Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review
Abstract
1. Introduction
1.1. Physical Inactivity among Pregnant Women
1.2. Benefits of Physical Activity during Pregnancy
1.3. Intervention on Prenatal Physical Activities
1.4. Objectives
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Summary
2.4. Critical Appraisal on Methodological Quality of Included Studies
3. Results
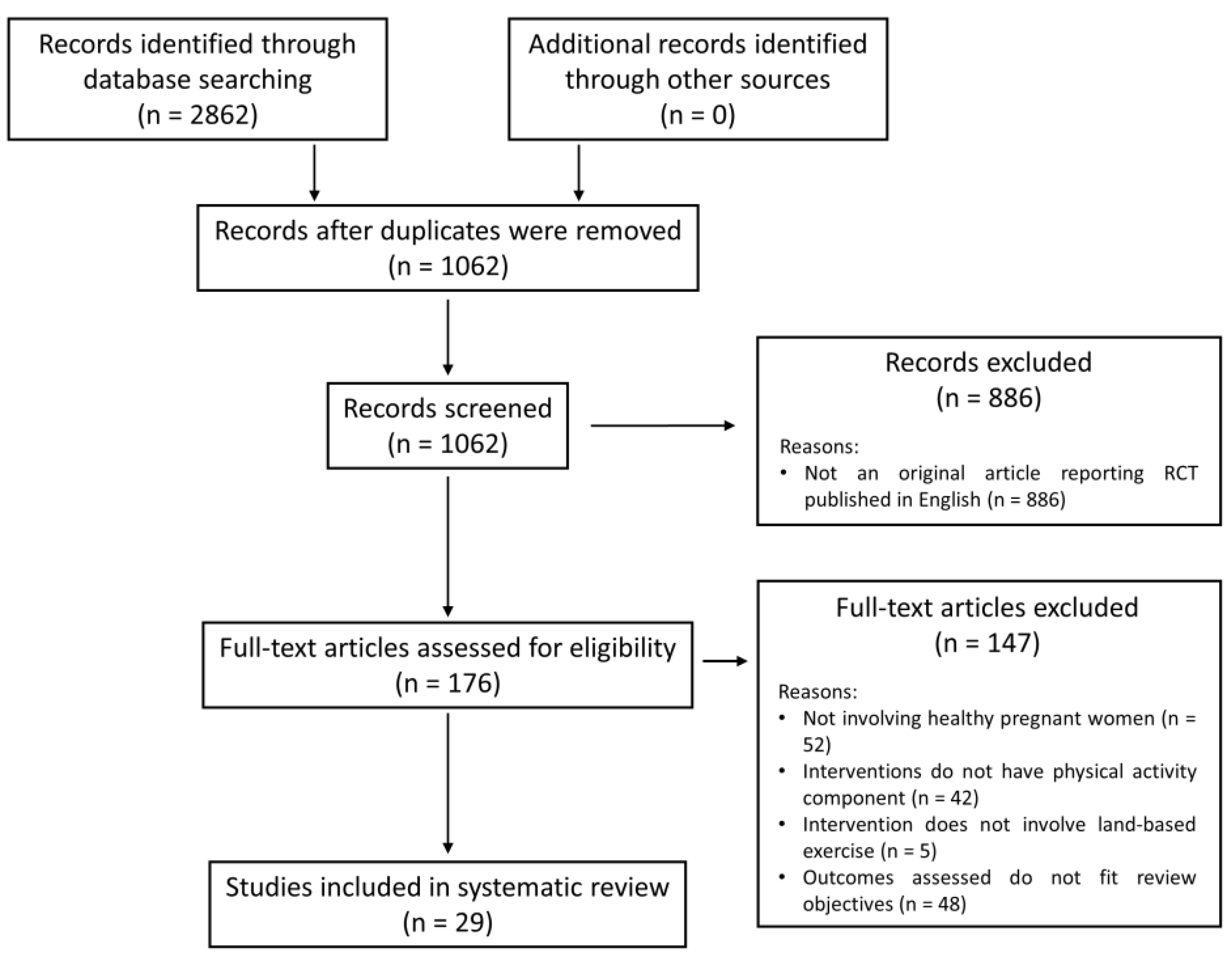
3.1. Search Results
3.1.1. Methodological Quality of Included Studies
3.1.2. Characteristics of Included Studies
3.1.3. Effects of Physical Activity Interventions on Pregnancy-Related Outcomes
Intervention Content
Intervention Dosage
4. Discussion
Implications for Practice and Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benefits of Regular Physical Activity. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/benefits-of-regular-physical-activity (accessed on 15 March 2019).
- Global Recommendations on Physical Activity for Health: 18–64 Years Old. Available online: http://www.who.int/dietphysicalactivity/physical-activity-recommendations-18-64years.pdf?ua=1 (accessed on 15 March 2019).
- Global Recommendations on Physical Activity for Health. Available online: http://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf;jsessionid=712481861C37570B9F9C546472F8F3F4?sequence=1 (accessed on 15 March 2019).
- Level of Physical Activity by WHO Recommendations. Available online: https://www.chp.gov.hk/en/statistics/data/10/280/6626.html (accessed on 15 March 2019).
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [PubMed]
- Downs, D.S.; Chasan-Taber, L.; Evenson, K.R.; Leiferman, J.; Yeo, S. Physical activity and pregnancy: Past and present evidence and future recommendations. Res. Q. Exerc. Sport 2012, 83, 485–502. [Google Scholar] [CrossRef]
- Ruifrok, A.E.; Althuizen, E.; Oostdam, N.; van Mechelen, W.; Mol, B.W.; de Groot, C.J.; van Poppel, M.N. The relationship of objectively measured physical activity and sedentary behaviour with gestational weight gain and birth weight. J. Pregnancy 2014, 2014, 567379. [Google Scholar] [PubMed]
- Padmapriya, N.; Shen, L.; Soh, S.E.; Shen, Z.; Kwek, K.; Godfrey, K.M.; Gluckman, P.D.; Chong, Y.S.; Saw, S.M.; Müller-Riemenschneider, F. Physical activity and sedentary behavior patterns before and during pregnancy in a multi-ethnic sample of Asian women in Singapore. Matern. Child Health J. 2015, 19, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Gaston, A.; Cramp, A. Exercise during pregnancy: A review of patterns and determinants. J. Sci. Med. Sport 2011, 14, 299–305. [Google Scholar]
- Fazzi, C.; Saunders, D.H.; Linton, K.; Norman, J.E.; Reynolds, R.M. Sedentary behaviours during pregnancy: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 32. [Google Scholar] [CrossRef]
- Evenson, K.R.; Savitz, D.A.; Huston, S.L. Leisure-time physical activity among pregnant women in the US. Paediatr. Perinat. Epidemiol. 2004, 18, 400–407. [Google Scholar] [PubMed]
- Löf, M. Physical activity pattern and activity energy expenditure in healthy pregnant and non-pregnant Swedish women. Eur. J. Clin. Nutr. 2011, 65, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Meijer, O.G.; Uegaki, K.; Mens, J.M.; van Dieën, J.H.; Wuisman, P.I.; Ostgaard, H.C. Pregnancy-related pelvic girdle pain (PPP), I: Terminology, clinical presentation, and prevalence. Eur. Spine J. 2004, 13, 575–589. [Google Scholar] [PubMed]
- Littleton, H.L.; Breitkopf, C.R.; Berenson, A.B. Correlates of anxiety symptoms during pregnancy and association with perinatal outcomes: A meta-analysis. Am. J. Obstet. Gynecol. 2007, 196, 424–432. [Google Scholar]
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Lardon, E.; St-Laurent, A.; Babineau, V.; Descarreaux, M.; Ruchat, S.M. Lumbopelvic pain, anxiety, physical activity and mode of conception: A prospective cohort study of pregnant women. BMJ Open 2018, 8, e022508. [Google Scholar] [CrossRef] [PubMed]
- De Wit, L.; Jelsma, J.G.; van Poppel, M.N.; Bogaerts, A.; Simmons, D.; Desoye, G.; Corcoy, R.; Kautzky-Willer, A.; Harreiter, J.; van Assche, A.; et al. Physical activity, depressed mood and pregnancy worries in European obese pregnant women: Results from the DALI study. BMC Pregnancy Childbirth 2015, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Moos, M.K.; Carrier, K.; Siega-Riz, A.M. Perceived barriers to physical activity among pregnant women. Matern. Child Health J. 2009, 13, 364–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Zuo, J.; Hu, X.; Zhang, H.; Zhao, Y. Physical activity level of urban pregnant women in Tianjin, China: A cross-sectional study. PLoS ONE 2014, 9, e109624. [Google Scholar] [CrossRef]
- Lee, D.T.; Ngai, I.S.; Ng, M.M.; Lok, I.H.; Yip, A.S.; Chung, T.K. Antenatal taboos among Chinese women in Hong Kong. Midwifery 2009, 25, 104–113. [Google Scholar] [CrossRef]
- Clarke, P.E.; Rousham, E.K.; Gross, H.; Halligan, A.W.; Bosio, P. Activity patterns and time allocation during pregnancy: A longitudinal study of British women. Ann. Hum. Biol. 2005, 32, 247–258. [Google Scholar] [CrossRef]
- Fell, D.B.; Joseph, K.S.; Armson, B.A.; Dodds, L. The impact of pregnancy on physical activity level. Matern. Child Health J. 2009, 13, 597–603. [Google Scholar] [CrossRef]
- Ning, Y.; Williams, M.A.; Dempsey, J.C.; Sorensen, T.K.; Frederick, I.O.; Luthy, D.A. Correlates of recreational physical activity in early pregnancy. J. Matern. Fetal Neonatal Med. 2003, 13, 385–393. [Google Scholar] [CrossRef]
- Pereira, M.A.; Rifas-Shiman, S.L.; Kleinman, K.P.; Rich-Edwards, J.W.; Peterson, K.E.; Gillman, M.W. Predictors of change in physical activity during and after pregnancy: Project viva. Am. J. Prev. Med. 2007, 32, 312–319. [Google Scholar] [CrossRef]
- Schmidt, M.D.; Pekow, P.; Freedson, P.S.; Markenson, G.; Chasan-Taber, L. Physical activity patterns during pregnancy in a diverse population of women. J. Women’s Health (Larchmt) 2006, 15, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Phelan, S. Pregnancy: A “teachable moment” for weight control and obesity prevention. Am. J. Obstet. Gynecol. 2010, 202, 135.e1–135.e8. [Google Scholar] [CrossRef] [PubMed]
- Streuling, I.; Beyerlein, A.; Rosenfeld, E.; Hofmann, H.; Schulz, T.; von Kries, R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG 2011, 118, 278–284. [Google Scholar] [CrossRef]
- Dempsey, J.C.; Butler, C.L.; Sorensen, T.K.; Lee, I.M.; Thompson, M.L.; Miller, R.S.; Frederick, I.O.; Williams, M.A. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2004, 66, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Mørkrid, K.; Jenum, A.K.; Berntsen, S.; Sletner, L.; Richardsen, K.R.; Vangen, S.; Holme, I.; Birkeland, K.I. Objectively recorded physical activity and the association with gestational diabetes. Scand. J. Med. Sci. Sports 2014, 24, e389–e397. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Middleton, P.; Crowther, C.A. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2012, 7, CD009021. [Google Scholar] [CrossRef]
- Van Poppel, M.N.; Ruchat, S.M.; Mottola, M.F. Physical activity and gestational diabetes mellitus. Med. Sport Sci. 2014, 60, 104–112. [Google Scholar]
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Physical activity and the risk of preeclampsia: A systematic review and meta-analysis. Epidemiology 2014, 25, 331–343. [Google Scholar] [CrossRef]
- Kasawara, K.T.; do Nascimento, S.L.; Costa, M.L.; Surita, F.G.; e Silva, J.L. Exercise and physical activity in the prevention of pre-eclampsia: Systematic review. Acta Obstet. Gynecol. Scand. 2012, 91, 1147–1157. [Google Scholar] [CrossRef]
- Davenport, M.H.; Marchand, A.A.; Mottola, M.F.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Sobierajski, F.; James, M.; Meah, V.L.; et al. Exercise for the prevention and treatment of low back, pelvic girdle and lumbopelvic pain during pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 90–98. [Google Scholar] [CrossRef]
- Hinckley, A.F.; Bachand, A.M.; Reif, J.S. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ. Health Perspect. 2005, 113, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Montejo, R.; Luaces, M.; Zakynthinaki, M. Exercise during pregnancy improves maternal health perception: A randomized controlled trial. Am. J. Obstet. Gynecol. 2011, 204, e402–e407. [Google Scholar] [CrossRef] [PubMed]
- Gaston, A.; Prapavessis, H. Tired, moody and pregnant? Exercise may be the answer. Psychol. Health 2013, 28, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Fitzgerald, E.M.; Cardinal, B.J. Physical activity and depression symptoms among pregnant women from the National Health and Nutrition Examination Survey 2005–2006. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 227–235. [Google Scholar] [CrossRef]
- Perales, M.; Refoyo, I.; Coteron, J.; Bacchi, M.; Barakat, R. Exercise during pregnancy attenuates prenatal depression: A randomized controlled trial. Eval. Health Prof. 2015, 38, 59–72. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Montejo, R.; Refoyo, I.; Coteron, J. Exercise throughout pregnancy does not cause preterm delivery: A randomized, controlled trial. J. Phys. Act. Health 2014, 11, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Haakstad, L.A.; Bø, K. Exercise in pregnant women and birth weight: A randomized controlled trial. BMC Pregnancy Childbirth 2011, 11, 66. [Google Scholar] [CrossRef]
- Evenson, K.R.; Barakat, R.; Brown, W.J.; Dargent-Molina, P.; Haruna, M.; Mikkelsen, E.M.; Mottola, M.F.; Owe, K.M.; Rousham, E.K.; Yeo, S. Guidelines for Physical Activity during Pregnancy: Comparisons From Around the World. Am. J. Lifestyle Med. 2014, 8, 102–121. [Google Scholar] [CrossRef]
- Harrison, C.L.; Lombard, C.B.; Teede, H.J. Limiting postpartum weight retention through early antenatal intervention: The HeLP-her randomised controlled trial. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 134. [Google Scholar] [CrossRef]
- Hawkins, M.; Chasan-Taber, L.; Marcus, B.; Stanek, E.; Braun, B.; Ciccolo, J.; Markenson, G. Impact of an exercise intervention on physical activity during pregnancy: The behaviors affecting baby and you study. Am. J. Public Health 2014, 104, e74–e81. [Google Scholar] [CrossRef]
- Lewis, B.A.; Martinson, B.C.; Sherwood, N.E.; Avery, M.D. A pilot study evaluating a telephone-based exercise intervention for pregnant and postpartum women. J. Midwifery Women’s Health 2011, 56, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Gaston, A.; Prapavessis, H. Maternal-fetal disease information as a source of exercise motivation during pregnancy. Health Psychol. 2009, 28, 726–733. [Google Scholar] [CrossRef]
- Currie, S.; Sinclair, M.; Murphy, M.H.; Madden, E.; Dunwoody, L.; Liddle, D. Reducing the decline in physical activity during pregnancy: A systematic review of behavior change interventions. PLoS ONE 2013, 8, e66385. [Google Scholar] [CrossRef]
- Huang, T.T.; Yeh, C.Y.; Tsai, Y.C. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: A randomised controlled trial. Midwifery 2011, 27, 257–264. [Google Scholar] [CrossRef]
- Luoto, R.; Kinnunen, T.I.; Aittasalo, M.; Kolu, P.; Raitanen, J.; Ojala, K.; Mansikkamäki, K.; Lamberg, S.; Vasankari, T.; Komulainen, T.; et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: A cluster-randomized controlled trial. PLoS Med. 2011, 8, e1001036. [Google Scholar] [CrossRef]
- Callaway, L.K.; Colditz, P.B.; Byrne, N.M.; Lingwood, B.E.; Rowlands, I.J.; Foxcroft, K.; McIntyre, H.D.; BAMBINO Group. Prevention of gestational diabetes: Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010, 33, 1457–1459. [Google Scholar] [CrossRef]
- Guelinckx, I.; Devlieger, R.; Mullie, P.; Vansant, G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 373–380. [Google Scholar] [CrossRef]
- Hui, A.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Murray, R.; Morris, M.; Shen, G.X. Community-based exercise and dietary intervention during pregnancy: A pilot study. Can. J. Diabetes 2006, 30, 1–7. [Google Scholar] [CrossRef]
- Grunebaum, A.; Dudenhausen, J.W.; Ross, C.M. Compliance with the institute of medicine gestational weight gain recommendations in teenage pregnancies. Obstet. Gynecol. 2015, 125 (Suppl. 5), 37S. [Google Scholar] [CrossRef]
- Evans, N.; Lasen, M.; Tsey, K. A Systematic Review of Rural Development Research, 1st ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 45–50. [Google Scholar]
- Picot, J.; Hartwell, D.; Harris, P.; Mendes, D.; Clegg, A.J.; Takeda, A. The effectiveness of interventions to treat severe acute malnutrition in young children: A systematic review. Health Technol. Assess. 2012, 16, 121–126. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.A.; Romundstad, P.R.; Stuge, B.; Mørkved, S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2012, 91, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Songoygard, K.M.; Stafne, S.N.; Evensen, K.A.; Salvesen, K.A.; Vik, T.; Morkved, S. Does exercise during pregnancy prevent postnatal depression? A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2012, 91, 62–67. [Google Scholar] [CrossRef]
- Gustafsson, M.K.; Stafne, S.N.; Romundstad, P.R.; Mørkved, S.; Salvesen, K.; Helvik, A.S. The effects of an exercise program during pregnancy on health-related quality of life in pregnant women: A Norwegian randomised controlled trial. BJOG 2016, 123, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.H.; Stuge, B.; Mowinckel, P.; Jensen, K.S.; Hagen, K.B. Can supervised group exercises including ergonomic advice reduce the prevalence and severity of low back pain and pelvic girdle pain in pregnancy? A randomized controlled trial. Phys. Ther. 2012, 92, 781–790. [Google Scholar] [CrossRef]
- Sagedal, L.R.; Øverby, N.C.; Bere, E.; Torstveit, M.K.; Lohne-Seiler, H.; Småstuen, M.; Hillesund, E.R.; Henriksen, T.; Vistad, I. Lifestyle intervention to limit gestational weight gain: The Norwegian Fit for Delivery randomised controlled trial. BJOG 2017, 124, 97–109. [Google Scholar] [CrossRef]
- Haakstad, L.A.; Vistad, I.; Sagedal, L.R.; Lohne-Seiler, H.; Torstveit, M.K. How does a lifestyle intervention during pregnancy influence perceived barriers to leisure-time physical activity? The Norwegian fit for delivery study, a randomized controlled trial. BMC Pregnancy Childbirth 2018, 18, 127. [Google Scholar] [CrossRef]
- Haakstad, L.A.; Bø, K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: A randomised controlled trial. Eur. J. Contracept. Reprod. Health Care 2011, 16, 116–125. [Google Scholar] [CrossRef]
- Haakstad, L.A.; Bø, K. Effect of a regular exercise program on pelvic girdle and low back pain in previously inactive pregnant women: A randomized controlled trial. J. Rehabil. Med. 2015, 47, 229–234. [Google Scholar] [CrossRef]
- Haakstad, L.A.; Torset, B.; Bø, K. What is the effect of regular group exercise on maternal psychological outcomes and common pregnancy complaints? An assessor blinded RCT. Midwifery 2016, 32, 81–86. [Google Scholar] [CrossRef]
- Ozdemir, S.; Bebis, H.; Ortabag, T.; Acikel, C. Evaluation of the efficacy of an exercise program for pregnant women with low back and pelvic pain: A prospective randomized controlled trial. J. Adv. Nurs. 2015, 71, 1926–1939. [Google Scholar] [CrossRef]
- Aşcı, O.; Rathfisch, G. Effect of lifestyle interventions of pregnant women on their dietary habits, lifestyle behaviors, and weight gain: A randomized controlled trial. J. Health Popul. Nutr. 2016, 35, 7. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Perales, M.; Pelaez, M.; Lopez, C.; Lucia, A.; Barakat, R. Supervised exercise-based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef]
- Kokic, I.S.; Ivanisevic, M.; Uremovic, M.; Kokic, T.; Pisot, R.; Simunic, B. Effect of therapeutic exercises on pregnancy-related low back pain and pelvic girdle pain: Secondary analysis of a randomized controlled trial. J. Rehabil. Med. 2017, 49, 251–257. [Google Scholar] [CrossRef]
- Kinnunen, T.I.; Pasanen, M.; Aittasalo, M.; Fogelholm, M.; Hilakivi-Clarke, L.; Weiderpass, E.; Luoto, R. Preventing excessive weight gain during pregnancy—A controlled trial in primary health care. Eur. J. Clin. Nutr. 2007, 61, 884–891. [Google Scholar] [CrossRef]
- Ronnberg, A.K.; Ostlund, I.; Fadl, H.; Gottvall, T.; Nilsson, K. Intervention during pregnancy to reduce excessive gestational weight gain—A randomised controlled trial. BJOG 2015, 122, 537–544. [Google Scholar] [CrossRef]
- Miquelutti, M.A.; Cecatti, J.G.; Makuch, M.Y. Evaluation of a birth preparation program on lumbopelvic pain, urinary incontinence, anxiety and exercise: A randomized controlled trial. BMC Pregnancy Childbirth 2013, 13, 154. [Google Scholar] [CrossRef]
- Da Silva, S.G.; Hallal, P.C.; Domingues, M.R.; Bertoldi, A.D.; Silveira, M.F.D.; Bassani, D.; da Silva, I.C.M.; da Silva, B.G.C.; Coll, C.V.N.; Evenson, K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 175. [Google Scholar] [CrossRef]
- Hui, A.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.; Sellers, E.; McGavock, J.; Morris, M.; Bruce, S.; et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG 2012, 119, 70–77. [Google Scholar] [CrossRef]
- Arizabaleta, A.V.M.; Buitrago, L.O.; de Plata, A.C.A.; Escudero, M.M.; Ramirez-Velez, R. Aerobic exercise during pregnancy improves health-related quality of life: A randomised trial. J. Physiother. 2010, 56, 253–258. [Google Scholar] [CrossRef]
- Robledo-Colonia, A.F.; Sandoval-Restrepo, N.; Mosquera-Valderrama, Y.F.; Escobar-Hurtado, C.; Ramírez-Vélez, R. Aerobic exercise training during pregnancy reduces depressive symptoms in nulliparous women: A randomised trial. J. Physiother. 2012, 58, 9–15. [Google Scholar] [CrossRef]
- Marquez-Sterling, S.; Perry, A.C.; Kaplan, T.A.; Halberstein, R.A.; Signorile, J.F. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med. Sci. Sports Exerc. 2000, 32, 58–62. [Google Scholar] [CrossRef]
- Garshasbi, A.; Faghih Zadeh, S. The effect of exercise on the intensity of low back pain in pregnant women. Int. J. Gynaecol. Obstet. 2005, 88, 271–275. [Google Scholar] [CrossRef]
- Ghodsi, Z.; Asltoghiri, M. Effects of aerobic exercise training on maternal and neonatal outcome: A randomized controlled trial on pregnant women in Iran. J. Pak. Med. Assoc. 2014, 64, 1053–1056. [Google Scholar]
- Gau, M.L.; Chang, C.Y.; Tian, S.H.; Lin, K.C. Effects of birth ball exercise on pain and self-efficacy during childbirth: A randomised controlled trial in Taiwan. Midwifery 2011, 27, e293–e300. [Google Scholar] [CrossRef]
- Kluge, J.; Hall, D.; Louw, Q.; Theron, G.; Grové, D. Specific exercises to treat pregnancy-related low back pain in a South African population. Int. J. Gynaecol. Obstet. 2011, 113, 187–191. [Google Scholar] [CrossRef]
- Suputtitada, A.; Wacharapreechanont, T.; Chaisayan, P. Effect of the “sitting pelvic tilt exercise” during the third trimester in primigravidas on back pain. J. Med. Assoc. Thail. 2002, 85 (Suppl. 1), S170–S179. [Google Scholar]
- Khodaveisi, M.; Omidi, A.; Farokhi, S.; Soltanian, A.R. The effect of pender’s health promotion model in improving the nutritional behavior of overweight and obese women. Int. J. Community Based Nurs. Midwifery 2017, 5, 165–174. [Google Scholar] [CrossRef]
- Laitakari, J.; Asikainen, T.M. How to promote physical activity through individual counseling—A proposal for a practical model of counseling on health-related physical activity. Patient Educ. Couns. 1998, 33 (Suppl. 1), S13–S24. [Google Scholar] [CrossRef]
- Physical Activity and Exercise during Pregnancy and the Postpartum Period. Available online: https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Physical-Activity-and-Exercise-During-Pregnancy-and-the-Postpartum-Period (accessed on 15 March 2019).
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Maes, M. High incidence of body image dissatisfaction in pregnancy and the postnatal period: Associations with depression, anxiety, body mass index and weight gain during pregnancy. Sex. Reprod. Healthc. 2017, 13, 103–109. [Google Scholar] [CrossRef]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef]
- Cid, M.; González, M. Potential benefits of physical activity during pregnancy for the reduction of gestational diabetes prevalence and oxidative stress. Early Hum. Dev. 2016, 94, 57–62. [Google Scholar] [CrossRef]
- Padmapriya, N.; Bernard, J.Y.; Liang, S.; Loy, S.L.; Shen, Z.; Kwek, K.; Godfrey, K.M.; Gluckman, P.D.; Chong, Y.S.; Saw, S.M.; et al. Association of physical activity and sedentary behavior with depression and anxiety symptoms during pregnancy in a multiethnic cohort of Asian women. Arch. Womens Ment. Health 2016, 19, 1119–1128. [Google Scholar] [CrossRef]
- Shakeel, N.; Richardsen, K.R.; Martinsen, E.W.; Eberhard-Gran, M.; Slinning, K.; Jenum, A.K. Physical activity in pregnancy and postpartum depressive symptoms in a multiethnic cohort. J. Affect. Disord. 2018, 236, 93–100. [Google Scholar] [CrossRef]
- Goodwin, A.; Astbury, J.; McMeeken, J. Body image and psychological well-being in pregnancy. A comparison of exercisers and non-exercisers. Aust. N. Z. J. Obstet. Gynaecol. 2000, 40, 442–447. [Google Scholar] [CrossRef]
- Bisson, M.; Alméras, N.; Dufresne, S.S.; Robitaille, J.; Rhéaume, C.; Bujold, E.; Frenette, J.; Tremblay, A.; Marc, I. A 12-week exercise program for pregnant women with obesity to improve physical activity levels: An open randomised preliminary study. PLoS ONE 2015, 10, e0137742. [Google Scholar] [CrossRef]
- Engberg, E.; Tikkanen, H.O.; Koponen, A.; Hägglund, H.; Kukkonen-Harjula, K.; Tiitinen, A.; Peltonen, J.E.; Pöyhönen-Alho, M. Cardiorespiratory fitness and health-related quality of life in women at risk for gestational diabetes. Scand. J. Med. Sci. Sports 2018, 28, 203–211. [Google Scholar] [CrossRef]
- Chan, D.N.; So, W.K. A systematic review of randomised controlled trials examining the effectiveness of breast and cervical cancer screening interventions for ethnic minority women. Eur. J. Oncol. Nurs. 2015, 19, 536–553. [Google Scholar] [CrossRef]
| ‘Pregnant women’ OR ‘Pregnancy’ OR ‘Prenatal’ OR ‘Antenatal’ OR ‘Gestation’ OR ‘Maternal’ |
| AND |
| ‘Intervention’ OR ‘Program’ OR ‘Program’ OR ‘Therapy’ OR ‘Education’ OR ‘Web-based’ OR ‘E-health’ |
| AND |
| ‘Physical activity’ OR ‘Exercise’ OR ‘Land-base exercise’ OR ‘Nurse-led’ |
| AND |
| ‘Weight gain’ OR ‘Weight control’ OR ‘Self-efficacy’ OR ‘Depression’ OR ‘Psychological’ OR ‘Pain’ OR ‘Sleep disturbance’ OR ‘Sleep difficulties’ OR ‘Functional ability’ OR Functional status’ OR ‘Sick leave’ |
| Author/Year | Methodological Quality Rating (EPHPP) | ||||||
|---|---|---|---|---|---|---|---|
| Selection Bias | Study Design | Confounders | Blinding | Data Collection Method | Withdrawals and Dropouts | Overall | |
| Kinnunen et al. 2007 | Moderate | Strong | Strong | Weak | Strong | Moderate | Moderate |
| Huang et al. 2011 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Ozdemir et al. 2015 | Moderate | Strong | Strong | Weak | Strong | Strong | Moderate |
| Garshasbi and Faghih Zadeh 2005 | Weak | Strong | Strong | Weak | Weak | Strong | Weak |
| Ronnberg et al. 2014 | Moderate | Strong | Strong | Weak | Strong | Strong | Moderate |
| Stafne et al. 2012 | Moderate | Strong | Strong | Weak | Strong | Strong | Moderate |
| Songoygard et al. 2012 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Gustafsson et al. 2016 | Moderate | Strong | Strong | Weak | Weak | Strong | Weak |
| Eggen et al. 2012 | Weak | Strong | Weak | Moderate | Strong | Moderate | Weak |
| Miquelutti et al. 2013 | Moderate | Strong | Strong | Weak | Strong | Moderate | Moderate |
| Sagedal et al. 2017 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Haakstad et al. 2018 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Montoya Arizabaleta et al. 2010 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Robledo-Colonia et al. 2012 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Marquez-Sterling et al. 2000 | Weak | Weak | Strong | Weak | Strong | Moderate | Weak |
| Suputtitada et al. 2002 | Weak | Weak | Strong | Weak | Strong | Moderate | Weak |
| Hui et al. 2006 | Weak | Weak | Strong | Weak | Weak | Moderate | Weak |
| Hui et al. 2012 | Strong | Strong | Strong | Weak | Strong | Moderate | Moderate |
| Haakstad and Bo 2011 | Weak | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Haakstad and Bo 2015 | Weak | Strong | Strong | Moderate | Weak | Moderate | Weak |
| Haakstad et al. 2016 | Weak | Strong | Strong | Moderate | Weak | Weak | Weak |
| Perales et al. 2015 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| da Silva et al. 2017 | Moderate | Strong | Strong | Weak | Strong | Strong | Moderate |
| Kluge et al. 2011 | Weak | Strong | Weak | Weak | Strong | Strong | Weak |
| Gau et al. 2011 | Moderate | Strong | Strong | Weak | Strong | Weak | Weak |
| Aşcı and Rathfisch 2016 | Moderate | Strong | Strong | Strong | Strong | Strong | Strong |
| Ruiz et al. 2013 | Moderate | Strong | Strong | Weak | Strong | Strong | Moderate |
| Sklempe Kokic et al. 2017 | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Ghodsi and Asltoghiri 2014 | Weak | Weak | Strong | Weak | Weak | Weak | Weak |
| Author/Year/Country | Study Design/Settings | Participant Characteristics/Sample Size/Number of Withdrawals | Intervention Components | Interveners | Assessed Outcomes on Pain/Data Collection Time Points | Assessment tools for Outcome Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Ozdemir et al. 2015; Turkey | Randomized controlled trial; Local hospital in Ankara | Adult pregnant women at 20–35 weeks of gestation n = 96 (Intervention: 48, control: 48) Withdrawals Intervention: 0, control: 0 | Intervention group: Counselling
| Nurse |
|
| Within-group comparison
|
| Garshasbi and Faghih Zadeh 2005; Iran | Randomized controlled trial; a local hospital in Tehran | Adult first-time pregnant women at 17–22 weeks of gestation n = 266 (Intervention: 161, control: 105) Withdrawals Intervention: 0, control: 0 | Intervention group: Supervised exercise program
| Midwife |
|
| Within-group comparison
|
| Stafne et al. 2012; Norway | Randomized controlled trial; Local hospitals in Trondheim and Stavanger | Adult women with singleton pregnancy, at 18th–22nd week of pregnancy n = 855 (Intervention: 429, control: 426) Withdrawals Intervention: 33, control: 61 | Intervention group: Supervised exercise program
| Physiothera-pists |
|
| Between-group comparison
|
| Eggen et al. 2012; Norway | Randomized controlled trial; Two local maternity primary care centers in Southeast Norway | Adult pregnant women, before the 20th week of gestation n = 257 (Intervention: 129, control: 128) Withdrawals Intervention: 26, control: 21 | Intervention group: Supervised exercise program
| Physiothera-pists |
|
| Between-group comparison Prevalence of low back pain and pelvic girdle pain
|
| Miquelutti et al. 2013; Brazil | Randomized controlled trial; A local hospital and four primary healthcare centers in Sao Paulo | Adult women with singleton pregnancy, at 18–24 weeks of gestation n = 205 (Intervention: 103, control: 102) Withdrawals Intervention: 3, control: 1 | Intervention group: Supervised exercise program
| Physiothera-pists |
|
| Between-group comparison Lumbopelvic pain
|
| Suputtitada et al. 2002; Thailand | Randomized controlled trial; Prenatal clinic of a local hospital | Adult first-time pregnant women, at the 26th–30th week of gestation n = 84 (Intervention: 42, control: 42) Withdrawals Total: 7. Number of withdrawals in each group were not specified. | Intervention group: Supervised exercise program
| Exercise instructors |
|
| Between-group comparison
|
| Haakstad and Bo 2015; Norway | Secondary analysis of randomized controlled trial; Local community | First-time pregnant women before the 24th week of pregnancy n = 105 (Intervention: 52, control: 53) Withdrawals Intervention: 10, control: 11 | Intervention group: Supervised exercise program
| Aerobic instructors |
|
| Between-group comparison Intention-to-treat analysis
|
| Kluge et al. 2011; South Africa | Randomized controlled trial; prenatal clinics at two local hospitals in Western Cape | Adult pregnant women with a gestational age of 16–24 weeks, who were experiencing low back pain n = 50 (Intervention: 26, control: 24) Withdrawals Intervention: 2, control: 2 | Intervention group: Information dissemination
| Biokineticist and the investigator of the study |
|
| Within-group comparison
|
| Gau et al. 2011; Taiwan | Randomized controlled trial; Local hospital and medical center in Taiwan | Adult women with singleton pregnancy, at 30–32 weeks of gestation n = 188 (Intervention: 94, control: 94) Withdrawals Intervention: 46, control: 55 | Intervention group: Home-based exercise program
| Investigators of the study |
|
| Between-group comparisons
|
| Sklempe Kokic et al. 2017; Croatia | Secondary analysis of randomized controlled trial; Two local hospitals in Zagreb | Adult pregnant women before 30 weeks of gestation n = 45 (Intervention: 22, control: 23) Withdrawals Intervention: 2, control: 1 | Intervention group: Supervised exercise program
| Not specified |
|
| Between-group comparison Lumbopelvic pain
|
| Author/Year/Country | Study Design/Settings | Participant Characteristics/Sample Size/Number of Withdrawals | Intervention Components | Interveners | Assessed Outcomes on Gestational Weight Gain/Data Collection Time Points | Assessment Tools for Outcome Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Kinnunen et al. 2007; Finland | Controlled clinical trial; Six maternity clinics in southern Finland | Adult, first-time pregnant women n = 122 (Intervention: 69, control: 53) Withdrawals Intervention: 20, control: 7 | Comprising dietary and physical activity components Physical activity components Intervention group: Counselling
| Public health nurses |
|
| Between-group comparisons
|
| Huang et al. 2011; Taiwan | Three-group randomized controlled trial; clinic at a local medical center in northern Taiwan | Adult women before the 16th week of gestation n = 240 (First intervention group (EP): 80, second intervention group (EPP): 80, control group: 80) Withdrawals EP: 19, EPP: 16control: 16 | Comprising dietary and physical activity components Physical activity components First intervention group (EP) Counselling and information dissemination
| Nurse |
|
| Between-group comparisons
|
| Garshasbi and Faghih Zadeh 2005; Iran | Randomized controlled trial; a local hospital in Tehran | Adult first-time pregnant women at 17–22 weeks of gestation n = 266 (Intervention: 161, control: 105) Withdrawals Intervention: 0, control: 0 | Intervention group: Supervised exercise program
| Midwife |
|
| Between-group comparison
|
| Ronnberg et al. 2014; Sweden | Randomized controlled trial; antenatal clinics in the Orebro County of Sweden | Adult pregnant women on or before their 16th week of pregnancy n = 445 (Intervention: 221, control: 224) Withdrawals Intervention: 29, control: 42 | Intervention group: Information dissemination
| Midwife |
|
| Between-group comparisons
|
| Sagedal et al. 2017; Norway | Randomized controlled trial; Eight healthcare clinics in southern Norway | Adult women with a singleton pregnancy at no more than 20 weeks of gestation n = 606 (Intervention: 303, control: 303) Withdrawals Intervention: 34, control: 39 | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Physiotherapists and students at fitness centers |
|
| Between-group comparisons Gestational weight gain from pre-pregnancy to term delivery
|
| Marquez-Sterling et al. 2000; USA | Randomized controlled trial; Local community | Adult women during their second trimester of pregnancy n = 20 (Intervention: 10, Control: 10) Withdrawals Intervention: 1, control: 4 | Intervention group: Supervised exercise program
| Aerobic instructors |
|
| Between-group comparisons
|
| Hui et al. 2006; Canada | Pilot randomized controlled trial; Local community in urban Winnipeg | Pregnant women before the 26th week of pregnancy n = 52 (Numbers of participants randomized into the two groups are not reported) Withdrawals Intervention: Not reported, control: Not reported | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Fitness instructors |
|
| Between-group comparison
|
| Hui et al. 2012; Canada | Randomized controlled trial; Local community in Winnipeg | Pregnant women before the 26th week of pregnancy n = 224 (Intervention: 112, control: 112) Withdrawals Intervention: 10, control: 24 | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Fitness instructors |
|
| Between-group comparison
|
| Haakstad and Bo 2011; Norway | Randomized controlled trial; Local community | Adult women within the first 24 weeks of pregnancy n = 105 (Intervention: 52, Control: 53) Withdrawals Intervention: 10, control: 11 | Intervention group: Supervised exercise program
| Aerobic instructors |
|
| Between-group comparisons Intent-to-treat analysis
|
| Perales et al. 2015; Spain | Randomized controlled trial; University Hospital of Fuenlabrada in Madrid | Adult women with uncomplicated and singleton gestations n = 184 (Intervention: 101, control: 83) Withdrawals Intervention: 11, control: 6 | Intervention group: Supervised exercise program
| Qualified fitness specialists |
|
| Between-group comparisons
|
| da Silva et al. 2017; Brazil | Randomized controlled trial; Health facilities offering antenatal care in Pelotas, Brazil | Adult pregnant women living in urban areas n = 639 (Intervention: 213, control: 426) Withdrawals Intervention: 15, control: 30 | Intervention group: Supervised exercise program
| Trained physical education professionals |
|
| Between-group comparisons
|
| Aşcı and Rathfisch 2016; Turkey | Randomized controlled trial; Local family health center in Istanbul | Adult pregnant women who were pregnant for less than three months n = 102 (Intervention: 51, control: 51) Withdrawals Intervention: 6, control: 6 | Comprising dietary and physical activity components Physical activity components Intervention group: Counselling
| Investigator of the study |
|
| Between-group comparison Gestational weight gain
|
| Ruiz et al. 2013; Spain | Randomized controlled trial; Local primary care medical centers in Madrid | Women with singleton pregnancy, at the 5th–6th week of gestation n = 962 (Intervention: 481, control: 481) Withdrawals Intervention: 70, control: 68 | Intervention group: Supervised exercise program
| Not specified |
|
| Between-group comparison
|
| Ghodsi and Asltoghiri 2014; Iran | Randomized controlled trial; Prenatal clinics and delivery centers in Hamedan, Iran | Adult pregnant women at 20–26 weeks of gestation n = 80 (Intervention: 40, control: 40) Withdrawals Intervention: Not reported control: Not reported | Intervention group: Home-based exercise program
| Not specified |
|
| Between-group comparison
|
| Author/Year/Country | Study Design/Settings | Participant Characteristics/Sample Size/Number of Withdrawals | Intervention Components | Interveners | Assessed Outcomes on Gestational Weight Gain/Data Collection Time Points | Assessment Tools for Outcome Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Huang et al. 2011; Taiwan | Three-group randomized controlled trial; clinic at a local medical center in northern Taiwan | Adult women before the 16th week of gestation n = 240 (First intervention group (EP): 80, second intervention group (EPP): 80, control group: 80) Withdrawals EP: 19, EPP: 16 control: 16 | Comprising dietary and physical activity components Physical activity components First intervention group (EP) Counselling and information dissemination
| Nurse |
|
| Between-group comparisons
|
| Songoygard et al. 2012; Norway | Randomized controlled trial; Local hospitals in Trondheim and Stavanger | Adult pregnant women attending ultrasound examination during the 18th week of pregnancy n = 855 (Intervention: 429, control: 426) Withdrawals Intervention: 50, control: 86 | Intervention group: Supervised exercise program
| Physiotherapists |
|
| Between-group comparisons
|
| Gustafsson et al. 2016; Norway | Randomized controlled trial; Local hospitals in Trondheim and Stavanger | Adult pregnant women attending ultrasound examination during the 18th week of pregnancy n = 855 (Intervention: 429, control: 426) Withdrawals Intervention: 33, control: 61 | Intervention group: Supervised exercise program
| Physiotherapists |
|
| Between-group comparisons
|
| Miquelutti et al. 2013; Brazil | Randomized controlled trial; A local hospital and four primary healthcare centers in Sao Paulo | Adult women with singleton pregnancy, at 18–24 weeks of gestation n = 205 (Intervention: 103, control: 102) Withdrawals Intervention: 3, control: 1 | Intervention group: Supervised exercise program
| Physiotherapists |
|
| Between-group comparison
|
| Robledo-Colonia et al. 2012; Columbia | Randomized controlled trial; Three local hospitals in Cali | Adult pregnant women at 16–20 weeks of gestation n = 80 (Intervention: 40, control: 40) Withdrawals Intervention: 3, control: 3 | Intervention group: Supervised exercise program
| Physiotherapists and physicians |
|
| Between-group comparisons
|
| Haakstad et al. 2016; Norway | Secondary analysis of randomized controlled trial; Local community | Adult women within the first 24 weeks of pregnancy n = 105 (Intervention: 52, Control: 53) Withdrawals Intervention: 0, control: 0 | Intervention group: Supervised exercise program
| Aerobics instructors |
|
| Between-group comparisons Intention-to-treat analysis
|
| Perales et al. 2015; Spain | Randomized controlled trial; University Hospital of Fuenlabrada in Madrid | Adult women with uncomplicated and singleton gestations n = 184 (Intervention: 101, control: 83) Withdrawals Intervention: 11, control: 6 | Intervention group: Supervised exercise program
| Qualified fitness specialists |
|
| Within-group comparisons
|
| Author/Year/Country | Study Design/Settings | Participant Characteristics/Sample Size/Number of Withdrawals | Intervention Components | Interveners | Assessed Outcomes on Gestational Weight Gain/Data Collection Time Points | Assessment Tools for Outcome Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Montoya Arizabaleta et al. 2010; Columbia | Randomized controlled trial; Three local hospitals in Cali | Adult pregnant women at 16–20 weeks of gestation n = 64 (Intervention: 33, control: 31) Withdrawals Intervention: 9, control: 5 | Intervention group: Supervised exercise program
| Physiotherapists and physicians |
|
| Between-group comparisons
|
| Haakstad et al. 2016; Norway | Secondary analysis of randomized controlled trial; Local community | Adult women within the first 24 weeks of pregnancy n = 105 (Intervention: 52, Control: 53) Withdrawals Intervention: 0, control: 0 | Intervention group: Supervised exercise program
| Aerobics instructors |
|
| Between-group comparisons Intention-to-treat analysis
|
| Author/Year/Country | Study Design/Settings | Participant Characteristics/Sample Size/Number of Withdrawals | Intervention Components | Interveners | Assessed Outcomes on Gestational Weight Gain/Data Collection Time Points | Assessment Tools for Outcome Assessment | Findings |
|---|---|---|---|---|---|---|---|
| Kinnunen et al. 2007; Finland | Controlled clinical trial; Six maternity clinics in southern Finland | Adult, first-time pregnant women n = 122 (Intervention: 69, control: 53) Withdrawals Intervention: 20, control: 7 | Comprising dietary and physical activity components Physical activity components Intervention group: Counselling
| Public health nurses |
|
| Between-group comparisons
|
| Huang et al. 2011; Taiwan | Three-group randomized controlled trial; clinic at a local medical center in northern Taiwan | Adult women before the 16th week of gestation n = 240 (First intervention group (EP): 80, second intervention group (EPP): 80, control group: 80) Withdrawals EP: 19, EPP: 16 control: 16 | Comprising dietary and physical activity components Physical activity components First intervention group (EP) Counselling and information dissemination
| Nurse |
|
| Between-group comparisons
|
| Miquelutti et al. 2013; Brazil | Randomized controlled trial; A local hospital and four primary healthcare centers in Sao Paulo | Adult women with singleton pregnancy, at 18–24 weeks of gestation n = 205 (Intervention: 103, control: 102) Withdrawals Intervention: 3, control: 1 | Intervention group: Supervised exercise program
| Physiotherapists |
|
| Between-group comparison
|
| Sagedal et al. 2017; Norway | Randomized controlled trial; Eight healthcare clinics in southern Norway | Adult women with a singleton pregnancy at no more than 20 weeks of gestation n = 606 (Intervention: 303, control: 303) Withdrawals Intervention: 34, control: 39 | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Physiotherapists and students at fitness centers |
|
| Between-group comparisons
|
| Haakstad et al. 2018; Norway | Secondary analysis of randomized controlled trial; Eight healthcare clinics in southern Norway | Adult women with singleton pregnancy within the first 20 weeks of gestation n = 606 (Intervention: 303, control: 303) Withdrawals Intervention: 8, control: 9 | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Physiotherapists and students at fitness centers |
|
| Within-group comparisons
|
| Hui et al. 2006; Canada | Pilot randomized controlled trial; Local community in urban Winnipeg | Pregnant women before the 26th week of pregnancy n = 52 (Numbers of participants randomized into the two groups are not reported) Withdrawals Intervention: Not reported, control: Not reported | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Fitness instructors |
|
| Between-group comparison
|
| Hui et al. 2012; Canada | Randomized controlled trial; Local community in Winnipeg | Pregnant women before the 26th week of pregnancy n = 224 (Intervention: 112, control: 112) Withdrawals Intervention: 10, control: 24 | Comprising dietary and physical activity components Physical activity components Intervention group: Supervised exercise program
| Fitness instructors |
|
| Between-group comparison
|
| Aşcı and Rathfisch 2016; Turkey | Randomized controlled trial; Local family health center in Istanbul | Adult pregnant women who were pregnant for less than three months n = 102 (Intervention: 51, control: 51) Withdrawals Intervention: 6, control: 6 | Comprising dietary and physical activity components Physical activity components Intervention group: Counselling
| Investigator of the study |
|
| Between-group comparison
|
| Sklempe Kokic et al. 2017; Croatia | Secondary analysis of randomized controlled trial; Two local hospitals in Zagreb | Adult pregnant women before 30 weeks of gestation n = 45 (Intervention: 22, control: 23) Withdrawals Intervention: 2, control: 1 | Intervention group: Supervised exercise program
| Not specified |
|
| Between-group comparison
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.W.H.; Au Yeung, E.; Law, B.M.H. Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1840. https://doi.org/10.3390/ijerph16101840
Chan CWH, Au Yeung E, Law BMH. Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(10):1840. https://doi.org/10.3390/ijerph16101840
Chicago/Turabian StyleChan, Carmen W. H., Elce Au Yeung, and Bernard M. H. Law. 2019. "Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 10: 1840. https://doi.org/10.3390/ijerph16101840
APA StyleChan, C. W. H., Au Yeung, E., & Law, B. M. H. (2019). Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review. International Journal of Environmental Research and Public Health, 16(10), 1840. https://doi.org/10.3390/ijerph16101840





