1. Background
The history of chocolate began with the Maya, who were probably the first people in South America to cultivate the cocoa plant [
1]. For the Maya, chocolate was a cocoa drink prepared with hot water and often flavored with cinnamon and pepper. It was called the “Food of the Gods” and was presented at the table of Emperor Moctezuma II by the Aztecs [
1].
In 1502, Christopher Columbus was the first European to encounter cocoa. He captured a canoe that contained cocoa beans, which were considered “mysterious-looking almonds” and identified as a form of currency in Mesoamerica [
2,
3].
Cocoa appeared in Europe in 1528, when the Spanish conquistador Hernán Cortés brought samples of cocoa to King Charles of Spain, spreading the great effects of the beverage prepared from this “brown gold” [
3,
4]. It was in 1753 that the Swedish scientist Carl Linnaeus named the cocoa plant
Theobroma cacao, from the Latin name Theobroma [literally ‘food of the Gods’], and the Aztec word xocolatl [i.e., xococ (bitter) and atl (water)] [
5].
The characteristics of chocolate were long ignored in Europe owing to difficulties with an environment unfavorable to its growth. The natural habitat of the cocoa tree is the lower level of an evergreen rain forest. Cocoa plants respond well to relatively high temperatures (with a maximum annual average of 30–32 °C and minimum average of 18–21 °C) and generally high relative humidity: often as much as 100% during the day, falling to 70–80% at night [
6]. According to the latest published data of the International Cocoa Organization (ICCO), the total world production of cocoa beans in 2016–17 was 4,739,000 tons, principally from Africa (3,622,000 tons) [
7].
Demand for organic cocoa products is also expanding, as consumers are increasingly concerned about food security and other environmental issues. However, the organic cocoa market still represents a very small share of the total cocoa market, estimated at less than 0.5% of total production [
8].
In this review, we will discuss the main evidence relating to cocoa and chocolate, exploring the possible effects on human health related to their consumption.
2. Chocolate Varieties
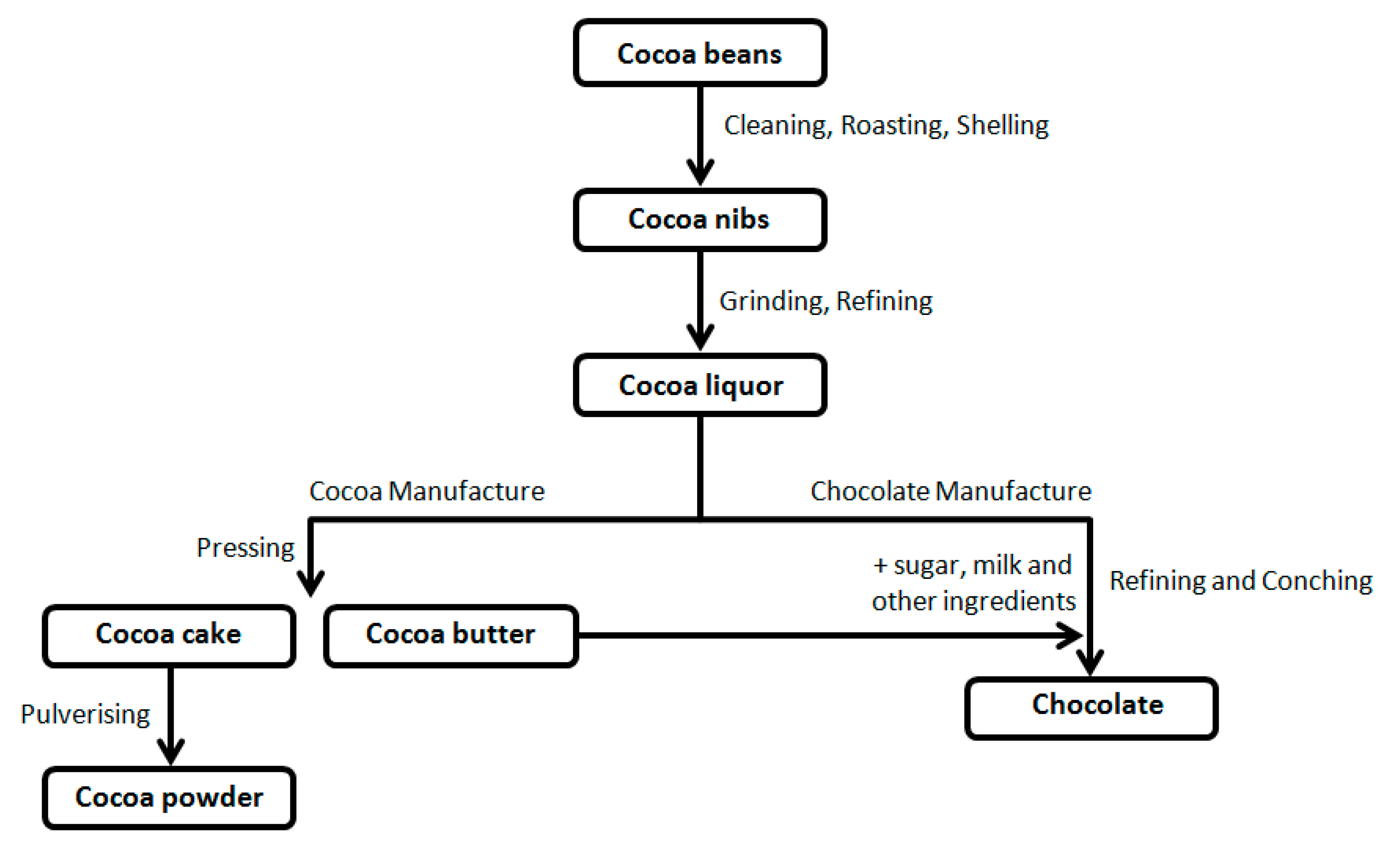
Starting from cocoa beans, through various processes of transformation (
Figure 1), the food industry produces different types of chocolate with defined ingredients and characteristics [
1,
9,
10,
11].
(1) Dark chocolate contains cocoa bean solids (up to 80% of the total weight) and cocoa butter. With the intense, persistent aroma of cocoa, it melts in the mouth, leaving a pleasant, bitter aftertaste. Its quality depends on the percentage of cocoa. Most of the health benefits attributable to chocolate are associated with consuming the dark type.
(2) Gianduja chocolate is a combination of hazelnuts, cocoa, and sugar; it is brown.
(3) Milk chocolate contains cocoa butter, sugar, milk powder, lecithin, and cocoa (the latter not less than 20–25%). With a bright appearance, it has an intense, persistent aroma and sweet taste with a slightly bitter accent of cocoa.
(4) White chocolate contains cocoa butter, milk, and sugar with no cocoa solids; it has a sweet, pleasant taste.
3. Nutritional Aspects
Cocoa, the basic ingredient in chocolate, contains a significant amount of fat (40–50% as cocoa butter, with approximately 33% oleic acid, 25% palmitic acid, and 33% stearic acid). It also contains polyphenols, which constitute about 10% of a whole bean’s dry weight [
12]. Cocoa bean is one of the best-known sources of dietary polyphenols, containing more phenolic antioxidants than most foods [
13]. Three groups of polyphenols can be identified in cocoa beans: catechins (37%), anthocyanidins (4%), and proanthocyanidins (58%); these flavonoids are the most abundant phytonutrients in cocoa beans [
14,
15,
16]. However, the bitterness caused by polyphenols makes unprocessed cocoa beans rather unpalatable. Manufacturers have, therefore, developed processing techniques for eliminating the bitterness. Such processes decrease the polyphenol content by up to 10-fold: for consumers the product is markedly different, mainly owing to the low-polyphenol content [
12,
15] and the other substances added during the processing phase (e.g., sugar, emulsifiers such as soy lecithin). It is well known that polyphenols are associated with beneficial effects, therefore cocoa (rich in polyphenols) and dark chocolate (with a high percentage of cocoa and higher phenolic antioxidant compounds compared to the other chocolate varieties [
13]) have assumed significant importance [
17].
The nitrogenous compounds of cocoa include both proteins and methylxanthines (theobromine and caffeine) [
18]. Cocoa is also rich in minerals: potassium, phosphorus, copper, iron, zinc, and magnesium [
18]. The nutritional values of cocoa and two types of chocolate appear in
Table 1 [
13,
19,
20].
4. Lights and Shadows in Chocolate and Cocoa Consumption
Chocolate consumption has recently increased around the world; dark chocolate, in particular, has become very popular for its high concentrations of cocoa and beneficial effects on human health compared with normal or milk chocolate [
21,
22,
23,
24]. In addition, milk chocolate could be associated with adverse effects due to its sugar content.
Therefore, only dark chocolate, with high percentages of cocoa, flavonoids, and theobromine and low content of sugar, differently from milk chocolate or other types of chocolate, would be associated with health-promoting effects [
11], including the prevention of cardiovascular disease. Similarly, cocoa induces positive effects on blood pressure, insulin resistance, and vascular function. It increases production of nitric oxide (NO) and has antioxidant effects, e.g., delayed oxidation of low-density lipoprotein (LDL) cholesterol and inhibiting ultraviolet-induced DNA oxidation [
25,
26].
The advantages and disadvantages of chocolate and cocoa consumption are discussed in the following sections, according to in vivo or in vitro studies.
4.1. Cardiovascular Effects
A series of beneficial effects on the cardiovascular system might occur following regular intake of cocoa-containing foods and beverages. Benefits include effects on blood pressure, insulin resistance, and vascular and platelet function [
25].
Polyphenols, abundant in cocoa and dark chocolate, activate endothelial NO synthase; that leads to generation of NO [
27], which lowers blood pressure by promoting vasodilation [
28,
29,
30,
31,
32,
33]. Indeed, following the consumption of dark chocolate, effects include improvement of the pulse wave speed and of the atherosclerotic score index, with parietal relaxation of large arteries and dilation of small and medium-sized peripheral arteries. Higher concentrations of plasma epicatechins help release endothelium-derived vasodilators and increase the concentration of plasma procyanidins, which leads to greater NO production and bioavailability [
32]. Once released, NO also activates the prostacyclin synthesis pathway, which acts as a vasodilator in synergy with NO, thereby contributing to thrombosis protection [
17]. Further, the anti-inflammatory and vasoprotective properties of prostacyclin are enhanced by its ability to reduce plasma leukotrienes [
17,
34,
35].
A meta-analysis of randomized trials report that both acute and chronic chocolate and cocoa ingestion effectively increased flow-mediated vasodilatation, reduced systolic and diastolic blood pressure, and reduced serum insulin levels [
36]. In young and healthy adults, a daily ingestion of 20 g of higher cocoa chocolate (90%) for a 30-day period improved vascular function by reducing central brachial artery pressures and promoting vascular relaxation [
37]. A Swedish prospective study linked chocolate consumption (≥3–4 servings/week) with lower risk of myocardial infarction and ischemic heart disease [
38]. On the other hand, a large prospective study exploring data from 83,310 postmenopausal women free of pre-existing major chronic diseases found no association between chocolate consumption and risk of coronary heart disease, stroke, or both combined. Conversely, an increased risk existed among women less than 65 years, in the highest quintile of chocolate consumption [
39]. A lack of association between chocolate intake and risk of atrial fibrillation was also reported in a large cohort of United States male physicians [
40]. Another population-based, prospective study on 20,992 participants failed to demonstrate an association between high chocolate intake (up to 100 g/day) and incident heart failure [
41]. A systematic review suggested that regular chocolate use (<100 g/week) may be linked with reduced cardiovascular risk, and that the most appropriate dose of chocolate consumption was 45 g/week, since higher levels might counteract the health benefits due to adverse effects linked with elevated sugar consumption [
42]. These findings were similar to results from a large cohort of Swedish men, which showed a J-shaped association between chocolate consumption and incidence of heart failure, with protective effects absent in subjects consuming ≥1 serving per day [
43].
Cocoa plays also a role in treating cerebral conditions, such as stroke; in fact, cocoa intake is associated with increased cerebral blood flow [
44]. In the same way, daily chocolate consumption may reduce the likelihood of a stroke attack [
18,
45]. However, a large Japanese population-based, prospective cohort study reported an association between chocolate consumption and lower risk of stroke in women but not in men [
26].
Table 2 shows the studies on cardiovascular effects related to cocoa or chocolate consumption.
4.2. Glucose Homeostasis
Cocoa components offer potential as antidiabetic agents, especially with type 2 diabetes mellitus (T2D). This aspect is of particular relevance owing to the emerging worldwide epidemic of metabolic syndrome, including obesity, T2D, and dyslipidemia [
46].
Cocoa and flavonols improve glucose homeostasis by slowing carbohydrate digestion and absorption in the gut [
47,
48]. Indeed, cocoa extracts and procyanidins dose-dependently inhibit pancreatic α-amylase, pancreatic lipase, and secreted phospholipase A2 [
48,
49]. Cocoa and its flavonols improve insulin sensitivity by regulating glucose transport and insulin signaling proteins in insulin-sensitive tissues (liver, adipose tissue, and skeletal muscle) preventing in these tissues oxidative and inflammatory damage associated with the disease [
47]. In younger and normal body-weight men, the results from the Physicians’ Health Study reported an inverse relation of chocolate consumption with incident diabetes [
50]. In a multiethnic United States cohort, authors found a lower risk of developing T2D in subjects with the highest intake of chocolate products and cocoa-derived flavonoids [
51]. A dose-response meta-analysis, however, suggested a nonlinear association between chocolate consumption and the risk of T2D, with a peak protective effect at 2 servings/week and no benefit recorded when increasing consumption was above 6 servings/week [
52].
A prospective study in a large number of Japanese pregnant women also showed a lower risk of gestational diabetes in subjects in the highest quartile of chocolate consumption [
53].
The observed effects on glucose homeostasis seem to be strongly dependent on the amount of polyphenols. In fact, a single-blind randomized placebo-controlled cross-over study showed, after 4 weeks, negative metabolic effects (i.e., raised fasting insulin, insulin resistance, and salivary cortisol) in subjects consuming 20 g/day dark chocolate with negligible polyphenol content but not in those consuming the same amount of polyphenol-rich (500 mg) chocolate [
54].
Therefore, the daily consumption of small quantities of flavonols from cocoa or chocolate, associated with a dietary intake of flavonoids, would constitute a natural and economic approach to prevent or potentially contribute to the treatment of T2D with minimal toxicity and negative side effects [
47]. However, most commercially available soluble cocoa products or chocolates contain low amount of flavonols and are rich in sugar and calories. Therefore, high consumption of chocolate will induce paradoxical consequences, i.e., weight gain and impaired glucose homeostasis, especially in T2D patients and obese individuals [
48].
Table 3 shows the studies on glucose homeostasis effects related to cocoa or chocolate use.
4.3. Cancer
Results regarding the effects of cocoa/chocolate consumption on cancer are rather controversial. Early studies suggested that excess chocolate intake could be a predisposing factor to tumor development (as colorectal and breast cancer) [
55,
56].
According to other in vitro studies, cocoa inhibits the growth of cancer cells; however, the exact anticancer mechanisms are poorly understood [
57,
58].
Some authors demonstrated that cocoa liquor procyanidins significantly reduced the incidence and multiplicity of lung carcinomas and decreased thyroid adenomas developed in male rats, and inhibited mammary and pancreatic tumorigenesis in female rats [
59,
60]. Cocoa procyanidins also reduced vascular endothelial growth factor activity and angiogenic activity associated with tumor, determining down-regulation of tyrosine kinase ErbB2 [
61].
In the last years, the treatment of different ovarian cancer cell lines with various concentrations of cocoa procyanidin-rich extract, inducing cytotoxicity and chemosensitization, showed a significant percentage of cells in sub-G1/G0 (hypodiploid) phase, which increased with increasing concentration, and a significant accumulation of cells in the S phase was seen [
62]. This effect is probably due to an increase in intracellular levels of reactive oxygen species (ROS) [
63]. In an animal study, a diet containing dark chocolate reduced the total number of aberrant crypt foci in the colon. The effect was associated with down-regulation in the transcription levels of both COX-2 and ReIA [
64]. In addition, cocoa significantly decreased the tumor incidence and size in mice with colitis-associated cancer [
65].
At present, further translational and prospective studies need to explore the intrinsic mechanisms of cocoa’s anticancer action to support its use as a co-adjuvant in preventing and treating cancer [
18].
Table 4 shows the studies on cancer related to cocoa or chocolate use.
4.4. Obesity and Lipid Metabolism
Recently, some studies have investigated the preventive or therapeutic effects of cocoa and cocoa constituents against obesity and metabolic syndrome [
66]. Administering cocoa to rats decreased visceral adipose tissue [
67]. DNA analysis conducted on the liver and mesenteric fat tissue provided interesting clues. In that study, the authors observed decreased expression of various genes associated with fatty acid transport and synthesis in the liver and mesenteric fat as well as increased expression of genes associated with thermogenesis [
18,
67].
In a clinical study, smelling dark chocolate was assessed to evaluate an appetite response. Chocolate produced a satiation response and reduced appetite; thus, it could be helpful in preventing weight gain [
68]. Further, flavonoids can produce metabolic events that induce reduction of lipogenesis, induction of lipolysis, and increased adiponectin secretion; such events reduce lipid deposition and insulin resistance, thus mitigating obesity [
17].
A study reported a significantly greater and dose-dependent weight gain over time in subjects with more frequent chocolate consumption. However, no information was provided about the consumer profile of enrolled subjects and the type of chocolate consumed (in particular, the specific amount of dark chocolate) [
69].
A recent meta-analysis reported the lack of effects of cocoa or dark chocolate on weight, body mass index (BMI), and waist circumference. However, a subgroup analysis showed reduced weight and BMI following cocoa/dark chocolate supplementation ≥ 30 g chocolate per day in trials between 4–8 weeks, pointing to the relevant role of the consumed dose and trial duration [
70].
Dark chocolate might also operate in combination with other nutraceuticals, and have positive effects on lipid profile. Our group has recently reported distinct effects of 24 g almond varieties on organoleptic features and on gastrointestinal function (gallbladder and gastric emptying, orocecal transit) in healthy subjects [
71]. One 4-week crossover feeding trial among 31 overweight or obese adults determined that daily consumption of almonds (42 g/day) alone or combined with dark chocolate was beneficial for total cholesterol, low-density (LDL) lipoprotein cholesterol, and apolipoprotein B concentrations. The authors concluded that incorporating almonds, dark chocolate, and cocoa into a diet without exceeding energy needs could reduce the risk of coronary heart disease [
72].
A meta-analysis showed that, in the short term (2–12 weeks), dark chocolate/cocoa consumption can significantly lower total and LDL cholesterol levels, but has no effect on high-density lipoprotein HDL and triglycerides [
73]. Similar results derive from a placebo-controlled cross-over study, in which daily consumption of cocoa flavonol-containing dark chocolate bars with added plant sterols significantly reduced serum total and LDL cholesterol [
74].
Normal weight obese syndrome consists of an excessive body fat associated with a normal BMI, and a higher risk for cardiovascular morbidity and mortality. A group of normal weight obese women consuming dark chocolate (100 g/day, 70% cocoa) for a short period (one week) displayed a rise in the HDL cholesterol levels, and a decrease of the LDL/HDL cholesterol ratio and abdomen circumference. The authors concluded that the regular consumption of dark chocolate would help in maintaining a good atherogenic profile, due to the favorable effects on HDL cholesterol, lipoprotein ratios, and possibly on inflammation markers [
75].
Table 5 shows the studies on obesity and lipid metabolism related to cocoa or chocolate use.
4.5. Intestinal Microbiota
In recent years, there is a growing interest in the study of intestinal microbiota and its changes as result of a particular diet. The human gut harvests the intestinal microbiota, a huge collection of microbes with a key role in energy storage and metabolic disorders [
76]. Whereas flavonol monomers and dimers are absorbed in the small intestine, procyanidins undergo metabolization by colonic microbiota, with production of phenolic acids, subsequently absorbed, metabolized in the liver, and eliminated in the urine or in feces [
77,
78,
79,
80]. Thus, gut microbiota is responsible for the metabolization of polyphenols in other bio-active compounds (i.e., valerolactones [
81], and various phenolic acids [
82]) with potential anti-inflammatory properties [
17].
A study conducted on rats fed with cocoa diet for 6 weeks highlighted a significant reduction of percent of
Bacteroides,
Clostridium, and
Staphylococcus, changes of tool-like reception expression, and a reduction of immunoglobulin A intestinal secretion, significantly correlated with the decrease in the proportion of the
Clostridium and
Streptococcus [
78].
In pigs, cocoa consumption, in addition to determining changes in metabolites in biofluids and tissues, as the increase in O-methyl-epicatechin glucuronide conjugates in serum, urine, and visceral adipose tissue, induced a significant increase of the abundance of
Lactobacillus species from the
casei group in feces and
Bifidobacterium species in proximal colon contents [
83].
Tzounis et al. [
79] conducted the first human-intervention study designed to investigate the influence of high cocoa flavanol intake on the growth of the human fecal microbiota. In particular, these authors assessed that the intake of 494 mg of cocoa flavonoids/ day for 4 weeks had a significant effect on intestinal microbiota growth.
Table 6 shows the studies on intestinal microbiota related to cocoa or chocolate use.
4.6. Immune System
In vivo and in vitro studies showed that cocoa has regulatory properties on the immune cells implicated in both innate and acquired immunity. In animals, these effects are present at systemic and intestinal level [
84,
85]. In Lewis rats a 10% cocoa diet or a 0.25% theobromine diet were both able, after one week, to lower serum concentrations of IgG, IgM, IgA, and intestinal IgA, as compared with control diet. Both cocoa and theobromine modified the thymocyte composition increasing CD4-CD8- and CD4+CD8- proportions, and changed the composition of mesenteric lymph node (reduced percentage of T-helper) and spleen (increased proportion of T-helper). Taken together, the data suggest that theobromine is the agent mediating the major immunoregulatory effects of cocoa [
86]. Dark chocolate consumption was found having anti-inflammatory effects in a 4-week randomized clinical trial, which was especially visible in the reduced post-challenge responses of cytokines, vascular markers, white blood cells, and leukocyte-activation markers [
87,
88].
Regular cocoa consumption could be related to preventing or improving health imbalance induced by allergic processes [
89]. The positive effects of cocoa flavonoids on the immune system (related to several allergic mechanisms) are known, such as reducing the release of mediators, restoring the balance of T-helper 1 and T-helper 2 cells [
90], and down-regulation of IgE production [
89,
91]. By contrast, chocolate is one of the main potentially allergenic foods that is also capable of causing hypersensitivity reactions, manifesting different clinical symptoms (e.g., fatigue, irritability, insomnia, headache, asthma, and diarrhea) which appear in a few hours or days after food intake [
92].
Table 7 shows the studies on the immune system related to cocoa or chocolate use.
4.7. Central Nervous System
There is evidences of some beneficial effects on the central nervous system, but larger, prospective studies are missing, so far.
In healthy volunteers, the ingestion of 100 g dark chocolate (72% cocoa) increased [
18F] fluorodeoxyglucose (
18F-FDG) uptake in the visual cortex, in somatosensory, motor, and pre-frontal cortices, as shown by combined positron emission tomography-computed tomography (PET-CT) [
22]. These findings point to dark chocolate-dependent acute effects on cerebral function [
22]. The polyphenols in dark chocolate could act on the central nervous system (CNS) and neurological functions through the production of NO [
11,
17]. Vasodilation and increased cerebral blood flow provide oxygen and glucose to neurons, leading to increased formation of blood vessels in the hippocampus [
11,
93]. The polyphenol-dependent antioxidant potential could contribute to amelioration of some neurodegenerative disorders [
11,
93,
94]. This inference is based on the fact that age-related cognitive impairment and disorders, such as Alzheimer’s and Parkinson’s diseases, are related to the accumulation of reactive oxygen species in the brain [
11,
94,
95].
The effect of cocoa bioactives on signaling pathways in neurocytes may provide another support for linking dark chocolate with regulation of brain function [
11]. Cocoa flavonols and methylxanthines can activate the cascade pathways of such molecules as rapamycin that play a crucial role in synaptic function, neuronal growth, memory mechanisms, and the pathogenesis of neurodegenerative disorders [
96].
A prospective study on elderly subjects (age ≥65 years) with normal mini-mental state examination at entry showed that chocolate intake was linked with a decreased risk of cognitive decline during a median follow up of 48 months [
97]. Results from a cross-sectional analysis in subjects aged 23–98 years showed a better cognitive performance in those consuming chocolate more frequently. However, following a prospective observation, a relationship between cognitive function and chocolate intake was not confirmed when measured up to 18 years later [
98].
4.8. Psychological Aspects
The social and psychological context of everyday life affects metabolic health, emotions, and moods; it can play a role in determining dietary choices [
99,
100]. In some cases, chocolate consumption can be indirectly associated with a form of depression: hysteroid dysphoria. This condition involves frequent episodes of depression in response to feeling inadequate or socially rejected, which culminates in true bulimic attacks for confectionery and chocolate. A true chocolate addiction (being chocoholic) is akin to alcoholism and nicotine dependence; it affects 40% of the female and 15% of the male population in Western countries [
101]. The symptoms involve being responsive to drugs that enhance serotonin transmission; this suggests that central serotonin pathways may be involved in chocolate consumption. The presence of serotonin could explain why sugar and confectionery are strongly desired during chocolate bulimic crises. The ingestion of carbohydrates (e.g., bread and chocolate) increases the relationship between plasma tryptophan and other neutral amino acids; consequently, the transport of tryptophan through the blood–brain barrier is activated, with an increase in cerebral serotonin synthesis, which produces a feeling of energy and pleasure [
102].
4.9. Sexual Aspects
Chocolate exerts several effects on human sexuality, mainly acting as an aphrodisiac [
103]. Cocoa powder and chocolate contain three unsaturated N-acylethanolamines, which, acting as cannabinoid mimics, could activate cannabinoid receptors or increase anandamide concentrations [
103,
104]. The latter, in conjunction with other components of chocolate (such as caffeine and theobromine), produces a transient feeling of well-being. Anandamide enhances sexual performance in male rats [
103,
105]. Moreover, serotonin has been found in several regions of the female genital tract in humans and other animals, where it acts on vasoconstriction and vasodilatation. The principal component of sexual arousal is peripheral vasocongestion of genital tissues; thus, serotonin could be involved in the process of sexual stimulation [
103].
Table 8 shows the studies on the nervous system, and psychological and sexual aspects related to cocoa or chocolate use.
5. Conclusions
Cocoa and chocolate act as functional foods, since both carry a number of substances contributing to beneficial health effects. Chocolate combines some organoleptic characteristics with aphrodisiac and antidepressant properties, extending its effects beyond the cardiovascular system, metabolic diseases, CNS diseases, and psychological profiles.
We should stress that several studies evaluated the health-promoting properties of cocoa and not of chocolate itself.
Moreover, because in chocolate processing, cocoa loses some of the polyphenol compounds (the main constituents responsible for the beneficial effects on health), we think that the role of chocolate on human health cannot be completely compared to that of cocoa. Despite the availability of a number of in vitro and experimental reports, epidemiological studies assessing possible beneficial effects of chocolate (in particular dark chocolate) are still scarce. One should keep in mind the presence of a number of confounders (i.e., other diet components, lifestyle, environmental exposures, exact consumption of chocolate, chocolate composition, duration of observation, and other risk factors). Such conditions strongly limit the strength of evidences.
In conclusion, further translational studies need to evaluate all possible effects related to consuming chocolate and to verify in humans the effects hitherto demonstrated only in vitro and on animals. This approach could suggest how best to consume (in terms of dose, mode, and time) chocolate in the daily diet, considering eating habits and lifestyle.