Relationship between Autism Spectrum Disorder and Pesticides: A Systematic Review of Human and Preclinical Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Study Selection and Data Collection Process
2.5. Risk of Bias in Individual Studies
3. Results
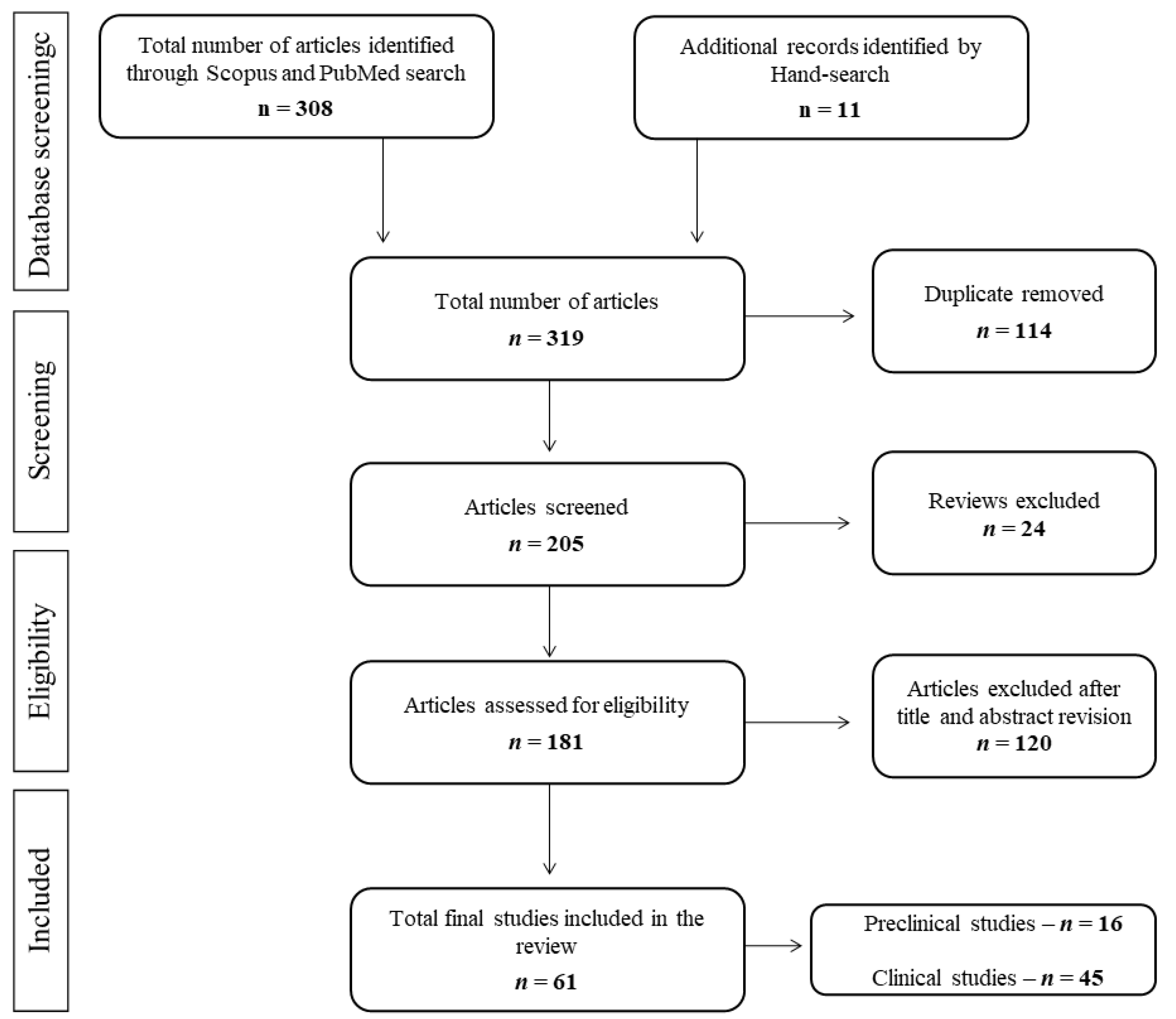
3.1. Selection of Studies
3.2. Search Results and General Quality
3.3. Pesticide Exposure and ASD-Like Outcomes: Preclinical Studies
3.3.1. Organophosphates Compounds
3.3.2. Other Potential Neurotoxic Compounds
3.4. Pesticide Exposure, Cognitive and Behavioral Alteration Related to ASD: Clinical Studies
3.4.1. Organophosphate Compounds
3.4.2. Organochloride Compounds
3.4.3. Pyrethroid Compounds
3.4.4. Mixtures of Pesticides and Other Potential Neurotoxic Agents
4. Discussion
4.1. Preclinical Studies and ASD
4.2. Clinical Studies and ASD
4.3. Relationship between Preclinical and Clinical Studies Concerning ASD
5. Conclusions
- -
- The relation between exposure to different pesticides and the ASD-like phenotype concerning the core symptomatology of autism is relatively under-explored in preclinical research. Even in the case of those compounds for which there is a significant amount of empirical research regarding sociability and/or communicative outcomes (e.g., CPF), the considerable differences between studies regarding exposure protocols (e.g., gestational vs. postnatal or early vs. medium vs. late gestational) make it impossible, in the end, for us to draw any solid conclusions.
- -
- There is a significant gap in the literature as only one study included in the review used rats. Although the relevance of the use of mice is unquestionable, it is known that rat models are closer to humans in terms of genetic background and behavioral regulation, particularly with regard to social behaviors [96].
- -
- Future preclinical research should focus on a more in-depth analysis of exposure to developmental CPF and other pesticides concerning the core (sociability and USVs) and secondary (e.g., neuromotor development) clinical signs of ASD, with a special emphasis on the gestational period around GD12, whilst it will also be necessary to include rat models along with the work carried out with mice.
- -
- The study on wild-type mice should be complemented with the systematic analyses of the interactions of this exposure with the various genetic backgrounds of vulnerability associated with the ASD-like phenotype.
- -
- It is difficult to draw solid conclusions as there are a wide variety of studies that differ in many aspects such as route, age, or source of exposure.
- -
- The study of exposure to a single pesticide in humans lacks ecological validity, due to the fact that humans are constantly exposed to a wide range of pesticides through a range of routes such as diet, house fumigation, or agriculture. This wide variability of compounds and environmental exposure could contribute to the heterogeneity of results found in the literature.
- -
- Pesticide exposure appears to co-exist with other factors that may be harmful or beneficial for the development of the nervous system. Examples of other factors that could explain the association between pesticides and ASD are lifestyle, socioeconomic or educational status as well as ethnicity or gender. Moreover, maternal age is an important factor to consider, as the concentration of pesticides in the body increases with age, and so higher maternal ages are more strongly associated with an increased risk of autism in their offspring [107].
- -
- Pesticide exposure did not always show harmful effects when authors considered different covariates, suggesting the existence of certain genetic polymorphisms which could interact with environmental factors and amplify the adverse effects of pesticides in relation to ASD (gene-environment interaction).
- -
- Further clinical research is needed to homogenize exposure in human studies, particularly in terms of exposure to specific pesticides, consideration of other risk factors, as well as the use of a more well-defined follow-up period and validated tools for measuring behavioral outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Barrio, V. Diagnostic and statistical manual of mental disorders. In The Curated Reference Collection in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128093245. [Google Scholar]
- Whyatt, C.; Craig, C. Sensory-motor problems in Autism. Front. Integr. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Keehn, B.; Westerfield, M.; Müller, R.-A.; Townsend, J. Autism, Attention, and Alpha Oscillations: An Electrophysiological Study of Attentional Capture. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Harris, L.; Pollick, F.; Melville, C. A meta-analysis of working memory in individuals with autism spectrum disorders. PLoS ONE 2019, 14, e0216198. [Google Scholar] [CrossRef]
- Christ, S.E.; Holt, D.D.; White, D.A.; Green, L. Inhibitory Control in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2007, 37, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder Analysis method B. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, M.T.; Golshani, P. The utility of rodent models of autism spectrum disorders. Curr. Opin. Neurol. 2015, 28, 103–109. [Google Scholar] [CrossRef][Green Version]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Børglum, A.D. Identification of common genetic risk variants for autism spectrum disorder HHS Public Access Author manuscript. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef]
- Sinzig, J. Autism Spectrum. Monatsschrift Kinderheilkd 2015, 163, 673–680. [Google Scholar] [CrossRef]
- Hansen, S.N.; Schendel, D.E.; Parner, E.T. Explaining the Increase in the Prevalence of Autism Spectrum Disorders. JAMA Pediatr. 2015, 169, 56. [Google Scholar] [CrossRef]
- Karimi, P.; Kamali, E.; Mousavi, S.; Karahmadi, M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal Valproate Exposure and Risk of Autism Spectrum Disorders and Childhood Autism. JAMA 2013, 309, 1696. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.E.; Bolden, A.L.; Kwiatkowski, C.F. Environmental Chemicals and Autism: A Scoping Review of the Human and Animal Research. Environ. Health Perspect. 2019, 127, 046001. [Google Scholar] [CrossRef]
- Roberts, J.R.; Dawley, E.H.; Reigart, J.R. Children’s low-level pesticide exposure and associations with autism and ADHD: A review. Pediatr. Res. 2019, 85, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- DeBord, D.G.; Carreón, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef]
- Coats, J.R. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ. Health Perspect. 1990, 87, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Suppiramaniam, V.; Abdel-Rahman, E.A.; Buabeid, M.A.; Parameshwaran, K. Ion Channels. In Comprehensive Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 11, pp. 129–171. [Google Scholar]
- Gupta, R. Toxicology of Organophosphate & Carbamate Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2006; ISBN 9780120885237. [Google Scholar]
- Casida, J.E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the Toxicology of Chlorpyrifos With an Emphasis on Human Exposure and Neurodevelopment. Crit. Rev. Toxicol. 2008, 38, 1–125. [Google Scholar] [CrossRef] [PubMed]
- Becerra, T.A.; Von Ehrenstein, O.S.; Heck, J.E.; Olsen, J.; Arah, O.A.; Jeste, S.S.; Rodriguez, M.; Ritz, B. Autism spectrum disorders and race, ethnicity, and nativity: A population-based study. Pediatrics 2014, 134, 63–71. [Google Scholar] [CrossRef]
- Silva, M.H. Effects of low-dose chlorpyrifos on neurobehavior and potential mechanisms: A review of studies in rodents, zebrafish, and Caenorhabditis elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev. Esp. Nutr. Humana Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. NOS for assessing quality of nonrandomised studies. The Ottawa Hospital: Research Institude. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 June 2020).
- Venerosi, A.; Cutuli, D.; Colonnello, V.; Cardona, D.; Ricceri, L.; Calamandrei, G. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol. Teratol. 2008, 30, 468–474. [Google Scholar] [CrossRef]
- Venerosi, A.; Ricceri, L.; Scattoni, M.L.; Calamandrei, G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in cd-1 mouse pups. Environ. Health A Glob. Access Sci. Source 2009, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Ricceri, L.; Rungi, A.; Sanghez, V.; Calamandrei, G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology 2010, 208, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Tait, S.; Stecca, L.; Chiarotti, F.; De Felice, A.; Cometa, M.F.; Volpe, M.T.; Calamandrei, G.; Ricceri, L. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring—A mouse study. Environ. Health 2015, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- De Felice, A.; Venerosi, A.; Ricceri, L.; Sabbioni, M.; Scattoni, M.L.; Chiarotti, F.; Calamandrei, G. Sex-dimorphic effects of gestational exposure to the organophosphate insecticide chlorpyrifos on social investigation in mice. Neurotoxicol. Teratol. 2014, 46, 32–39. [Google Scholar] [CrossRef]
- De Felice, A.; Scattoni, M.L.; Ricceri, L.; Calamandrei, G. Prenatal Exposure to a Common Organophosphate Insecticide Delays Motor Development in a Mouse Model of Idiopathic Autism. PLoS ONE 2015, 10, e0121663. [Google Scholar] [CrossRef] [PubMed]
- De Felice, A.; Greco, A.; Calamandrei, G.; Minghetti, L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J. Neuroinflamm. 2016, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Kalimian, M.; Amram, B.; Kofman, O. Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environ. Health A Glob. Access Sci. Source 2017, 16. [Google Scholar] [CrossRef]
- Lan, A.; Stein, D.; Portillo, M.; Toiber, D.; Kofman, O. Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav. Brain Funct. 2019, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mullen, B.R.; Khialeeva, E.; Hoffman, D.B.; Ghiani, C.A.; Carpenter, E.M. Decreased Reelin Expression and Organophosphate Pesticide Exposure Alters Mouse Behaviour and Brain Morphology. ASN Neuro 2013, 5, e00106. [Google Scholar] [CrossRef] [PubMed]
- Basaure, P.; Guardia-Escote, L.; Biosca-Brull, J.; Blanco, J.; Cabré, M.; Peris-Sampedro, F.; Sánchez-Santed, F.; Domingo, J.L.; Colomina, M.T. Exposure to chlorpyrifos at different ages triggers APOE genotype-specific responses in social behavior, body weight and hypothalamic gene expression. Environ. Res. 2019, 178, 108684. [Google Scholar] [CrossRef] [PubMed]
- Ouardi, F.Z.; Anarghou, H.; Malqui, H.; Ouasmi, N.; Chigr, M.; Najimi, M.; Chigr, F. Gestational and Lactational Exposure to Malathion Affects Antioxidant Status and Neurobehavior in Mice Pups and Offspring. J. Mol. Neurosci. 2019, 69, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, R. Neurotoxic impact of organophosphate pesticide phosphomedon on the albino rat. J. Environ. Biol. 2014, 35, 427–430. [Google Scholar]
- Laugeray, A.; Herzine, A.; Perche, O.; Hébert, B.; Aguillon-Naury, M.; Richard, O.; Menuet, A.; Mazaud-Guittot, S.; Lesné, L.; Briault, S.; et al. Pre- and Postnatal Exposure to Low Dose Glufosinate Ammonium Induces Autism-Like Phenotypes in Mice. Front. Behav. Neurosci. 2014, 8, 390. [Google Scholar] [CrossRef]
- Dong, T.; Guan, Q.; Hu, W.; Zhang, M.; Zhang, Y.; Chen, M.; Wang, X.; Xia, Y. Prenatal exposure to glufosinate ammonium disturbs gut microbiome and induces behavioral abnormalities in mice. J. Hazard. Mater. 2020, 389. [Google Scholar] [CrossRef]
- Laugeray, A.; Herzine, A.; Perche, O.; Richard, O.; Montecot-Dubourg, C.; Menuet, A.; Mazaud-Guittot, S.; Lesné, L.; Jegou, B.; Mortaud, S. In utero and lactational exposure to low-doses of the pyrethroid insecticide cypermethrin leads to neurodevelopmental defects in male mice—An ethological and transcriptomic study. PLoS ONE 2017, 12, e0184475. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Wu, C.; Lv, S.; Lu, D.; Qi, X.; Jiang, S.; Feng, C.; Yu, H.; Liang, W.; et al. Associations of prenatal and childhood chlorpyrifos exposure with Neurodevelopment of 3-year-old children. Environ. Pollut. 2019, 251, 538–546. [Google Scholar] [CrossRef]
- Philippat, C.; Barkoski, J.; Tancredi, D.J.; Elms, B.; Barr, D.B.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int. J. Hyg. Environ. Health 2018, 221, 548–555. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ji, L.; Hu, Y.; Zhang, J.; Wang, C.; Ding, G.; Chen, L.; Kamijima, M.; Ueyama, J.; et al. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ. Int. 2017, 108, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, S.; Liang, D.; Shi, X.; Wang, F.; Liu, W.; Zhang, L.; Chen, L.; Gu, Y.; Tian, Y. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: A birth cohort study in Shenyang, China. PLoS ONE 2014, 9, e88491. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Donauer, S.; Altaye, M.; Xu, Y.; Sucharew, H.; Succop, P.; Calafat, A.M.; Khoury, J.C.; Lanphear, B.; Yolton, K. An Observational Study to Evaluate Associations between Low-Level Gestational Exposure to Organophosphate Pesticides and Cognition during Early Childhood. Am. J. Epidemiol. 2016, 184, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.M.; Wetmur, J.; Chen, J.; Zhu, C.; Barr, D.B.; Canfield, R.L.; Wolff, M.S. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ. Health Perspect. 2011, 119, 1182–1188. [Google Scholar] [CrossRef]
- Eskenazi, B.; Huen, K.; Marks, A.; Harley, K.G.; Bradman, A.; Barr, D.B.; Holland, N. PON1 and Neurodevelopment in Children from the CHAMACOS Study Exposed to Organophosphate Pesticides in Utero. Environ. Health Perspect. 2010, 118, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.A.; Engel, S.M.; Barr, D.B.; Wolff, M.S. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ. Int. 2014, 70, 125–131. [Google Scholar] [CrossRef] [PubMed]
- González-Alzaga, B.; Hernández, A.F.; Rodríguez-Barranco, M.; Gómez, I.; Aguilar-Garduño, C.; López-Flores, I.; Parrón, T.; Lacasaña, M. Pre- and postnatal exposures to pesticides and neurodevelopmental effects in children living in agricultural communities from South-Eastern Spain. Environ. Int. 2015, 85, 229–237. [Google Scholar] [CrossRef]
- Kongtip, P.; Techasaensiri, B.; Nankongnab, N.; Adams, J.; Phamonphon, A.; Surach, A.; Sangprasert, S.; Thongsuksai, A.; Srikumpol, P.; Woskie, S. The impact of prenatal organophosphate pesticide exposures on thai infant neurodevelopment. Int. J. Environ. Res. Public Health 2017, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, C.; Chang, X.; Qi, X.; Zheng, M.; Zhou, Z. Adverse associations of both prenatal and postnatal exposure to organophosphorous pesticides with infant neurodevelopment in an agricultural area of Jiangsu Province, China. Environ. Health Perspect. 2016, 124, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Millenson, M.E.; Braun, J.M.; Calafat, A.M.; Barr, D.B.; Huang, Y.-T.; Chen, A.; Lanphear, B.P.; Yolton, K. Urinary organophosphate insecticide metabolite concentrations during pregnancy and children’s interpersonal, communication, repetitive, and stereotypic behaviors at 8 years of age: The home study. Environ. Res. 2017, 157, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Harris, M.H.; Gunier, R.B.; Kogut, K.R.; Harley, K.G.; Deardorff, J.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal Organophosphate Pesticide Exposure and Traits Related to Autism Spectrum Disorders in a Population Living in Proximity to Agriculture. Environ. Health Perspect. 2018, 126, 047012. [Google Scholar] [CrossRef]
- Silver, M.K.; Shao, J.; Zhu, B.; Chen, M.; Xia, Y.; Kaciroti, N.; Lozoff, B.; Meeker, J.D. Prenatal naled and chlorpyrifos exposure is associated with deficits in infant motor function in a cohort of Chinese infants. Environ. Int. 2017, 106, 248–256. [Google Scholar] [CrossRef] [PubMed]
- van den Dries, M.A.; Guxens, M.; Pronk, A.; Spaan, S.; El Marroun, H.; Jusko, T.A.; Longnecker, M.P.; Ferguson, K.K.; Tiemeier, H. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring attention-deficit hyperactivity disorder and autistic traits. Environ. Int. 2019, 131, 105002. [Google Scholar] [CrossRef]
- Woskie, S.; Kongtip, P.; Thanasanpaiboon, W.; Kiatdamrong, N.; Charoonrungsirikul, N.; Nankongnab, N.; Surach, A.; Phamonphon, A. A pilot study of maternal exposure to organophosphate pesticides and newborn neurodevelopment in Thailand. Int. J. Occup. Environ. Health 2017, 23, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Medina, L.A.; Torres-Sánchez, L.; Schnaas, L.; Cebrián, M.E.; Chávez, C.H.; Osorio-Valencia, E.; Hernández, R.M.G.; López-Carrillo, L. Neonatal neurodevelopment and prenatal exposure to dichlorodiphenyldichloroethylene (DDE): A cohort study in Mexico. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 609–614. [Google Scholar] [CrossRef]
- Brown, A.S.; Cheslack-Postava, K.; Rantakokko, P.; Kiviranta, H.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Surcel, H.-M.; Sourander, A. Association of Maternal Insecticide Levels With Autism in Offspring From a National Birth Cohort. Am. J. Psychiatry 2018, 175, 1094–1101. [Google Scholar] [CrossRef]
- Kim, S.; Eom, S.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D.; et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age- CHECK cohort study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalkbrenner, A.E.; Just, A.C.; Yolton, K.; Calafat, A.M.; Sjödin, A.; Hauser, R.; Webster, G.M.; Chen, A.; Lanphear, B.P. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: The HOME study. Environ. Health Perspect. 2014, 122, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jeddy, Z.; Kordas, K.; Allen, K.; Taylor, E.V.; Northstone, K.; Dana Flanders, W.; Namulanda, G.; Sjodin, A.; Hartman, T.J. Prenatal exposure to organochlorine pesticides and early childhood communication development in British girls. Neurotoxicology 2018, 69, 121–129. [Google Scholar] [CrossRef]
- Kao, C.-C.; Que, D.E.; Bongo, S.J.; Tayo, L.L.; Lin, Y.-H.; Lin, C.-W.; Lin, S.-L.; Gou, Y.-Y.; Hsu, W.-L.; Shy, C.-G.; et al. Residue Levels of Organochlorine Pesticides in Breast Milk and Its Associations with Cord Blood Thyroid Hormones and the Offspring’s Neurodevelopment. Int. J. Environ. Res. Public Health 2019, 16, 1438. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.A.; Sjödin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Perspect. 2017, 125, 474–480. [Google Scholar] [CrossRef]
- Hamra, G.B.; Lyall, K.; Windham, G.C.; Calafat, A.M.; Sjödin, A.; Volk, H.; Croen, L.A. Prenatal Exposure to Endocrine-disrupting Chemicals in Relation to Autism Spectrum Disorder and Intellectual Disability. Epidemiology 2019, 30, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, L.; Schnaas, L.; Rothenberg, S.J.; Cebrián, M.E.; Osorio-Valencia, E.; Hernández, M.d.C.; García-Hernández, R.M.; López-Carrillo, L. Prenatal p,p’-DDE Exposure and Neurodevelopment among Children 3.5–5 Years of Age. Environ. Health Perspect. 2013, 121, 263–268. [Google Scholar] [CrossRef]
- Puertas, R.; Lopez-Espinosa, M.J.; Cruz, F.; Ramos, R.; Freire, C.; Pérez-García, M.; Abril, A.; Julvez, J.; Salvatierra, M.; Campoy, C.; et al. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. Neurotoxicology 2010, 31, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Simard, M.-N.; Muckle, G.; Rouget, F.; Kadhel, P.; Bataille, H.; Chajès, V.; Dallaire, R.; Monfort, C.; Thomé, J.-P.; et al. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology 2013, 35, 162–168. [Google Scholar] [CrossRef]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef]
- Viel, J.-F.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Limon, G.; Rouget, F.; Monfort, C.; Durand, G.; Cordier, S.; Chevrier, C. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother–child cohort. Environ. Int. 2015, 82, 69–75. [Google Scholar] [CrossRef]
- Viel, J.-F.; Rouget, F.; Warembourg, C.; Monfort, C.; Limon, G.; Cordier, S.; Chevrier, C. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: The PELAGIE mother–child cohort. Occup. Environ. Med. 2017, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.K.; Rundle, A.; Camann, D.E.; Boyd Barr, D.; Rauh, V.A.; Whyatt, R.M. Impact of Prenatal Exposure to Piperonyl Butoxide and Permethrin on 36-Month Neurodevelopment. Pediatrics 2011, 127, e699–e706. [Google Scholar] [CrossRef]
- Watkins, D.J.; Fortenberry, G.Z.; Sánchez, B.N.; Barr, D.B.; Panuwet, P.; Schnaas, L.; Osorio-Valencia, E.; Solano-González, M.; Ettinger, A.S.; Hernández-Ávila, M.; et al. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: Distribution and relationships with child neurodevelopment. Environ. Res. 2016, 147, 307–313. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Wu, C.; Qi, X.; Jiang, S.; Lu, D.; Feng, C.; Liang, W.; Chang, X.; Zhang, Y.; et al. Exposure to carbamate and neurodevelopment in children: Evidence from the SMBCS cohort in China. Environ. Res. 2019, 177, 108590. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.M.; Córdoba, L.; Cano, J.C.; Hernandez-Bonilla, D.; Pardo, L.; Schnaas, L.; Smith, D.R.; Menezes-Filho, J.A.; Mergler, D.; Lindh, C.H.; et al. Prenatal Mancozeb Exposure, Excess Manganese, and Neurodevelopment at 1 Year of Age in the Infants’ Environmental Health (ISA) Study. Environ. Health Perspect. 2018, 126, 057007. [Google Scholar] [CrossRef]
- Keil, A.P.; Daniels, J.L.; Hertz-Picciotto, I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: The CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Environ. Heal. A Glob. Access Sci. Source 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Debes, F.; Wohlfahrt-Veje, C.; Murata, K.; Grandjean, P. Occupational pesticide exposure in early pregnancy associated with sex-specific neurobehavioral deficits in the children at school age. Neurotoxicol. Teratol. 2015, 47, 1–9. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ 2019, 364, l962. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; An, S.; Rauch, S.A.; Coker, E.S.; Maphula, A.; Obida, M.; Crause, M.; Kogut, K.R.; Bornman, R.; Chevrier, J. Prenatal Exposure to DDT and Pyrethroids for Malaria Control and Child Neurodevelopment: The VHEMBE Cohort, South Africa. Environ. Health Perspect. 2018, 126, 047004. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.A.; Herring, A.; Buckley, J.P.; Goldman, B.D.; Daniels, J.L.; Engel, L.S.; Wolff, M.S.; Chen, J.; Wetmur, J.; Barr, D.B.; et al. Prenatal exposure to organophosphorus pesticides and childhood neurodevelopmental phenotypes. Environ. Res. 2017, 158, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Gunier, R.B.; Bradman, A.; Castorina, R.; Holland, N.T.; Avery, D.; Harley, K.G.; Eskenazi, B. Residential proximity to agricultural fumigant use and IQ, attention and hyperactivity in 7-year old children. Environ. Res. 2017, 158, 358–365. [Google Scholar] [CrossRef] [PubMed]
- McCanlies, E.C.; Ma, C.C.; Gu, J.K.; Fekedulegn, D.; Sanderson, W.T.; Ludeña-Rodriguez, Y.J.; Hertz-Picciotto, I. The CHARGE study: An assessment of parental occupational exposures and autism spectrum disorder. Occup. Environ. Med. 2019, 76, 644–651. [Google Scholar] [CrossRef]
- Ostrea Jr, E.M.; Reyes, A.; Villanueva-Uy, E.; Pacifico, R.; Benitez, B.; Ramos, E.; Bernardo, R.C.; Bielawski, D.M.; Delaney-Black, V.; Chiodo, L.; et al. Fetal exposure to propoxur and abnormal child neurodevelopment at 2 years of age. Neurotoxicology 2012, 33, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Kogan, V.; Shelton, J.F.; Delwiche, L.; Hansen, R.L.; Ozonoff, S.; Ma, C.C.; McCanlies, E.C.; Bennett, D.H.; Hertz-Picciotto, I.; et al. Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ. Health Perspect. 2017, 125, 097007. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Gunier, R.B.; Bradman, A.; Harley, K.G.; Kogut, K.; Eskenazi, B. Prenatal Residential Proximity to Agricultural Pesticide Use and IQ in 7-Year-Old Children. Environ. Health Perspect. 2017, 125, 057002. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, Y.; Wen, X.; Smith, S.S.; Nizomov, J.; Fishe, J.; Hogan, W.R.; Shenkman, E.A.; Bian, J. An external exposome-wide association study of COVID-19 mortality in the United States. Sci. Total Environ. 2021, 768, 144832. [Google Scholar] [CrossRef] [PubMed]
- Berger-Sweeney, J. The cholinergic basal forebrain system during development and its influence on cognitive processes: Important questions and potential answers. Neurosci. Biobehav. Rev. 2003, 27, 401–411. [Google Scholar] [CrossRef]
- Williams, T.; Borchelt, D.R.; Chakrabarty, P. Therapeutic approaches targeting Apolipoprotein E function in Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 8. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Thabtah, F.; Peebles, D. Early autism screening: A comprehensive review. Int. J. Environ. Res. Public Health 2019, 16, 3502. [Google Scholar] [CrossRef]
- Engel, S.M.; Bradman, A.; Wolff, M.S.; Rauh, V.A.; Harley, K.G.; Yang, J.H.; Hoepner, L.A.; Barr, D.B.; Yolton, K.; Vedar, M.G.; et al. Prenatal Organophosphorus Pesticide Exposure and Child Neurodevelopment at 24 Months: An Analysis of Four Birth Cohorts. Environ. Health Perspect. 2016, 124, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Alcalá, L.; Mehta, S.; Eskenazi, B. Pyrethroid Pesticide Exposure and Parental Report of Learning Disability and Attention Deficit/Hyperactivity Disorder in U.S. Children: NHANES 1999–2002. Environ. Health Perspect. 2014, 122, 1336–1342. [Google Scholar] [CrossRef]
- Oulhote, Y.; Bouchard, M.F. Urinary Metabolites of Organophosphate and Pyrethroid Pesticides and Behavioral Problems in Canadian Children. Environ. Health Perspect. 2013, 121, 1378–1384. [Google Scholar] [CrossRef]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Roza, S.J.; van Batenburg-Eddes, T.; Steegers, E.A.P.; Jaddoe, V.W.V.; Mackenbach, J.P.; Hofman, A.; Verhulst, F.C.; Tiemeier, H. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br. J. Nutr. 2010, 103, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tancredi, D.J.; Tassone, F.; Hertz-Picciotto, I. Prenatal Vitamins, One-carbon Metabolism Gene Variants, and Risk for Autism. Epidemiology 2011, 22, 476–485. [Google Scholar] [CrossRef]
- Lu, C.; Bravo, R.; Caltabiano, L.M.; Irish, R.M.; Weerasekera, G.; Barr, D.B. The Presence of Dialkylphosphates in Fresh Fruit Juices: Implication For Organophosphorus Pesticide Exposure and Risk Assessments. J. Toxicol. Environ. Health Part A 2005, 68, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, R.C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 2008, 20, 775–803. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Tancredi, D.J.; Hertz-Picciotto, I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010, 3, 30–39. [Google Scholar] [CrossRef] [PubMed]
| Study, Year (Reference) | Strain/Age at Evaluation/Sex | Exposure Agent/Dose/Age/Route | Exposure Control | Behavioral Tests | Behavioral/Pharmacological/Physiological Outcomes | Quality Index |
|---|---|---|---|---|---|---|
| Organophosphate compounds | ||||||
| Venerosi et al. [30] | Mice/PND > 40. Mums postpartum/Both | CPF. 3 mg/kg/d PND11-15 s.c. | Neuromotor battery | Three-chambers test. Maternal behavior. Nest building. Dark-light test | = sociability and reaction to social novelty. ↓ maternal care. ↓ anxiety in mums. ↓ maternal aggressive behavior. ↑ maternal social investigation. ↓ motricity | H |
| Lan et al. [37] | Mice/PND5 (maternal care) and PND90 (sociability)/M | CPF. 2.5–5 mg/kg/day GD12-15 Gavage | Weight, reflexes | Maternal behavior. Three-chambers test. Social conditioned place preference. NOR | ↓ Sociability. = maternal care. ↓ preference social conditioned place. = NOR | H |
| Lan et al. [38] | Mice/PND90/Both | CPF. 2.5–5 mg/kg/day GD12-15 Gavage | N.A. | Three-chambers test. Social conditioned place preference. | ↓ Social preference males vs. the rest (5mg/kg). ↓ Social preference females vs. males (2.5 mg/kg). = Oxytocin mRNA levels at hypothalamus | H |
| Venerosi et al. [31] | Mice/PND4-15/Both | CPF. 6 mg/kg/day GD15-18 Gavage | AChE activity, weight, neurobehavioral battery, reflexes | Maternal behavior. USVs. Spontaneous motricity | Altered maternal behavior in CPF exposed mums (increased wall rearing and decreased digging). ↓ USVs (calls/min and duration) only in PND10. ↓ pivoting. ↑ immobility. | MH |
| Venerosi et al. [32] | Mice/>PND90. Mums postpartum/Both | CPF. 6 mg/kg/day GD15-18 Gavage | N.A. | Maternal aggression. Light-dark test | ↓ Maternal aggressive behavior. ↑ Anxiety (Females). = Depressive-like behaviors. Serotonergic hyposensitivity (challenged with fluvoxamine) | MH |
| De Felice et al. [34] | Mice/>PND70/Both | CPF. 6 mg/kg/day GD14-17 Gavage | Pups, sex ratio, weight | Social Discrimination test | ↑ Social investigation (Females). = Reaction to social novelty | MH |
| Venerosi et al. [33] | Mice/>PND70/Both | CPF. 6 mg/kg/day GD15-PND14 Diet | AChE activity, weight, litter size, sex ratio | Social Recognition test. Open field | ↑ Social investigation males (all phases) and females (second exposure to the same partner). ↓ reaction to social novelty (females). ↑ Estrogen Receptor β at Hypothalamus (Males). ↓ Oxytocin at Amygdala (males). ↑ Vasopressin receptor 1a at amygdala. = locomotor activity | MH |
| De Felice et al. [35] | BTBR Mice/PND4, 6, 8, 8 (USVs). >70 (Sociability and USVs)/Both | CPF. 6 mg/kg/day GD14-17 Gavage | Weight, litter size, sex ratio, mortality, reflexes | USVs. Social Interaction test | ↑ (trend) calls. ↑ USVs and social investigation (sniffing) (males to females). Altered developmental neuromotor functioning in exposed mice | MH |
| De Felice et al. [36] | Mice BTBR/PND1-21/Both | CPF. 6 mg/kg/day GD14-17 Gavage | Weight, litter size | Enzyme immunoassay | ↑ 15-F2t-IsoP in BTBR model (vs. wild-type). CPF both reduced (wild-type) and increased (BTBR) 15-F2t-IsoP brain levels in PND1. CPF 15-F2t-IsoP in BTBR animals (males) at PND21. CPF increased PGE2 brain levels in BTBR animals at PND21 (males) and PND70. | MH |
| Basaure et al. [40] | APOE3 and 4 Mice/PNM5/M | CPF. 1 mg/kg/day PND10-15 Oral 2mg/kg/d PNM5 Diet | Weight | Three chambers test | ↑ Sociability in adult exposed (both preweaning exposed and not) APOE3 mice. ↓ reaction to social novelty in APOE3 mice postnatally exposed to CPF. Adult exposure blocked this effect. ↑ reaction to social novelty in APOE4 mice postnatally exposed. Hypothalamus: ↑ Oxytocin mRNA in adult exposed APOE3, ↓ in adult exposed APOE4. Adult exposure increased low expression rates of Vasopressin in APOE3. Adult exposure decreased Vasopressin and vasopressin receptor 1a mRNA levels in APOE4. Adult exposure decreased Estrogen receptor 1, Proopiomelacortina in APOE4, amongst others. | MH |
| Mullen et al. [39] | Reeler Mice/PND7 (USVs), PND30 (Social interaction)/Both | CPO. 6 mg/mL GD13-Delivery Pump | AChE activity | USVs. Three-chambers test. Open field. MBT | ↑ USVs number in exposed +/−Reeler (males) from its vehicle. ↓ USVs number exposed +/+Reeler (males) from its vehicle. ↓ USVs number in exposed females (both genotypes). ↓ USVs duration. ↑ social interaction (sniffing, females, both exposed +/+ and +/−) | ML |
| Hazarika et al. [42] | Rats/adulthood/Both | PMD. 35 ppm for 30-, 45- and 60-days Adulthood Diet | Weight | Social Interaction test. Open field | = Sociability. ↑ Locomotion (longer exposure protocol). Multiple histopathological disruptions following the different exposure protocols. | ML |
| Ouardi et al. [41] | Mice/PND21/Both | MAL. 5–15 mg/kg/day GD6-PND21 Gavage | Weight, AChE activity | 3-Chambers test. Open field. | ↓ Sociability. ↓ Reaction to social novelty. ↑ anxiety (time in periphery, the highest dose). ↑ Brain MDA (PND21). ↓ brain CAT and SOD (PND5-21 for the high exposure, PND21 for the low exposure condition). ↓ brain GST (PND21) and GPX (PND15) | ML |
| Other families of pesticides | ||||||
| Laugeray et al. [43] | Mice/PND1-5 (USVs). >PND90 (sociability)/Both (pups) and M (adulthood) | GLA. 0.2–1 mg/kg/3 times per week GD?-PND14 Intranasal | Neurobehavioral battery, weight, reflexes, litter size | Social Interaction test. Three-chambers test. USVs. Plus-maze | ↓ USVs in exposed mice (highest dose). ↓ Sociability in the three-chambers test (highest dose). ↓ social interaction with females. = anxiety. ↑ relative gene expression of brain phosphatase and Pten (lowest dose). ↓ relative gene expression of brain phosphatase and Pten and Peg3 genes (highest dose). | MH |
| Laugeray et al. [45] | Mice/PND1-15 (USVs). >PND90 (sociability)/Both (pups) and M (adulthood) | CYP. 5–20 mg/kg/3 times per week GD6-PND15 Intranasal | Neurobehavioral battery, weight, reflexes, litter size | Social Interaction test. Three-chambers test. USVs. Maternal behavior. Open field. Plus-maze | ↓ Maternal behavior (highest dose). ↓ Sociability (lowest dose). = reaction to social novelty (lowest dose). ↓ self-grooming (lowest dose). = USVs. ↑ motricity (velocity in the highest exposed mice). = anxiety (lowest dose). Dysregulation of multiple genes | MH |
| Dong et al. [44] | Mice/PNW6-10/Both | GLA.12 ug/mL For 8 weeks (mums before mating to delivery) Water | Pregnancy rate, litter size, weight | Social Interaction test. 3-Chambers test. Open field. MBT | ↓ Locomotor activity. ↓ Social interaction, sociability, and reaction to social novelty. ↑ compulsivity/anxiety (MBT). ↓ Relative expression of cortical Nrxn1 gene. ↑ Relative abundance of Bacteroidetes bacteria in the gut. ↓ Relative abundance of Firmicutes in the gut. ↓ species diversity in the gut. Gut dysbiosis concerning multiple bacteria at genus level. ↓ Fatty acids biosynthesis. | MH |
| Study, Year (Reference) | Study Design Region | Age at Evaluation/Sex/ Sample Size | Type, Agent, and Source of Exposure Assessment | Neurobehavioral/ Neuropsychological Assessment in Children | Behavioral, Physiological Outcomes/Diagnostic | Quality Index |
|---|---|---|---|---|---|---|
| Organophosphate Compound | ||||||
| Guo et al. [46] | SMBCS Cohort/Shenyang (China) | 3 yo Both/N = 377 | Env; OP (TCPy) Prenatal (prior to delivery) and postnatal (3 yo) urine samples | Gesell Developmental Schedules | No relationship between prenatal TCPy exposure and neurodevelopment alterations. ↓ Motor and social development related to postnatal exposure mainly in boys | VH |
| Wang et al. [48] | LWBC Cohort/ Shandong (China) | 1–2 yo Both/N = 262 | B; OP (DAPs) Prenatal (delivery) and postnatal (1 and 2 yo) urine samples | Gesell Developmental Schedules | No association between prenatal or postnatal exposure was found in children at 1 yo. Prenatal exposure to DEs and DAPs was associated with a ↓ in social scores (among boys), while postnatal exposure to DMs and DAPs ↑ adaptive domain in children 2 yo | VH |
| Zhang et al. [49] | Chinese Cohort/ Shenyang (China) | 3 do Both/N = 249 | Env; OP (DAPs) Prenatal urine samples (prior to delivery) | Neonatal Behavioral Neurological Assessment | ↓ Overall neurodevelopment scores after prenatal exposure to OP measured by urine DAPs metabolites. DAPs concentrations, specially DEs was associated with lower scores on the behavior scale and DMs was associated with poorer scores in a passive tone, active tone, and primary reflex | VH |
| Bouchard et al. [50] | CHAMACOS Cohort/ California (USA) | 7 yo Both/N = 329 | Env; OP (DAPs) Prenatal (13 and 26 gw) and postnatal (6 mo, 1, 2, 3.5, 5 yo) urine samples | Wechsler Intelligence Scale for Children—4th edition | Prenatal DAPs exposure was associated with lower cognitive scores, especially, in IQ, verbal comprehension (DAPs), and processing speed (DEs). Postnatal urinary DAPs concentrations were not associated with cognitive scores | VH |
| Donauer et al. [51] | HOME Cohort/ Ohio (USA) | Annually from 1 to 5 yo/Both/N = 327 | Env; OP (DAPs) Prenatal urine samples (16 and 26 gw) | Bayley Scales of Infant Development—2nd edition/Clinical Evaluation of Language Fundamentals—Preschool, 2nd edition/Wechsler Preschool and Primary Scale of Intelligence—3rd edition | No effect on cognitive and neurodevelopmental performance | VH |
| Engel et al. [52] | Mount Sinai Environmental Health Cohort/New York (USA) | 1, 2, and 6-9 yo Both/N = 169 | Env; OP (DAPs) Maternal blood, cord blood, and prenatal urine samples (between 26 and 28 gw) | Bayley Scales of Infant Development—2nd edition/Wechsler Psychometric Intelligence Test/Wechsler Preschool and Primary Scale of Intelligence—3rd edition/Wechsler Intelligence for Children—4th edition | ↓ mental development by DAPs (1 and 2 yo) and DMs (1 yo, race/ethnicity). No association in DAPs and psychomotor development. DEs negatively associated with IQ, perceptual reasoning, and working memory in children 7–9 yo | VH |
| Eskenazi et al. [53] | CHAMACOS Cohort/ California (USA) | 2 yo Both/N = 353 | Oc; OP (DAPs) Prenatal urine samples (during pregnancy) | Bayley Scales of Infants Development—2nd edition | Maternal DAPs were negatively associated with cognitive and mental abilities as well as with PON1 polymorphism | VH |
| Kongtip et al. [56] | Cohort/Thailand | 5 mo Both/N = 50 | B; OP (DAPs) Prenatal urine samples (around 28 gw) | Bayley Scales of Infants Development—3rd edition | DEs exposure during 3rd trimester were associated with ↓ in cognitive and motor function, while DAPs maternal levels only affected motor scores | VH |
| Liu et al. [57] | Chinese Cohort/Shenyang (China) | 2 yo Both/N = 310 | B; OP (DAPs) Prenatal (prior to delivery) and postnatal (2 yo) urine samples | Gesell Developmental Schedules | Prenatal DEs exposure is associated with ↑ risk of being developmentally delayed (in boys). Postnatal DAPs and DEs exposure showed delays in development, especially in motor and social area among boys | VH |
| Millenson et al. [58] | HOME Cohort/ Ohio (USA) | 8 yo Both/N = 224 | Env; OP (DAPs) Prenatal urine samples (between 13 and 19 gw) | Social Responsiveness Scales | DAPs exposure was not associated with autism symptoms after adjusting for covariates. No evidence that PON1 polymorphism modified prenatal DAPs exposure and autism risk/ASD | VH |
| Sagiv et al. [59] | CHAMACOS Cohort/ California (USA) | 1, 2, 5, 7, 9, 10.5, 12, and 14 yo/Both N = 333 | Env; OP (DAPs) Prenatal urine samples (13 and 26 wo) | Social Responsiveness Scales/Behavioral Assessment Scales for Children, Version 2/Infant Neuropsychological Evaluation Facial Expression Recognition Test/NEPSY-II Affect Recognition Test | Maternal DAPs were associated with an ↑ in autism-related traits in childhood and adolescence. However, no association was observed on facial recognition test in children 9 and 12 yo/ASD | VH |
| Van den Dries et al. [61] | Generation R Cohort/ Rotterdam (Netherlands) | 6 yo Both/N = 622 | Env; OP (DAPs) Prenatal (early, mid-, and late pregnancy) and postnatal (6 yo) urine samples | Social Responsiveness Scales | No association between DAPs and autism symptomatology/Autistic traits | VH |
| Philippat et al. [47] | MARBLES Cohort/ California (USA) | 3 yo Both/N = 203 | Env; OP (DAPs) Prenatal urine samples (1st, 2nd, 3rd trimester) | Autism Diagnostic Observation Schedule/Social Communication Questionnaire/Mullen Scales of Early Learning | OP exposure assessed by DMTP metabolite concentrations tended to ↑ the risk of autism only in girls. No association were observed without sex-stratification/ASD | H |
| Furlong et al. [54] | Mount Sinai Environmental Health Cohort/New York (USA) | 1, 2, 4, 6, 7–9 yo Both/N = 136 | Env; OP (DAPs) Prenatal urine samples (3rd trimester) | Social Responsiveness Scales | DEs levels were associated with poorer social responsiveness in black participants with a stronger effect on boys. No association was found with DAPs and DMs concentrations /ASD | H |
| González-Alzaga et al. [55] | Cohort/ Andalusia (Spain) | Between 6 and 11 yo Both/N = 256 | Env; OP (DAPs) Postnatal urine samples (between 6 and 11 yo) | Wechsler Intelligence Scale for Children—4th edition | DAPs levels associated with a ↓ in verbal comprehension, processing speed, and IQ among boys | H |
| Silver et al. [60] | Chinese Cohort/ Fuyang (China) | 6 wo and 9 mo Both/N = 199 | Env; OP Prenatal cord blood samples | Peabody Development Motor Scales | No significant findings were observed at 6 wo. Naled and CPF exposure associated with deficits in motor function, among girls at 9 mo | H |
| Woskie et al. [62] | Cohort/Thailand | Between 0 and 4 do Both/N = 82 | B; OP (DAPs) Prenatal urine samples (7 gm and prior to delivery) | Brazelton Neonatal Behavioral Assessment Scale | ↑ Score in the Range of state cluster score associated with maternal DEP metabolite levels and ↑ urinary DMP metabolite levels was associated with ↑ scores in Habituation cluster | MH |
| Organochlorine compounds | ||||||
| Bahena-Medina et al. [63] | Morelos Cohort/Mexico | 1 mo Both/N = 265 | Env; OC Prenatal blood samples (each trimester) | Brazelton Neonatal Behavioral Assessment Scale/Graham—Rosenblatt Scale/Bayley Scales of Infant Development | No effects on reflex, neurological or psychomotor development at 1 mo | VH |
| Brown et al. [64] | FiPS-A Case-Control/Finland | 0–7 yo/Both N = 1,556 | Env; OC Prenatal blood samples (each trimester) | Autism Diagnostic Interview—Revised | DDE ↑ odds of autism/ASD | VH |
| Kim et al. [65] | CHECK Cohort/ Seoul, Anyang, Ansan, and Jeju (Korea) | 13–24 mo Both/N = 140 | Env; 38 OC Prenatal blood (pregnancy) and breast milk (30 days after delivery) samples | Bayley Scales of Infant Development—2nd edition | No specific results related to OC pesticides exposure | VH |
| Puertas et al. [72] | INMA Cohort/ Granada (Spain) | 4 yo Both/N = 255 | Env; OC (Mirex) Placenta samples (at delivery) | McCarthy Scales of Children’s Abilities | ↓ Cognitive performance, especially, working memory and quantitative area (numerical memory, counting, and sorting). No effects were observed in perceptual-performance, verbal, and motor areas | VH |
| Boucher et al. [73] | Timoun Cohort/Guadeloupe | 18 mo Both/N = 204 | Env; OC (Chlordecone) Cord blood and breast milk (3 mo) samples | Ages and Stages Questionnaire/Bayley Scales of Infant Development—2nd edition | Prenatal exposure was associated with poorer motor ability among boys Postnatal exposure: no significant association with personal-social, communication, problem-solving, fine and gross motor scores | VH |
| Braun et al. [66] | HOME Cohort/ Ohio (USA) | 4 and 5 yo Both/N = 175 | Env; OC Prenatal and blood samples (2nd trimester and at delivery) | Social Responsiveness Scales | Maternal trans-nonachlor ↑ autistic behaviors/ASD | VH |
| Jeddy et al. [67] | ALSPAC Cohort/England | 15–38 mo Girls/N = 400 | Env; OC Prenatal blood samples (pregnancy) | Adapted versions of the MacArthur Communicative Development Inventory | No association between β-HCH or DDE and communication scores (15 and 38 mo). HCB ↓ vocabulary comprehension and production (15 mo) and ↓ intelligibility scores (38 mo). DDT was associated with a ↓ in communication scores (38 mo) | VH |
| Hamra et al. [70] | EMA Case-control/California (USA) | 4–9 yo Both/N = 864 | Env; OC Prenatal blood samples (2nd trimester) | Diagnostic and Statistical Manual of Mental Disorder—4th edition | No association between pesticides exposure and odds of autism/ASD | VH |
| Torres-Sanchez et al. [71] | Morelos Cohort/Mexico | 42–60 mo Both/N = 203 | Env; OC Prenatal blood samples (each trimester) | McCarthy Scales of Children’s Abilities | DDE exposure during the 3rd trimester was associated with ↓ cognition, verbal comprehension, and memory | VH |
| Kao et al. [68] | FiPS-A Cohort/Taiwan | 8–12 mo Both/N = 55 | Env; 20 OC Postnatal breast milk (between 2 wo and 1 mo) samples | Bayley Scales of Infant Development—3rd edition | DDT and trans-chlordane ↓ cognitive, language, social-emotional, and motor scores | H |
| Lyall et al. [69] | EMA Case-Control/California (USA) | 3–10 yo Both/N = 1144 | Env; 46 OC Prenatal blood samples (2nd trimester) | Diagnostic and Statistical Manual of Mental Disorders—4th edition, Text Revision | No clear evidence that higher levels of prenatal exposure to p,p’-DDE, and trans-nonachlor increased the risk of ASD/ASD | H |
| Pyrethroids compounds | ||||||
| Viel et al. [75] | PELAGIE Cohort/ Brittany (France) | 6 yo Both/N = 287 | Env; PT Prenatal (6–19 gw) and postnatal (6 yo) urine samples | Wechsler Intelligence Scale for Children—4th edition | No effect on neurocognitive scores after prenatal exposure ↓ Verbal comprehension and working memory associated with postnatal exposure to 3-PBA and cis-DBCA | VH |
| Viel et al. [76] | PELAGIE Cohort/ Brittany (France) | 6 yo Both/N = 287 | Env; PT Prenatal (6–19 gw) and postnatal (6 yo) urine samples | Strengths and Difficulties Questionnaire | No significant association between maternal urinary PT metabolites and child neurobehavioral deficits. Childhood urinary levels of 3-PBA and trans-DCCA associated with ↑ odds of behavioral disorders | VH |
| Furlong et al. [74] | Mount Sinai Children’s Environmental Health Cohort/New York (USA) | 1, 2, 4, 6, 7–9 yo Both/N = 162 | Env; PT Prenatal urine samples (3rd trimester) | Behavior Assessment System for Children/Behavior Rating Inventory of Executive Functioning | 3-PBA associated with depression, somatization, behavioral and emotional deficits. Cis-DCCA exposure was associated with behavioral regulation, emotional and externalizing problems, while, trans-DCCA was not associated with adverse effects | VH |
| Horton et al. [77] | CCEH Cohort/ New York (USA) | 3 yo Both/N = 342 | Env; PT (PBO and Permethrin) Prenatal air (3rd trimester), maternal or cord blood (delivery) samples | Bayley Scales of Infant Development—2nd edition | No association between permethrin air and blood samples with mental or motor development. ↓ in mental development after prenatal PBO exposure, while no association was found in motor development | VH |
| Watkins et al. [78] | ELEMENT Cohort/Mexico | 2–3 yo Both/N = 187 | Env; PT Prenatal urine samples (3rd trimester) | Bayley Scales for Infant Development—Spanish version, 2nd edition | Lower mental development in 1 yo children, being stronger in girls. No association between maternal 3-PBA and motor development at 2 or 3 years of age | H |
| Carbamates compounds | ||||||
| Zhang et al. [79] | SMBCS Cohort/ Shenyang (China) | 3 yo Both/N = 337 | Env; CM (Carbofuranphenol) Prenatal (prior to delivery) and postnatal (3 yo) urine samples | Gesell Developmental Schedules | Prenatal exposure associated with ↓ in social and adaptative behaviors Postnatal exposure associated with language and social behavior deficits | VH |
| Mora et al. [80] | ISA Cohort/ Matina (Costa Rica) | Pregnancy and 1 yo Both/N = 355 | B; CM (Mancozeb) Hair, blood, and prenatal urine samples (19, 30, and 33 gw) | Bayley Scales of Infants Development—3rd edition | ↓ Cognitive abilities in girls, while language and fine motor development were affected in boys. ↓ social-emotional scores in both sexes | VH |
| Neonicotinoids compounds | ||||||
| Keil et al. [81] | CHARGE Case-control/California (USA) | 3 and 4 yo Both/N = 669 | Env; NN (Imidacloprid) Prenatal household by maternal interviews | Autism Diagnostic Interview—Revised/Autism Diagnostic Observation Schedules/Mullen Scales of Early Learning/Vineland Adaptive Behavior Scales/Child Development and Social Communication Questionnaire | Association between autism and Imidacloprid exposure/ASD | VH |
| Mixture: pesticides and other potential neurotoxic | ||||||
| Andersen et al. [82] | Cohort/ Denmark | Between 6 and 11 yo Both/N = 177 | Oc; Insecticides, fungicides, and plant growth regulators Prenatal (1rst trimester) exposure No biomonitoring, estimation of exposure | BAEP/Finger Tapping Test/Conner/s Continuous Performance Test II/Wechsler Intelligence Scale for Children—Revised/Woodcock Intelligence Tests of Cognitive Abilities/Copying Test of the Stanford—Binet, 4th edition | ↑ Brainstem evoked potential (BAEP) latency (boys and girls). Impairment in neurobehavioral, language, motor speed, and short-term memory functions, only in girls | VH |
| Gunier et al. [91] | CHAMACOS Cohort/ California (USA) | 7 yo Both/N = 283 | Env; 15 OP, 6 CM, 2 Mn-fungicide, 8 PT, and 1 NN Prenatal house-dust samples | Wechsler Intelligence Scale of Children—4th edition | OP is associated with IQ and verbal comprehension deficits. OP and CM are associated with ↓ IQ. NN, PT, and Mn-fungicides are associated with ↓ in IQ, perceptual reasoning, and verbal comprehension | VH |
| Schmidt et al. [89] | CHARGE Case-Control/California (USA) | 2 and 5 yo Both/N = 516 | B; OP, PT, and CM Prenatal household (3 mo before conception and during pregnancy) | Autism Diagnostic Observation Schedule/Social Communication Questionnaire/Mullen Scales of Early Learning/Vineland Adaptive Behavior Scales | Exposure to OP, PT, and CM ↑ autism risk, while FA intake ↓ the risk/ASD | VH |
| Eskenazi et al. [84] | VHEMBRE Cohort/Limpopo (South Africa) | 1–2 yo Both/N = 705 | B; OC and PT Blood and urine (prior and post-delivery) samples | Bayley Scale of Infant Development—3rd edition | (1 yo) No effect of DDT/DDE exposure and neurodevelopment. Cis-DCCA, trans-DCC,A, and 3-PBA were associated with socio-emotional deficits. (2 yo) Motor problems associated with DDT, while DDE ↓ in communication and language. Cis-DBCA were associated with a ↓ in communication and language, among girls (1 yo) and both sexes (2 yo) | VH |
| Furlong et al. [85] | Mount Sinai Children’s Environmental Health Cohort/New York (USA) | 1, 2, 4–7, 9 yo Both/N = 404 | Env; OP and PT Prenatal (between 25 and 40 gw) urine samples | Behavior Rating Inventory of Executive Functioning/Behavior Assessment System for Children/Wechsler Preschool and Primary Scale of Intelligence—3rd edition/Wechsler Intelligence Scales—4th edition | DMs levels were associated with worse internalizing scores (anxiety scale) and ↑ working memory among black children, while DEs was associated with worse working memory scores. No association was observed with PON1 polymorphism | VH |
| McCanlies et al. [87] | CHARGE Case-control/California (USA) | 2 and 5 yo Both/N = 951 | Oc; Pesticides Postnatal mother/father interviews | Mullen Scales of Early Learning/Vineland Adaptive Behavior Scales/Autism Diagnostic Observation Schedule/Autism Diagnostic Interview—Revised/Social Communication Questionnaire | No association between pesticides and autism/ASD | VH |
| Ostrea et al. [88] | Cohort/ Bulacan (Philippines) | 2 yo Both/N = 754 | B; CM (Propoxur) and PT Prenatal maternal blood, hair and postnatal cord blood and children hair | Griffiths Test | Motor development was affected after Propoxur exposure among boys No association was observed between Propoxur exposure and social behavior | VH |
| Shelton et al. [90] | CHARGE Case-Control/California (USA) | Between 2 and 5 yo Both/N = 970 | B; OP, CM, PT, and OC Prenatal household (3 mo before conception and during pregnancy) | Autism Diagnostic Observation Schedule/Social Communication Questionnaire/Mullen Scales of Early Learning/Vineland Adaptive Behavior Scales | ↑ autism risk after prenatal OP pesticides (1st and 2nd trimester) and PT (3 mo before conception and 3rt trimester)/ASD | VH |
| Von Ehrenstein et al. [83] | Case-Control/ California (USA) | 1 yo/Both N = 38,331 | Env; Pesticides Prenatal (3 mo before conception and during pregnancy) and postnatal (first year of life) residential samples | Diagnostic and Statistical Manual of Mental Disorders—4th edition, revised | ↑ autism risk after prenatal exposure to pesticides such as Glyphosate, CPF, MAL, Diazinon, Avermectin, and Permethrin/ASD | VH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biosca-Brull, J.; Pérez-Fernández, C.; Mora, S.; Carrillo, B.; Pinos, H.; Conejo, N.M.; Collado, P.; Arias, J.L.; Martín-Sánchez, F.; Sánchez-Santed, F.; et al. Relationship between Autism Spectrum Disorder and Pesticides: A Systematic Review of Human and Preclinical Models. Int. J. Environ. Res. Public Health 2021, 18, 5190. https://doi.org/10.3390/ijerph18105190
Biosca-Brull J, Pérez-Fernández C, Mora S, Carrillo B, Pinos H, Conejo NM, Collado P, Arias JL, Martín-Sánchez F, Sánchez-Santed F, et al. Relationship between Autism Spectrum Disorder and Pesticides: A Systematic Review of Human and Preclinical Models. International Journal of Environmental Research and Public Health. 2021; 18(10):5190. https://doi.org/10.3390/ijerph18105190
Chicago/Turabian StyleBiosca-Brull, Judit, Cristian Pérez-Fernández, Santiago Mora, Beatriz Carrillo, Helena Pinos, Nelida Maria Conejo, Paloma Collado, Jorge L. Arias, Fernando Martín-Sánchez, Fernando Sánchez-Santed, and et al. 2021. "Relationship between Autism Spectrum Disorder and Pesticides: A Systematic Review of Human and Preclinical Models" International Journal of Environmental Research and Public Health 18, no. 10: 5190. https://doi.org/10.3390/ijerph18105190
APA StyleBiosca-Brull, J., Pérez-Fernández, C., Mora, S., Carrillo, B., Pinos, H., Conejo, N. M., Collado, P., Arias, J. L., Martín-Sánchez, F., Sánchez-Santed, F., & Colomina, M. T. (2021). Relationship between Autism Spectrum Disorder and Pesticides: A Systematic Review of Human and Preclinical Models. International Journal of Environmental Research and Public Health, 18(10), 5190. https://doi.org/10.3390/ijerph18105190











