón, B.P. Childhood obesity: Aetiology, comorbidities, and treatment. Diabetes/Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef]
- Cissé, A.H.; Lioret, S.; de Lauzon-Guillain, B.; Forhan, A.; Ong, K.K.; Charles, M.A.; Heude, B. Association between perinatal factors, genetic susceptibility to obesity and age at adiposity rebound in children of the EDEN mother–child cohort. Int. J. Obes. 2021, 1–9. [Google Scholar] [CrossRef]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect. 2004, 112, 524–531. [Google Scholar] [CrossRef]
- Kim, J.; Sun, Q.; Yue, Y.; Yoon, K.; Whang, K.-Y.; Clark, J.M.; Park, Y. 4,4′-Dichlorodiphenyltrichloroethane (DDT) and 4,4′-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture. Pestic. Biochem. Physiol. 2016, 131, 40–45. [Google Scholar] [CrossRef]
- Lee, D.-H.; Steffes, M.W.; Sjödin, A.; Jones, R.S.; Needham, L.L. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS ONE 2011, 6, e15977. [Google Scholar] [CrossRef]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 2014, 322, 1–13. [Google Scholar] [CrossRef]
- Slotkin, T.A. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod. Toxicol. 2011, 31, 297–301. [Google Scholar] [CrossRef]
- Haug, L.S.; Sakhi, A.K.; Cequier, E.; Casas, M.; Maitre, L.; Basagana, X.; Andrusaityte, S.; Chalkiadaki, G.; Chatzi, L.; Coen, M.; et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int. 2018, 121, 751–763. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Sargis, R.M.; Persky, V.; Turyk, M.E. Multiple organochlorine pesticide exposures and measures of sex steroid hormones in adult males: Cross-sectional findings from the 1999–2004 National Health and Nutrition Examination Survey. Int. J. Hyg. Environ. Health 2021, 231, 113609. [Google Scholar] [CrossRef]
- Siroux, V.; Agier, L.; Slama, R. The exposome concept: A challenge and a potential driver for environmental health research. Eur. Respir. Rev. 2016, 25, 124–129. [Google Scholar] [CrossRef]
- Jedynak, P.; Maitre, L.; Guxens, M.; Gützkow, K.B.; Julvez, J.; López-Vicente, M.; Sunyer, J.; Casas, M.; Chatzi, L.; Gražulevičienė, R.; et al. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age—An exposome-based approach in 5 European cohorts. Sci. Total Environ. 2021, 763, 144115. [Google Scholar] [CrossRef] [PubMed]
- Bialy, M.; Bogacki-Rychlik, W.; Przybylski, J.; Żera, T. The Sexual Motivation of Male Rats as a Tool in Animal Models of Human Health Disorders. Front. Behav. Neurosci. 2019, 13, 13. [Google Scholar] [CrossRef]
- Bornman, M.; Delport, R.; Farías, P.; Aneck-Hahn, N.; Patrick, S.; Millar, R.P.; de Jager, C. Alterations in male reproductive hormones in relation to environmental DDT exposure. Environ. Int. 2018, 113, 281–289. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Differential Disrupting Effects of Prolonged Low-Dose Exposure to Dichlorodiphenyltrichloroethane on Androgen and Estrogen Production in Males. Int. J. Mol. Sci. 2021, 22, 3155. [Google Scholar] [CrossRef]
- Hong, H.; Shen, J.; Ng, H.W.; Sakkiah, S.D.; Ye, H.; Ge, W.; Gong, P.; Xiao, W.; Tong, W. A Rat α-Fetoprotein Binding Activity Prediction Model to Facilitate Assessment of the Endocrine Disruption Potential of Environmental Chemicals. Int. J. Environ. Res. Public Health 2016, 13, 372. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Sledneva, Y.P.; Nazimova, S.V.; Obernikhin, S.S.; Yaglov, V.V. Sex Differences in the Production of SLC5A5, Thyroid Peroxidase, and Thyroid Hormones in Pubertal Rats Exposed to Endocrine Disruptor Dichlorodiphenyltrichloroethane (DDT) during Postnatal Ontogeny. Bull. Exp. Biol. Med. 2018, 164, 430–433. [Google Scholar] [CrossRef]
- Ma, S.; Jing, F.; Xu, C.; Zhou, L.; Song, Y.; Yu, C.; Jiang, D.; Gao, L.; Li, Y.; Guan, Q.; et al. Thyrotropin and Obesity: Increased Adipose Triglyceride Content Through Glycerol-3-Phosphate Acyltransferase 3. Sci. Rep. 2015, 5, 7633. [Google Scholar] [CrossRef] [PubMed]
- Aiceles, V.; Ramos, C.D.F. A link between hypothyroidism, obesity and male reproduction. Horm. Mol. Biol. Clin. Investig. 2016, 25, 5–13. [Google Scholar] [CrossRef]
- Kaspari, R.R.; Reyna-Neyra, A.; Jung, L.; Torres-Manzo, A.P.; Hirabara, S.M.; Carrasco, N. The paradoxical lean phenotype of hypothyroid mice is marked by increased adaptive thermogenesis in the skeletal muscle. Proc. Natl. Acad. Sci. USA 2020, 117, 22544–22551. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; White, N.M.; Toms, L.-M.; Mengersen, K. Application of ensemble methods to analyse the decline of organochlorine pesticides in relation to the interactions between age, gender and time. PLoS ONE 2019, 14, e0223956. [Google Scholar] [CrossRef]
- Ben Maamar, M.; King, S.E.; Nilsson, E.; Beck, D.; Skinner, M.K. Epigenetic transgenerational inheritance of parent-of-origin allelic transmission of outcross pathology and sperm epimutations. Dev. Biol. 2020, 458, 106–119. [Google Scholar] [CrossRef] [PubMed]
- King, S.; McBirney, M.; Beck, D.; Sadler-Riggleman, I.; Nilsson, E.; Skinner, M.K. Sperm epimutation biomarkers of obesity and pathologies following DDT induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2019, 5, dvz008. [Google Scholar] [CrossRef] [PubMed]
- Nantia, E.A.; Kada, A.S.; Manfo, F.P.; Tangu, N.N.; Mbifung, K.M.; Mbouobda, D.H.; Kenfack, A. Parastar insecticide induced changes in reproductive parameters and testicular oxidative stress biomarkers in Wistar male rats. Toxicol. Ind. Health 2018, 34, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Srivastava, P.; Shukla, R.K.; Gupta, R.; Singh, D.; Parmar, D.; Pant, A.B.; Khanna, V.K. Prenatal exposure to lambda-cyhalothrin alters brain dopaminergic signaling in developing rats. Toxicology 2017, 386, 49–59. [Google Scholar] [CrossRef]
- Pawar, N.N.; Badgujar, P.C.; Sharma, L.P.; Singh, K.P.; Telang, A.G. Oxidative impairment and histopathological alterations in kidney and brain of mice following subacute lambda-cyhalothrin exposure. Toxicol. Ind. Health 2016, 33, 277–286. [Google Scholar] [CrossRef]
- Palkhade, R.; Yadav, S.; Mishra, S.; Muhamed, J. Acute oral toxicity of pesticide combination (acephate 50% and imidacloprid 1.8% as active ingredients) in Sprague-Dawley rats. Vet. World 2018, 11, 1291–1297. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103–108. [Google Scholar] [CrossRef]
- Panzacchi, S.; Mandrioli, D.; Manservisi, F.; Bua, L.; Falcioni, L.; Spinaci, M.; Galeati, G.; Dinelli, G.; Miglio, R.; Mantovani, A.; et al. The Ramazzini Institute 13-week study on glyphosate-based herbicides at human-equivalent dose in Sprague Dawley rats: Study design and first in-life endpoints evaluation. Environ. Health 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Ganesan, S.; McGuire, B.C.; Keating, A.F. Absence of glyphosate-induced effects on ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 2020, 96, 156–164. [Google Scholar] [CrossRef]
- Cook, L.; Finger, B.J.; Green, M.P.; Pask, A.J. Exposure to atrazine during puberty reduces sperm viability, increases weight gain and alters the expression of key metabolic genes in the liver of male mice. Reprod. Fertil. Dev. 2019, 31, 920–931. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Chen, G.; Lin, X.; Miao, W.; Fu, Z. Exposure of mice to atrazine and its metabolite diaminochlorotriazine elicits oxidative stress and endocrine disruption. Environ. Toxicol. Pharmacol. 2014, 37, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; King, S.; McBirney, M.; Kubsad, D.; Pappalardo, M.; Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS ONE 2018, 13, e0202662. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Mittal, N.; Thompson, L.M.; Rodriguez-Santiago, M.; Duvauchelle, C.; Crews, D.; Gore, A.C. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Environ. Health Perspect. 2018, 126, 097005. [Google Scholar] [CrossRef] [PubMed]
- Ait-Bali, Y.; Ba-M’Hamed, S.; Gambarotta, G.; Sassoè-Pognetto, M.; Giustetto, M.; Bennis, M. Pre- and postnatal exposure to glyphosate-based herbicide causes behavioral and cognitive impairments in adult mice: Evidence of cortical ad hippocampal dysfunction. Arch. Toxicol. 2020, 94, 1703–1723. [Google Scholar] [CrossRef]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; Oliveira, P.V.; Rieg, C.E.H.; Leite, M.C.; Prediger, R.; Wendt, N.; Razzera, G.; et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Saunders, M.; Magnanti, B.L.; Carreira, S.C.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Von Krauss, M.K.; Keune, H.; et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11, S5. [Google Scholar] [CrossRef]
- Blanco, J.; Guardia-Escote, L.; Mulero, M.; Basaure, P.; Biosca-Brull, J.; Cabré, M.; Colomina, M.T.; Domingo, J.L.; Sánchez, D.J. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food Chem. Toxicol. 2020, 137, 111171. [Google Scholar] [CrossRef]
- Li, J.; Ren, F.; Li, Y.; Luo, J.; Pang, G. Chlorpyrifos Induces Metabolic Disruption by Altering Levels of Reproductive Hormones. J. Agric. Food Chem. 2019, 67, 10553–10562. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Cabré, M.; Basaure, P.; Reverte, I.; Domingo, J.L.; Colomina, M.T. Adulthood dietary exposure to a common pesticide leads to an obese-like phenotype and a diabetic profile in apoE3 mice. Environ. Res. 2015, 142, 169–176. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Blanco, J.; Cabré, M.; Basaure, P.; Guardia-Escote, L.; Domingo, J.L.; Sánchez, D.J.; Colomina, M.T. New mechanistic insights on the metabolic-disruptor role of chlorpyrifos in apoE mice: A focus on insulin- and leptin-signalling pathways. Arch. Toxicol. 2018, 92, 1717–1728. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Liu, J.; Ayoubi, P.; Pope, C. Dose-related gene expression changes in forebrain following acute, low-level chlorpyrifos exposure in neonatal rats. Toxicol. Appl. Pharmacol. 2010, 248, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Laporte, B.; Gay-Quéheillard, J.; Bach, V.; Villégier, A.-S. Developmental neurotoxicity in the progeny after maternal gavage with chlorpyrifos. Food Chem. Toxicol. 2018, 113, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cowell, W.J.; Wright, R.J. Sex-Specific Effects of Combined Exposure to Chemical and Non-chemical Stressors on Neuroendocrine Development: A Review of Recent Findings and Putative Mechanisms. Curr. Environ. Health Rep. 2017, 4, 415–425. [Google Scholar] [CrossRef]
- Gómez-Giménez, B.; Felipo, V.; Cabrera-Pastor, A.; Agustí, A.; Hernández-Rabaza, V.; Llansola, M. Developmental Exposure to Pesticides Alters Motor Activity and Coordination in Rats: Sex Differences and Underlying Mechanisms. Neurotox. Res. 2017, 33, 247–258. [Google Scholar] [CrossRef]
- Venerosi, A.; Ricceri, L.; Tait, S.; Calamandrei, G. Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: The case of chlorpyrifos. NeuroToxicology 2012, 33, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Swann, H.E.; Woodson, G.S.; Ballard, T.A. The Acute Toxicity of Intramuscular Parathion in Rats and the Relation of Weight, Sex and Sex Hormones to this Toxicity. Am. Ind. Hyg. Assoc. J. 1958, 19, 190–195. [Google Scholar] [CrossRef]
| Study, Year (Reference) | Study Design Region | Age at Evaluation/Sex/Sample Size/Rural vs. Urban | Type/Agent/Source of Exposure Assessment | Physiological Assessment in Children | Physiological Outcomes | Quality Index |
|---|---|---|---|---|---|---|
| Güil-Oumrait et al. [37] | INMA Cohort/Menorca (Spain) | 4, 6, 11, 14, 18 yo/ Both/N = 379/Rural | Env/p,p′-DDT, p,p′-DDE, HCB, PCBs/Cord blood | Birth weight, WC, HDL-C, LDL-C, triglycerides, insulin, and glucose. | Prenatal p,p′-DDT and HCB concentrations were significantly associated with increased BMI during childhood and adolescence (from 4- to 18-years-old), as well as WHtR during adolescence. Positive association between prenatal HCB and body fat % in adolescence. A continuous increase in HCB was associated with an elevated body fat % across all ages, and with higher CM-risk score and lipid biomarkers (total cholesterol, triglycerides and LDL-C at 14 years). p,p′-DDT exposure was associated with an increased CM-risk score, and ΣPCBs concentrations with LDL-C in all adolescents, and with total cholesterol only in girls. | 8 VH |
| Lee et al. [38] | CAS Cohort/Seoul and Gyeonggi (Korea) | 4 yo/ Both/N = 578/Urban | Env/Pyrethroid/Maternal urine (14–27 gw) and postnatal (4 yo) urine samples | zBMI | Prenatal urinary 3-phenoxybenzoic acid (3-PBA) concentration was not associated with height, weight, or zBMI at 4 years of age, regardless of sex. Postnatal childhood urinary 3-PBA concentration measured at 4 years of age was positively associated with zBMI in 4-year-old girls | 8 VH |
| Warner et al. [49] | CHAMACOS Cohort/California (USA) | 12 yo/ Both/N = 240/Rural | Env/DDT, DDE/ Gestational maternal blood (26 gw) or delivery blood samples | zBMI and zWC | Associations between prenatal exposure to DDT and DDE and several measures of obesity (zBMI, zWC) at 12 years of age in boys but not in girls | 8 VH |
| Xu et al. [52] | Cohort/Shengsi Islands (China) | Birth/ Both/N = 106/Rural | Env/DDT/ Cord blood (delivery) samples | Birth weight | Prenatal DDT levels were found to be significantly associated with an increase in neonatal birth weight in both sexes | 8 VH |
| Debost-Legrand et al. [53] | PELAGIE Cohort/Brittany (France) | Birth/ Both/N = 268/Rural | Env/DDE, βHCH, DAP/ Prenatal urine samples (1st trimester) Cord blood (delivery) samples | Insulin and adiponectin levels | A decrease in adiponectin levels was associated with an increase in DDE levels. Decrease in insulin levels was associated with an increase in DDE only in girls. A decrease in insulin levels was associated with low concentration of β-HCH in newborns. An increase in insulin levels with higher concentrations of DAP metabolites, specific with DM metabolites, reinforced by adjustment for BMIz scores at birth | 8 VH |
| Hervé et al. [54] | TIMOUN Cohort/Guadeloupe (FWI) | Birth/ Both/N = 593/Rural | Env/Chlordecone/ Cord blood (delivery) samples | Birth weight | No association between prenatal chlordecone exposure with birth weight | 8 VH |
| Agay-Shay et al. [55] | INMA Cohort/ Sabadell (Spain) | 7 yo/ Both/N = 470/ Urban | Env/DDE, HCB, βHCH/ Prenatal urine samples (1st and 3rd trimester) Maternal blood (1st trimester) | Overweight and zBMI | Positive associations between DDE and zBMI or overweight risk. An increase in zBMI and overweight was found with prenatal HCB exposure. | 8-VH |
| Tang-Peronad et al. [56] | Cohort/Odense (Denmark) | 8–10, 14–16, 20–22 yo/ Both/N = 278/Rural | Env/DDE, HCB/ Postnatal blood samples and breast milk | zBMI, WC and SFT | No relationship of postnatal HCB levels with weight gain was found in any age studied | 8 VH |
| Høyer et al. [57] | INUENDO Cohort/Warsaw (Poland) | 5–9 yo/ Both/N = 1109/Rural | Env/p,p′-DDE/ Gestational maternal blood (2nd–3rd trimester) | zBMI | No association with p,p′-DDE prenatal exposure and BMI | 8 VH |
| Tang-Peronad et al. [58] | Cohort/Faroe Islands (Denmark) | 5, 7,5 yo/ Both/N = 539/Rural | Env/DDE/ Gestational maternal blood (34 gw) and breast milk samples | BMI and WC | Positive association was reported among DDE prenatal exposure and BMI | 8 VH |
| Valvi et al. [39] | INMA Cohort/Sabadell, Valencia and Gipuzcoa (Spain) | 6, 14 mo/ Both/N = 136/ Urban | Env/DDT, DDE, HCB/ Gestational maternal blood (7th–26th gw) samples | zBMI | DDE and HCB was positively associated with overweight at 14 months of age. HCB was positively associated with overweight at 14 months of age | 8 VH |
| Warner et al. [40] | CHAMACOS Cohort/California (USA) | 9 yo/ Both/N = 261/Rural | Env/DDT, DDE/ Gestational maternal blood (26 gw) or delivery blood samples | zBMI and zWC | Associations between prenatal exposure to DDT and DDE and several measures of obesity (zBMI, zWC) at 9 years of age in boys but not in girls | 8 VH |
| Cupul-Uicab et al. [41] | Cohort/CPP (USA) | 7 yo/ Both/N = 1915/Both | Env/HCB, βHCH, p,p′-DDE, p,p′-DDT/ Gestational maternal blood (3rd trimester) samples | zBMI | No association with p,p′-DDE prenatal exposure and BMI. No association of HCB with zBMI in childhood | 8-VH |
| Warner et al. [42] | CHAMACOS Cohort/California (USA) | 7 yo/ Both/N = 270/Rural | Env/DDT, DDE/ Gestational maternal blood (26 gw) samples | zBMI | No association with DDE prenatal exposure and zBMI | 8 VH |
| Valvi et al. [43] | Asthma Multicenter Infants Cohort/Menorca (Spain) | 4, 6,5 yo/ Both/N = 344/Rural | Env/DDT, DDE, HCB/ Cord blood (delivery), postnatal blood (4 yo) samples | zBMI | Positive associations were reported between DDT/DDE prenatal exposure and BMI | 8 VH |
| Cupul-Uicab et al. [44] | Cohort/Chiapas (Mexico) | 13, 30 mean mo/ Males/N = 789/ Both | Env/DDE, DDT/ Maternal blood (delivery) samples | Heigh, weight SDS and BMI | No association with DDE prenatal exposure and BMI at 14 months | 8-VH |
| Smink et al. [45] | Asthma Multicenter Infants Cohort/Menorca (Spain) | 6,5 yo/ Both/N = 405/Rural | Env/HCB/ Cord blood (delivery) samples | zBMI | Increase in z BMI and overweight at age 5–7 was found with prenatal HCB exposure | 8 VH |
| Yang et al. [46] | Cohort/Wuhan (China) | Birth, 6, 12, 24 mo/N = 1039/Urban | Env/αHCH, βHCH, γHCH, p,p′-DDT, p,p′-DDD, p,p′-DDE/Cord blood | zBMI | Higher cord serum βHCH concentrations were associated with higher zBMI at 12 and 24 mo. Higher cord serum γHCH and p,p′-DDT were associated with higher zBMI at 6 and 12 mo. Cord serum βHCH was positively associated with the risk of overweight at 12 mo. Among girls, the effects of βHCH on zBMI and overweight were stronger than boys at 12 and 24 mo. | 7 VH |
| Vafeiadi et al. [47] | Rhea Cohort/Crete (Greece) | 4 yo/ Both/N = 689/Both | Env/DDE, HCB/ Gestational maternal blood (3rd–4th gw) postnatal blood samples | MBI, WC, SFT, leptin and adiponectin | Positive associations were reported among DDE prenatal exposure and BMI/WC. HCB was associated with excess adiposity | 7 VH |
| Delvaux et al. [48] | FLEHS Cohort/Flanders (Belgium) | 7 to 9 yo/ Both/N = 114/Both | Env/DDE, HCB/ Cord blood (delivery) samples | WC/abdominal obesity and zBMI | Positive associations were reported among DDE prenatal exposure and waist circumference/abdominal obesity, and waist/height ratio in only girls. No association of HCB with BMI in childhood | 7 VH |
| Burns et al. [50] | Russian Childrens’s study Cohort/ Chapaevsk (Russia) | Annually from 8–9 to 12–13 yo/ Males/N = 350/ Urban | Env/HCB, βHCH, p,p′-DDE/ Postnatal (8–9 yo) blood samples | zBMI | Boys with higher serum HCB, βHCH and p,p′-DDE had significantly lower mean zBMI | 7-VH |
| Mendez et al. [51] | INMA Cohort/Sabadell, (Spain) | Birth, 14 mo/N = 518/Urban | Env/DDE, HCB, βHCH, PCBs./Maternal blood (1st trimester) | zBMI | DDE exposure above the first quartile was associated with a doubling of the risk of rapid growth among children of normal-weight, but not overweight, mothers. DDE was associated with elevated BMI at 14 mo. | 7 VH |
| Burns et al. [59] | Russian Childrens’s study Cohort/ Chapaevsk (Russia) | Annually from 8–9 to 12–13 yo Males/N = 318/ Urban | Env/HCB, βHCH and p,p′-DDE/ Postnatal (biennially from 8–9 to 12–13 yo) blood samples | zBMI, Leptin and Homeostatic model assessment insuline resistence (HOMA-IR) | DDE postnatal exposure shows a significant relationship with other indicators related to obesity such as leptin serum. Higher prepubertal HCB concentrations were associated with greater ratios of insulin resistance, higher serum insulin, and homeostatic model assessment insulin resistance (HOMA-IR) levels. Postnatal exposure to β-HCH did not have an effect on obesity related parameters | 6-H |
| Parastar et al. [60] | Cross sectional study/Isfahan (Iran) | Between 6 and 18 yo/Both/N = 242/Urban | Env/CPs Postnatal urine samples | zBMI, WC, TC, LDL-C and HDL-C | Positive association between postnatal exposure to 2,5-DCP and zBMI, WC and obesity. Negative association with TC and HDL-C were detected at ages 6–18. 2,4-DCP showed an association with HDL-C. | 7 VH |
| Cabrera-Rodriguez et al. [61] | Cross sectional study/Canary Island (Spain) | Birth Both/N = 447/ Rural | Env/20 OCPs/ Cord blood (delivery) samples | Birth weight | Prenatal p,p′-DDE/p,p′-DDD and p,p′-DDT levels were found to be significantly associated with an increase in neonatal birth weight in girls. HCB was found to be significantly associated with an increase in neonatal birth weight, with a special emphasis on girls. Positive association between the proportion of newborns with small gestational age that have been exposed to ≥ 3 different OCPs among boys | 6-H |
| Study, Year (Reference) | Strain/Age at Evaluation/Sex | Exposure Agent/Dosage/Route/Duration of Exposure | Rimary Outcome: Body Weight Mesasures | Behavioral/Biochemical/ Physiological Outcomes | Quality Index |
|---|---|---|---|---|---|
| La Merrill et al. [65] | Mice (C57BL/6J)/PND5, PND21- 6 postnatal months (BW)/Both | Dichlorodiphenyltrichloroethane 1.7 mg/kg/d Gavage GD11.5-PND5 | ↓ decreased body weight in males (PND5) | ↓ body core temperature, ↑ energy expenditure in females = body core temperature in males | 15 H |
| Yan et al. [69] | CD-1 mice/PND1–15th postnatal week (BW), 15th postnatal week (BM, SS,OT)/M | Endosulfan sulfate 0.03 mg/kg Gavage GDO-PND21 | = body weight (PND1–42) | 14 H | |
| André et al. [71] | Rats (Long Evans)/PND1–20 (BW), PND60–80 (BT)/Both | Vinclozolin 0.1, 3, 6 or 12 mg/kg/d Gavage GD14-PND3 Vz was not administered on PND0 | = pup body weight | Disrupts extinction but not acquisition of a conditioned response in male rats. Male rats were more affected than female rats | 14 H |
| Lassiter et al. [63] | Rats (Sprague Dawley)/PND1–4, PND21–154 (BW,SS)/Both | Parathion 0.1 or 0.2 mg/kg/d s.c. PND1–4 | = body weight during PND1–4 ↑ body weight in low dose male group ↓ body weight in low female groups | 12 MH | |
| Ndonwi et al. [66] | Rats (Wistar)/PND0–71 (BW, SS)/Both | Imidacloprid 44 mg/kg/d, chlorpyrifos 13.5 mg/kg/d, imidacloprid + lambda cyhalothrin 5.6 + 5.6 mg/kg/d oxamyl 0.4 mg/kg/d Gavage GD0–21 | = body weight | ↑ aspartate transaminase and alanine transaminase (liver function enzymes), ↑ liver and kidney antioxidants and MDA levels in all the groups Changes in oxidative stress and lipid peroxidation in all the groups | 12 MH |
| Wang et al. [70] | Rats (Sprague Dawley)/pregnancy, lactation, offspring (BW)/1.5–3 postnatal months (BM, BT, SS, OT)/M | Atrazine 100 mg/kg/d Gavage Twice a week GD5—PND21 | = body weight (pregnancy, lactation, offspring) | Impaired spatial learning and memory in MWM Histomorphology alterations of hippocampal CA1 area ↓ gene levels of Wnt5a, JNK, PSD95, NR2B, PI3K, and c-fos mRNA in the hippocampus ↓ protein expression levels of Wnt5a, JNK, p-JNK, PSD95, NR2B, PI3 K, and c-fos in the hippocampus 28 days of exercise swimming trainning ameliorated the adverse effects of ATR | 12 MH |
| Lassiter et al. [64] | Rats (Sprague Dawley)/PND21–154 (BW), 22nd postnatal week (SS), 24th postnatal week (BM)/Both | Parathion 0.1 or 0.2 mg/kg/d s.c. PND1–4 | = body weight during PND1–4 ↑ body weight in low dose male group | 10 MH | |
| Lassiter and Brimijoin [67] | Rats (Long-evans)/PND 21–95 (BW, SS/Both | Chlorpyrifos 1, 2.5, or 4 mg/kg/d Gavage GD7-PND21 | ↑ weight gain in males beginning at PND51 ↑ body volume in males ↓ specific gravity in males | No effect on brain weight or RNA levels in pups = Serum leptin levels | 7 ML |
| Chen et al. [68] | Rats (Sprague Dawley)/PND37–38 (FST), PND43 (OF), PND46 (NSFT), PND48–52 (LH)/M | Chlorpyrifos 2.5, 5, 10 or 20 mg/kg/d s.c. PND 27–36 | = body weight | - FST: ↑ immobility time in the 10 mg/kg dose group - NSFT: ↑ feediLg latency at lower doses (2.5 and 5 mg/kg), ↓ latency at higher doses (10 and 20 mg/kg) - OF: No significant effects on locomotor activity and exploratory behavior measured, respectively by no. of crossings and rearings - LH: ↑ number of escape failures in the 20 mg/kg group | 7ML |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinos, H.; Carrillo, B.; Merchán, A.; Biosca-Brull, J.; Pérez-Fernández, C.; Colomina, M.T.; Sánchez-Santed, F.; Martín-Sánchez, F.; Collado, P.; Arias, J.L.; et al. Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7170. https://doi.org/10.3390/ijerph18137170
Pinos H, Carrillo B, Merchán A, Biosca-Brull J, Pérez-Fernández C, Colomina MT, Sánchez-Santed F, Martín-Sánchez F, Collado P, Arias JL, et al. Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(13):7170. https://doi.org/10.3390/ijerph18137170
Chicago/Turabian StylePinos, Helena, Beatriz Carrillo, Ana Merchán, Judit Biosca-Brull, Cristian Pérez-Fernández, María Teresa Colomina, Fernando Sánchez-Santed, Fernando Martín-Sánchez, Paloma Collado, Jorge L. Arias, and et al. 2021. "Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 13: 7170. https://doi.org/10.3390/ijerph18137170
APA StylePinos, H., Carrillo, B., Merchán, A., Biosca-Brull, J., Pérez-Fernández, C., Colomina, M. T., Sánchez-Santed, F., Martín-Sánchez, F., Collado, P., Arias, J. L., & Conejo, N. M. (2021). Relationship between Prenatal or Postnatal Exposure to Pesticides and Obesity: A Systematic Review. International Journal of Environmental Research and Public Health, 18(13), 7170. https://doi.org/10.3390/ijerph18137170











