Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
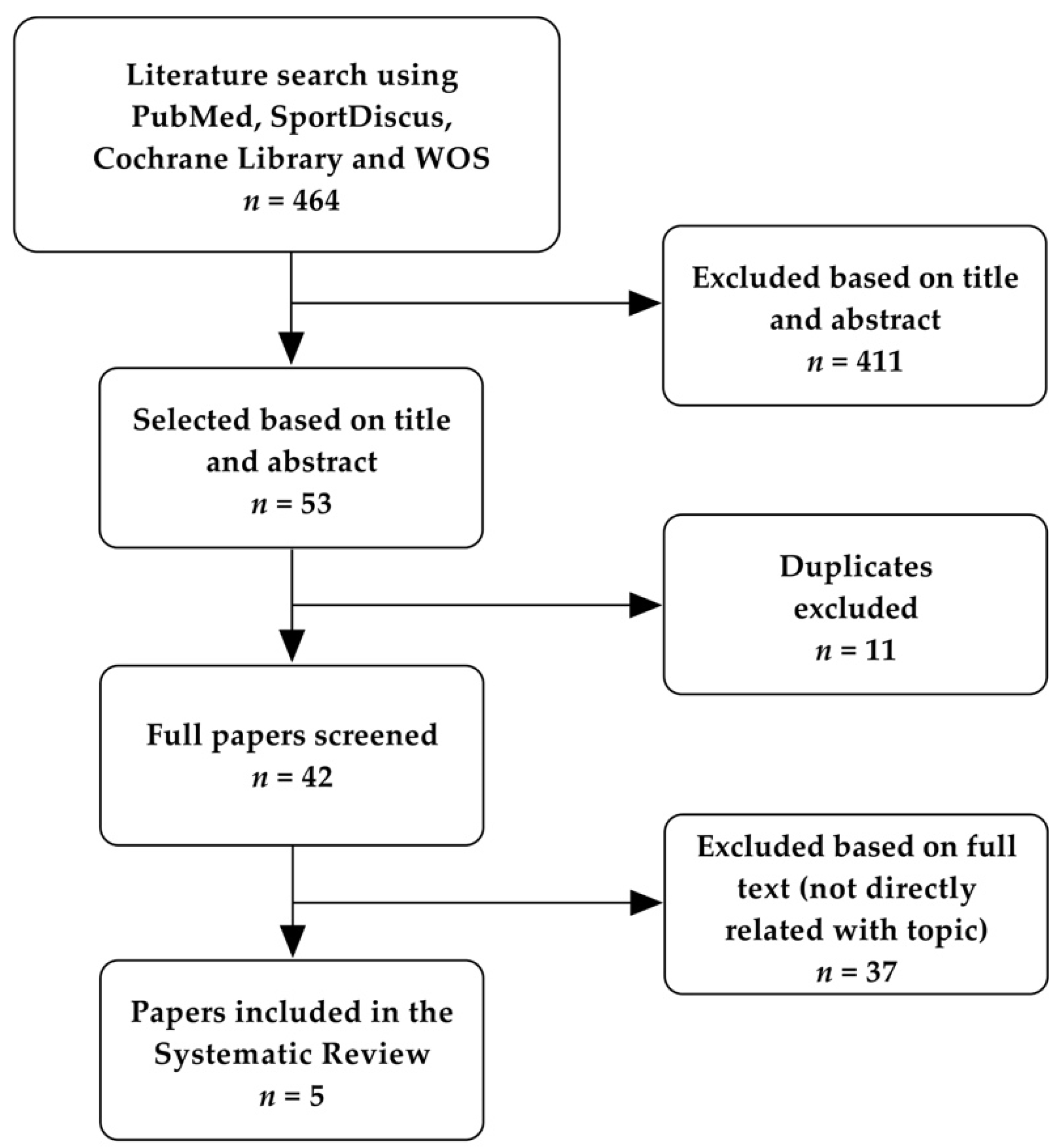
3.1. Study Selection
3.2. Description of Included Studies
3.3. Assessment of Main Variables
3.4. Risk of Bias Assessment
3.5. Risk of CD among Different PA Levels
4. Discussion
4.1. Physical Activity Dose and Risk of CD
4.2. Strengths and Limitations
4.3. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Han, X.; Zhang, X.; Wang, S. Spatiotemporal evolution of global population ageing from 1960 to 2017. BMC Public Health 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Makkar, S.R.; Crawford, J.D.; Thalamuthu, A.; Kochan, N.A.; Lima-costa, M.F.; Castro-costa, E.; Pinheireo Ferri, C.; Brayne, C.; Stephan, B.; et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: A COSMIC collaboration cohort study. PLoS Med. 2019, 16, 1–27. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Deep, C.A.; Jeste, D.V. Definitions and Predictors of Successful Aging: A Comprehensive Review of Larger Quantitative Studies. Am. J. Geriatr. Psychiatry 2006, 14, 6–20. [Google Scholar] [CrossRef]
- WHO. Decade of Healthy Ageing 2020–2030; World Health Organization: Madrid, Spain, 2020. [Google Scholar]
- Bousquet, J.; Malva, J.; Nogues, M.; Rodriguez, L.; Vellas, B.; Farrell, J. Operational Definition of Active and Healthy Aging (AHA): The European Innovation Partnership (EIP) on AHA Reference Site Questionnaire: Montpellier 20–21 October 2014, Lisbon 2 July 2015. J. Am. Med. Dir. Assoc. 2015, 16, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Geslani, D.M.; Tierney, C.; Szalai, J.P. Mild Cognitive Impairment: An Operational Defi nition and Its Conversion Rate to Alzheimer’ s Disease. Dement. Geriatr. Cogn. Disord. 2005, 19, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.A.; Clare, L.; Woods, R.T. What is the Relationship between Health, Mood, and Mild Cognitive Impairment? J. Alzheimer’s Dis. 2017, 55, 1183–1193. [Google Scholar] [CrossRef]
- Santabárbara, J.; Lopez-Anton, R.; Marcos, G.; De-la-Camara, C.; Lobo, E.; Saz, P.; Gracia-García, P.; Ventura, T.; Campayo, A.; Rodríguez-Mañas, L.; et al. Degree of cognitive impairment and mortality: A 17-year follow-up in a community study. Epidemiol. Psychiatr. Sci. 2015, 24, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Santabárbara, J.; Garcia-García, P.; Pírez, G.; López-antón, R.; Concepcion De La Cámara, M.; Ventura, T.; Pérez-Sastre, M.; Lobo, E.; Saz, P.; Marcos, G.; et al. Mortality in Mild Cognitive Impairment Diagnosed with DSM-5 Criteria and with Petersen’ s Criteria: A 17-Year Follow-Up in a Community Study. Am. J. Geriatr. Psychiatry 2016, 24, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Gracia-García, P.; López-antón, R.; Santabárbara, J.; Quintanilla, M.A.; De-la-Cámara, C.; Marcos, G.; Lobo, E.; Lobo, A.; The ZARADEMP Workgroup. Cognition and daily activities in a general population sample aged + 55. Aging Neuropsychol. Cogn. 2020, 4, 1–14. [Google Scholar] [CrossRef]
- Etgen, T.; Sander, D.; Bickel, H.; Förstl, H. Mild Cognitive Impairment and Dementia: The importance of modifiable risk factors. Dtsch. Aerzteblatt. Int. 2011, 108, 743–750. [Google Scholar]
- Czyz-Szypenbejl, K.; Medrzycka-Dabrowska, W.; Kwiecien-Jagus, K.; Lewandowska, K. The ocurrence of postoperative cognitive dysfunction (POCD)—Systematic Review. Psychiatr. Pol. 2019, 53, 145–160. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, C.; Zhao, K.; Ma, L.; Qiu, X.; Zhang, L.; Xiu, Y.; Chen, L.; Lu, W.; Huang, C.; et al. Depression as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Int J. Geriatr. Psychiatry 2013, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Köhler, S.; Oude Voshaar, R.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Rawle, M.J.; Davis, D.; Bendayan, R.; Wong, A. Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl. Psychiatry 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The distribution of alipoprotein E genotype over the adult lifespan and in relation to country of birth. Am. J. Epidemiol. 2015, 181, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Shah, T.; Prieto, D.; Zhang, W.; Price, J.; Fowkes, G.R.; Cooper, J.; Talmud, P.J.; Humphires, S.E.; Sundstrom, J.; et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: Systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int. J. Epidemiol. 2013, 42, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Liu, Y.; Li, Y.; Qiu, S.; Bai, Y.; Gu, Y.; Luo, J.; Cui, H.; Li, Y.; et al. Association between ApoE polymorphism and Hypertension: A Meta-analysis of 28 studies including 5898 cases and 7518 controls. Gene 2018, 30, 197–207. [Google Scholar] [CrossRef]
- Hayden, K.M.; Lutz, M.W.; Kuchibhatla, M. Effect of APOE and CD33 on Cognitive Decline. PLoS ONE 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Makkar, S.R.; Lipnicki, D.M.; Crawford, J.D.; Kochan, N.A.; Castro-costa, E.; Fernandez Lima-Costa, M.; Diniz, B.S.; Brayne, C.; Stephan, B.; Matthews, F.; et al. APOE e4 and the influence of sex, age, vascular risk factors, and ethnicity on cognitive decline. J. Gerontol. Ser. A 2020, 75, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Chia-Chen, L.; Takahisa, K.; Huaxi, X.; Guojun, B. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar]
- Gardener, H.; Wright, C.B.; Dong, C.; Cheung, K.; Derosa, J.; Nannery, M.; Stern, Y.; Elkind, M.S.V.; Sacco, R.L. Ideal Cardiovascular Health and Cognitive Aging in the Northern Manhattan Study. J. Am. Heart Assoc. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, U. Human cognitive aging: Corriger la fortune? Science 2014, 346, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.; Hardman, R.J.; MacPherson, H.; Scholey, A.B.; Pipingas, A. How Does Exercise Reduce the Rate of Age-Associated Cognitive Decline? A Review of Potential Mechanisms. J. Alzheimer’s Dis. 2017, 55, 1–18. [Google Scholar] [CrossRef]
- Macpherson, H.; Teo, W.-P.; Schneider, L.A.; Smith, A.E. A Life-Long Approach to Physical Activity for Brain Health. Front. Aging Neurosci. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H.; Alvarez, V.E.; Stein, T.D. The neuropathology of sport. Acta Neuropathol. 2014, 127, 29–51. [Google Scholar] [CrossRef]
- Elias, M.F.; Sullivan, L.M.; D’Agostino, R.B.; Elias, P.K.; Beiser, A.; Au, R.; Seshadri, S.; DeCarli, C.; Wolf, P.A. Framingham Stroke Risk Profile and Lowered Cognitive Performance. Stroke 2004, 35, 404–409. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Hohman, T.J.; Liu, D.; Haj-Hassan, S.; Gifford, K.A.; Benson, E.M.; Skinner, J.S.; Lu, Z.; Sparling, J.; Sumner, E.C.; et al. Adverse vascular risk is related to cognitive decline in older adults. J. Alzheimer’s Dis. 2015, 44, 1361–1373. [Google Scholar] [CrossRef]
- Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Wayne, W.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med. Sci. Sports Exerc. 2020, 51, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, 1–32. [Google Scholar] [CrossRef]
- Rebar, A.L.; Stanton, R.; Geard, D.; Short, C.; Duncan, M.J.; Vandelanotte, C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 2015, 9, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Osman, J.; Cabral, D.F.; Morris, T.P.; McInerney, K.; Cahalin, L.P.; Rundek, T.; Oliveira, A.; Pascual-Leone, A. Exercise for cognitive brain health in aging: A systematic review for an evolution of dose. Neurol. Clin. Pract. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Angevaren, M.; Vanhees, L.; Wendel-vos, W.; Verhaar, H.J.J.; Aufdemkampe, G.; Aleman, A.; Verschuren, W.M.M. Intensity, but not duration, of physical activities is related to cognitive function. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 825–830. [Google Scholar] [CrossRef]
- van Gelder, B.M.; Tijhuis, M.A.R.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Physical activity in relation to cognitive decline in elderly men: The FINE Study. Neurology 2004, 63, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Flicker, L.; Almeida, O.P.; Acres, J.; Le, M.T.; Tuohy, R.J.; Jamrozik, K.; Hankey, G.; Norman, P. Predictors of impaired cognitive function in men over the age of 80 years: Results. Age Ageing 2005, 34, 77–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical Activity and Risk of Cognitive Impairment and Dementia in Elderly Persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O´Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 October 2020).
- de Morton, N. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Krell-Roesch, J.; Pink, A.; Roberts, R.O.; Stokin, G.B.; Mielke, M.M.; Spangehl, K.A.; Bartley, M.M.; Knopman, D.S.; Christianson, T.J.H.; Petersen, R.C.; et al. Timing of Physical Activity, Apolipoprotein E ε4 Genotype, and Risk of Incident Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2016, 64, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Niti, M.; Yap, K.B.; Kua, E.H.; Tan, C.H.; Ng, T.P. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-ε4 genotype in Chinese older adults. Int. Psychogeriatr. 2008, 20, 237–251. [Google Scholar] [CrossRef]
- Shih, I.F.; Paul, K.; Haan, M.; Yu, Y.; Ritz, B. Physical activity modifies the influence of apolipoprotein E ε4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimer’s Dement. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Woodard, J.; Nielson, K.; Sugarman, M.; Smith, C.; Seidenberg, M.; Durgerian, S.; Butts, A.; Hantke, N.; Lancaster, M.; Matthews, M.A.; et al. Lifestyle and genetic contributions to cognitive decline and hippocampal integrity in healthy aging. Curr. Alzheimer Res. 2012, 9, 436–446. [Google Scholar] [CrossRef]
- Espeland, M.A.; Luchsinger, J.A.; Baker, L.D.; Neiberg, R.; Kahn, S.E.; Arnold, S.E.; Wing, R.R.; Blackburn, G.L.; Bray, G.; Evans, M.; et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology 2017, 88, 2026–2035. [Google Scholar] [CrossRef]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.; Cahalin, L.; Buman, M.; Ross, R. The Current State of Physical Activity Assessment Tools. Prog. Cardiovasc. Dis. 2015, 57, 387–395. [Google Scholar] [CrossRef]
- Warren, J.M.; Ekelund, U.; Besson, H.; Mezzani, A.; Geladas, N.; Vanhees, L. Assessment of physical activity—A review of methodologies with reference to epidemiological research: A report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Skender, S.; Ose, J.; Chang-claude, J.; Paskow, M.; Brühmann, B.; Siegel, E.M.; Steindorf, K.; Ulrich, C.M. Accelerometry and physical activity questionnaires—A systematic review. BMC Public Health 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Welk, G.J. Harmonizing Monitor- and Report-Based Estimates of Physical Activity through Calibration. Kinesiol. Rev. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Suri, S.; Heise, V.; Trachtenberg, A.J.; Mackay, C.E. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE e2. Neurosci. Biobehav. Rev. 2013, 37, 2878–2886. [Google Scholar] [CrossRef]
- Bangen, K.; Gu, Y.; Gross, A.; Schneider, B.; Skinner, J.; Benitez, A.; Sachs, B.C.; Shih, R.; Sisco, S.; Schupf, N.; et al. Relation of Type 2 Diabetes with Cognitive Change in a Multiethnic Elderly Cohort HHS Public Access. J. Am. Geriatr. Soc. 2015, 63, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.J.; Taddei, K.; Lui, J.K.; Laws, S.M.; Gupta, V.B.; Taddei, T.; Ward, V.K.; Rodrigues, M.A.; Burnham, S.; et al. Physical activity and amyloid- b plasma and brain levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry 2012, 18, 875–881. [Google Scholar] [CrossRef]
- Guure, C.B.; Ibrahim, N.A.; Adam, M.B.; Said, S.M. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. Biomed. Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schuit, A.J.; Feskens, E.J.M.; Launer, L.J.; Kromhout, D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med. Sci. Sports Exerc. 2001, 33, 772–777. [Google Scholar] [CrossRef]
- U.S. POINTER Alzheimer’s Association. Available online: https://uspointer.net/about.cfm (accessed on 17 November 2020).
- Erickson, K.I.; Grove, G.A.; Burns, J.M.; Hillman, C.H.; Kramer, A.F.; McAuley, E.; Vidoni, E.D.; Becker, J.T.; Butters, M.A.; Gray, K.; et al. Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE): Protocol. Contemp. Clin. Trials. 2019, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ganesh, A.B.; Berry, N.T.; Mobley, Y.P.; Karper, W.B.; Labban, J.D.; Wahlheim, C.N.; Williams, T.M.; Wideman, L.; Etnier, J.L. The effect of physical activity on cognition relative to APOE genotype (PAAD-2): Study protocol for a phase II randomized control trial. BMC Neurol. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
| Study, (Study Design) | Follow-Up, y, Mean, (Range) | Method to Assess PA | Method to Assess Cognitive Function | Adjudication of CD or MCI | Confounders | Study Subgroups | Main Results | Sample | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Female Sex, n (%) | Age, y, mean (SD), and/or Range | APOE e4, n, (e4e4, n) | No APOE e4, n | |||||||||
| Espeland, M. et al. (intervention) | 9.8 (8.4–11.1) |
Questionnaire |
| 3MSE test score fell below prespecified age- and education-specific cutoff points |
|
|
| 3802 | 2323 (61) | 45–76 | 724 (57) | 2407 | |
| Krell, J. et al. (observational) | 3.2 (1.9–4.7) |
|
| Petersen criteria for MCI 2004 |
|
| Midlife and LPA |
| 1830 | 919 (50.2) | 78 74–83 | 474 (NI) | 1347 |
| Midlife and MPA |
| ||||||||||||
| Midlife and VPA |
| ||||||||||||
| Late life and LPA |
| ||||||||||||
| Late life and MPA |
| ||||||||||||
| Late life and VPA |
| ||||||||||||
| Shih, I. et al. (observational) | 6.5 |
|
| Score 3MSE or SEVLT fell less than the 20th percentile/decreased ≥8 in 3MSE or ≥3 points in SEVLT and scores less than 20th percentile at follow-up |
| Groups by:
|
| 1438 | 840 (58) | 69.7 (6.2) | 201 (11) | 1237 | |
| Woodard, J.L. et al. (observational) | 1.5 |
|
| ≥1 SD reduction on at least one of the principal outcomes indices (DRS-2, RAVLT Sum of trials 1–5, RAVLT delayed word recall) | NI | Groups by:
|
| 78 | 57 (73) | 72.6 (5.0) | 26 (1) | 52 | |
| Study, (Study Design) | Follow-Up, y, Mean, (Range) | Method to Assess PA | Method to Assess Cognitive Function | Adjudication of CD or MCI | Confounders | Study Subgroups | Main Results | Sample | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n, (e4e4, n) | Female Sex, n (%) | Age, y, Mean (SD), and/or Range | |||||||||
| Krell, J. et al. (observational) | 3.2 (1.9–4.7) |
|
| Petersen criteria for MCI 2004 |
| Groups by:
| LPA |
| 474 (NI) | NI | NI |
| MPA |
| ||||||||||
| VPA |
| ||||||||||
| Niti, M. et al. (observational) | (1–2) |
|
| Decline ≥1 points in MMSE between baseline and follow-up |
| Groups by:
|
| 292 (NI) | NI | NI | |
| Woodard, J.L. et al. (observational) | 1.5 |
|
| ≥1 SD reduction on at least one of the principal outcomes indices (DRS-2, RAVLT Sum of trials 1–5, RAVLT delayed word recall) | NI | Groups by:
|
| 26 (1) | NI | NI | |
| Study | Quality Assessment of Cohort Studies with NOS | |||||||||||
| Selection | Comparability | Outcome | NOS QS | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| Krell, J. et al. | * | * | * | * | * | * | * | 7 | ||||
| Niti, M. et al. | * | * | * | * | * | * | 6 | |||||
| Shih, I. et al. | * | * | * | * | * | * | * | * | 8 | |||
| Woodard, J.L. et al. | * | * | * | * | * | 5 | ||||||
| Study | Quality Assessment of RCT Studies with PEDro Scale | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | PEDro QS | |
| Espeland, M. et al. | * | * | * | * | * | * | * | 7 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Lasierra, J.L.; Casajús, J.A.; Casasnovas, J.A.; Arbones-Mainar, J.M.; Lobo, A.; Lobo, E.; Moreno-Franco, B.; Gonzalez-Agüero, A. Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7238. https://doi.org/10.3390/ijerph18147238
Perez-Lasierra JL, Casajús JA, Casasnovas JA, Arbones-Mainar JM, Lobo A, Lobo E, Moreno-Franco B, Gonzalez-Agüero A. Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(14):7238. https://doi.org/10.3390/ijerph18147238
Chicago/Turabian StylePerez-Lasierra, Jose Luis, Jose Antonio Casajús, José Antonio Casasnovas, Jose Miguel Arbones-Mainar, Antonio Lobo, Elena Lobo, Belén Moreno-Franco, and Alejandro Gonzalez-Agüero. 2021. "Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 14: 7238. https://doi.org/10.3390/ijerph18147238
APA StylePerez-Lasierra, J. L., Casajús, J. A., Casasnovas, J. A., Arbones-Mainar, J. M., Lobo, A., Lobo, E., Moreno-Franco, B., & Gonzalez-Agüero, A. (2021). Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review. International Journal of Environmental Research and Public Health, 18(14), 7238. https://doi.org/10.3390/ijerph18147238









