Genome-Wide Identification of the VQ Protein Gene Family of Tobacco (Nicotiana tabacum L.) and Analysis of Its Expression in Response to Phytohormones and Abiotic and Biotic Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Stress Treatments
2.2. Identification of VQ Genes in Tobacco and Gene Structure Analysis
2.3. Multiple Alignment and Phylogenetic Analysis
2.4. Gene Duplication and Synonymous (Ks) and Nonsynonymous (Ka) Substitution Calculation
2.5. Promoter Analysis
2.6. RNA Extraction and Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
3. Results
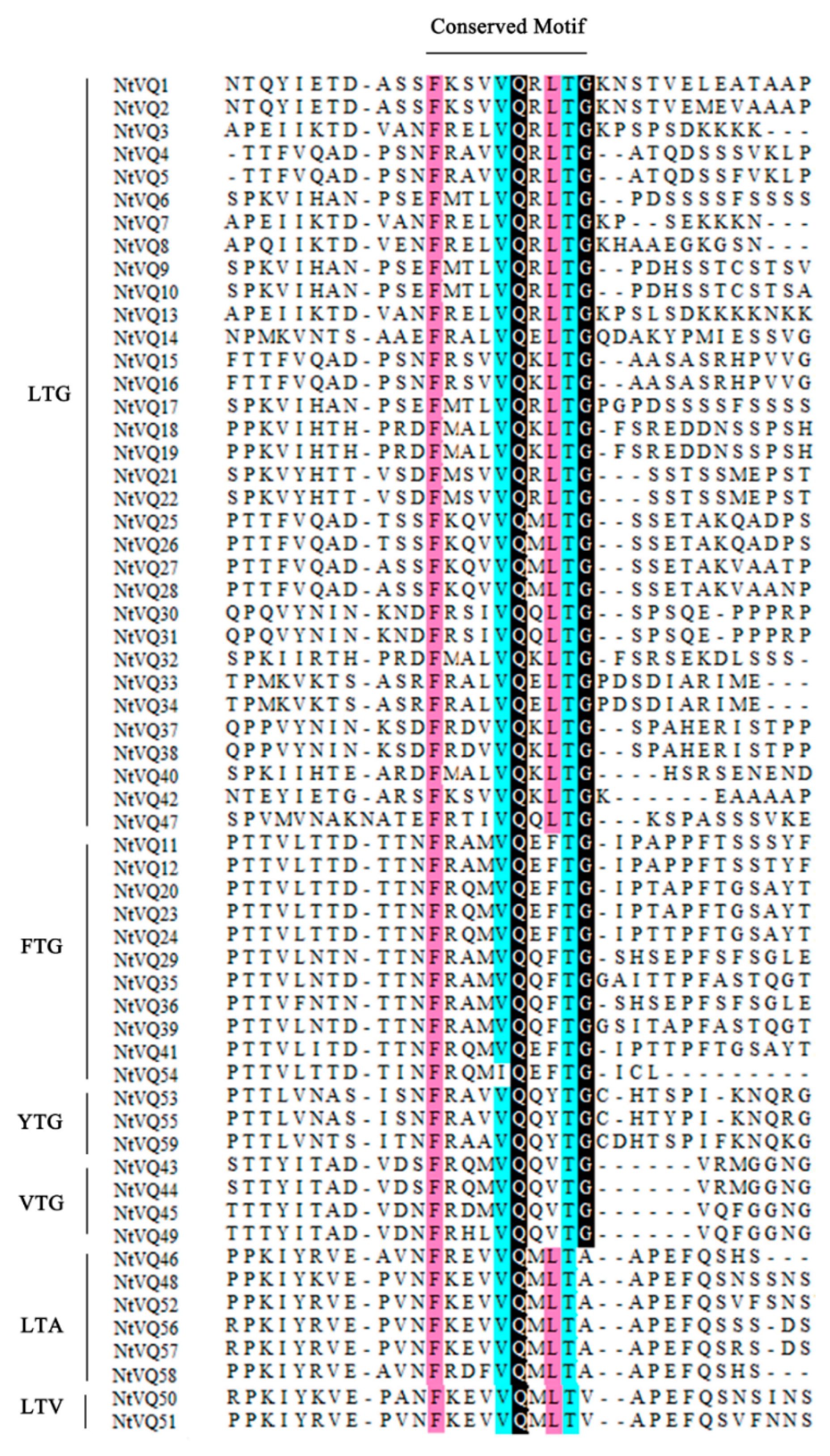
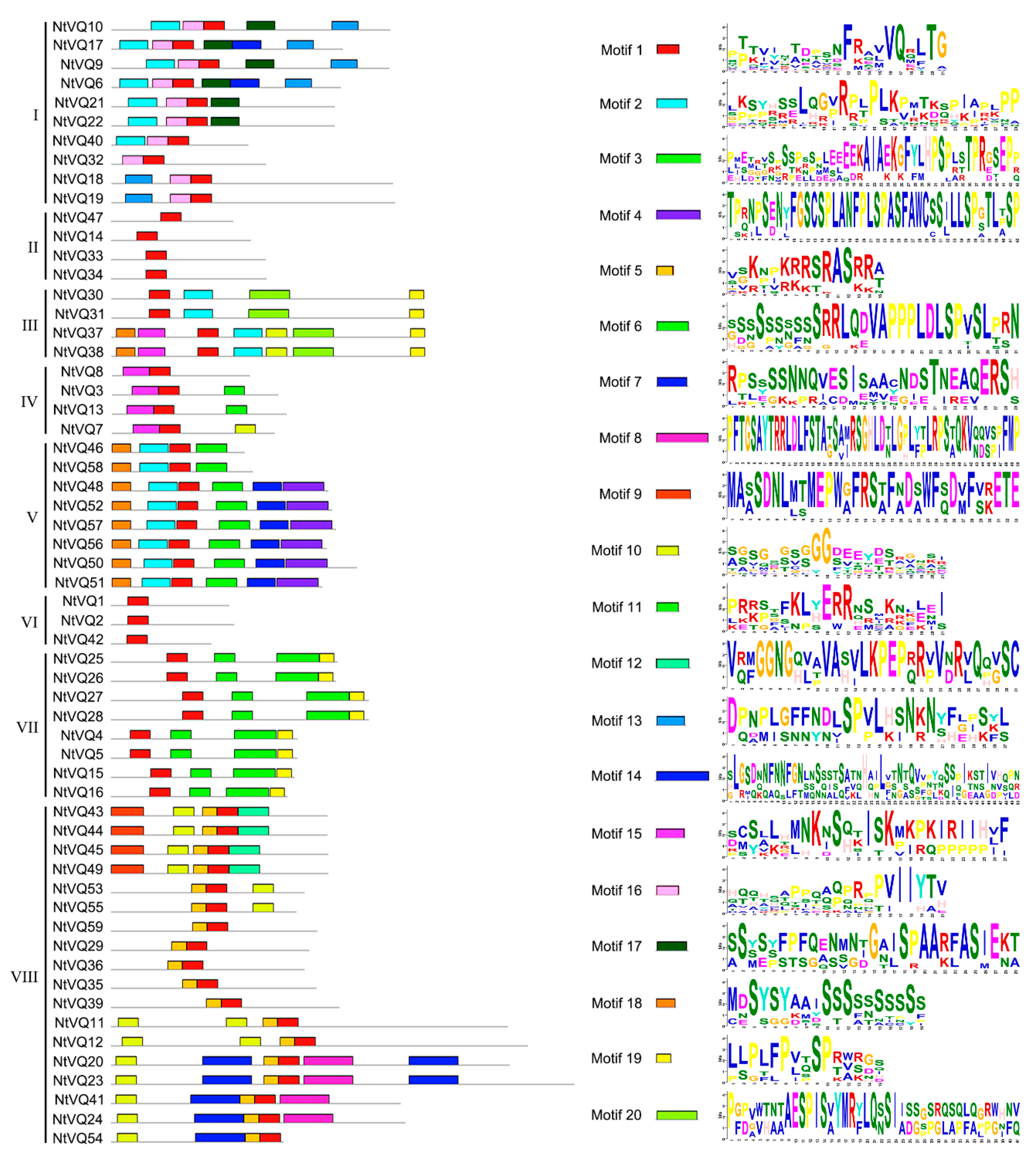
3.1. Identification of VQ Family Members and Sequence Analysis in Tobacco
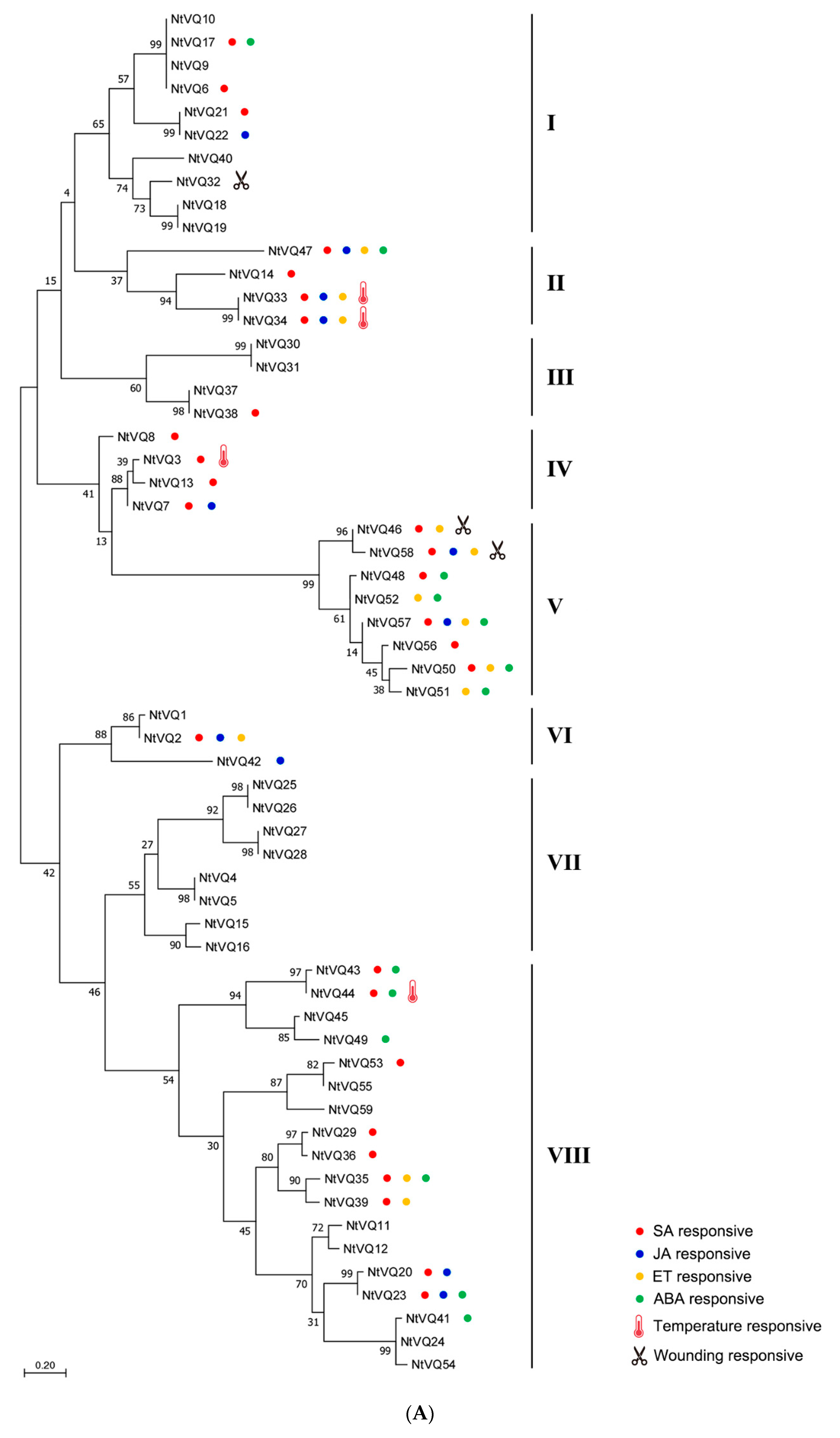
3.2. Evolution and Divergence of the VQ Gene Family in Tobacco and Arabidopsis
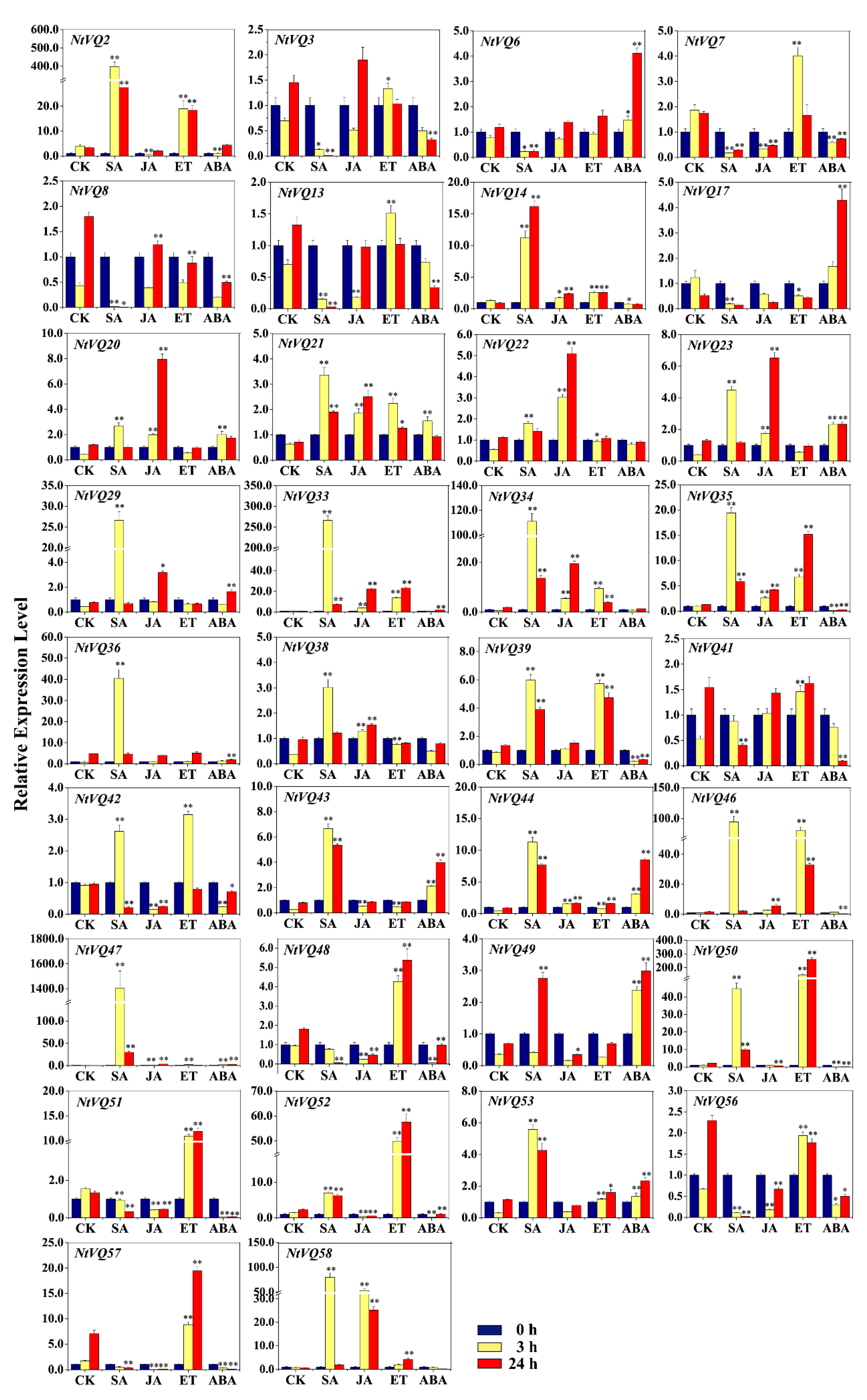
3.3. Differential Expression of NtVQ Genes in Response to Phytohormonal Treatments
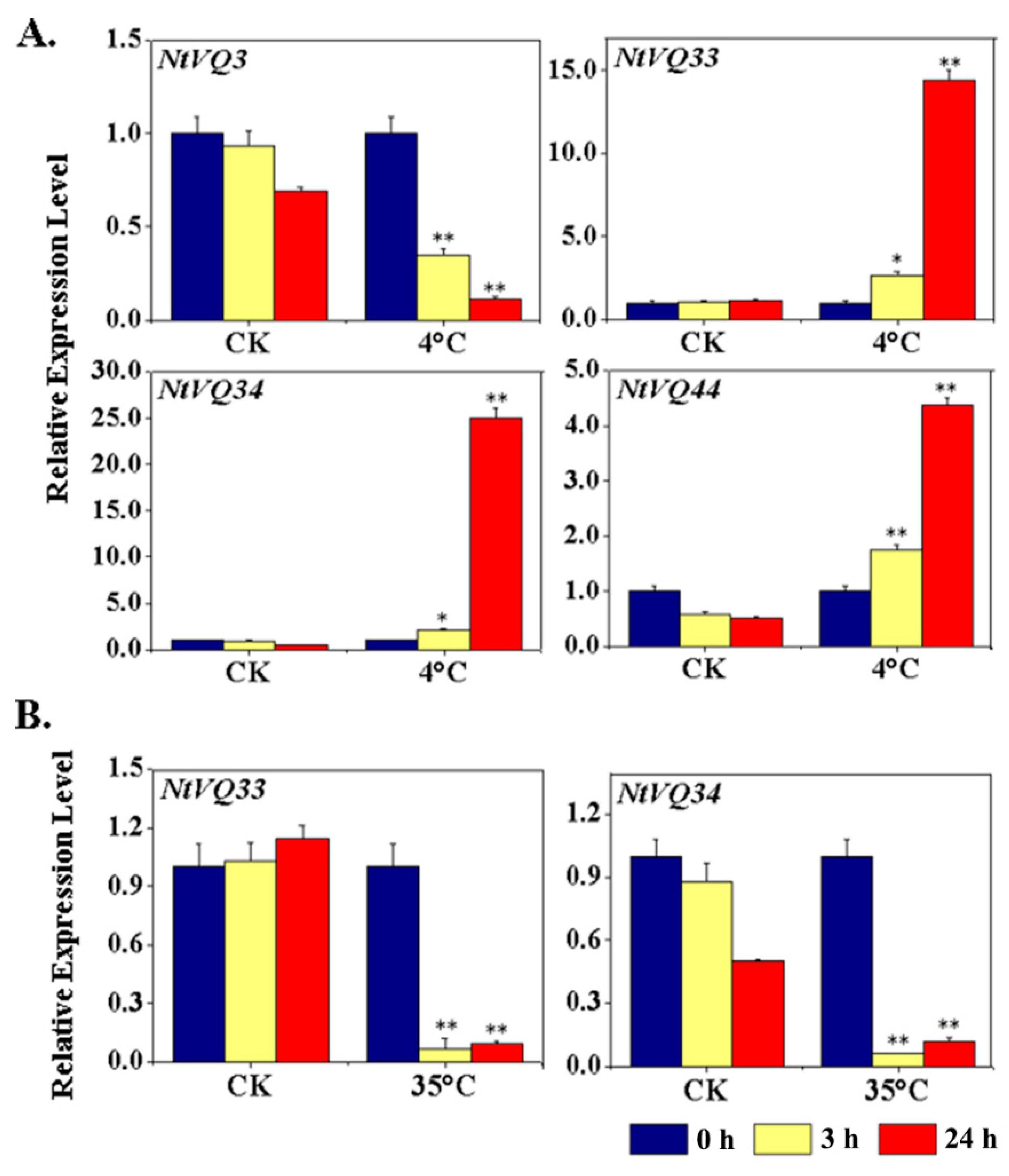
3.4. NtVQ Gene Expression Following Abiotic Treatments
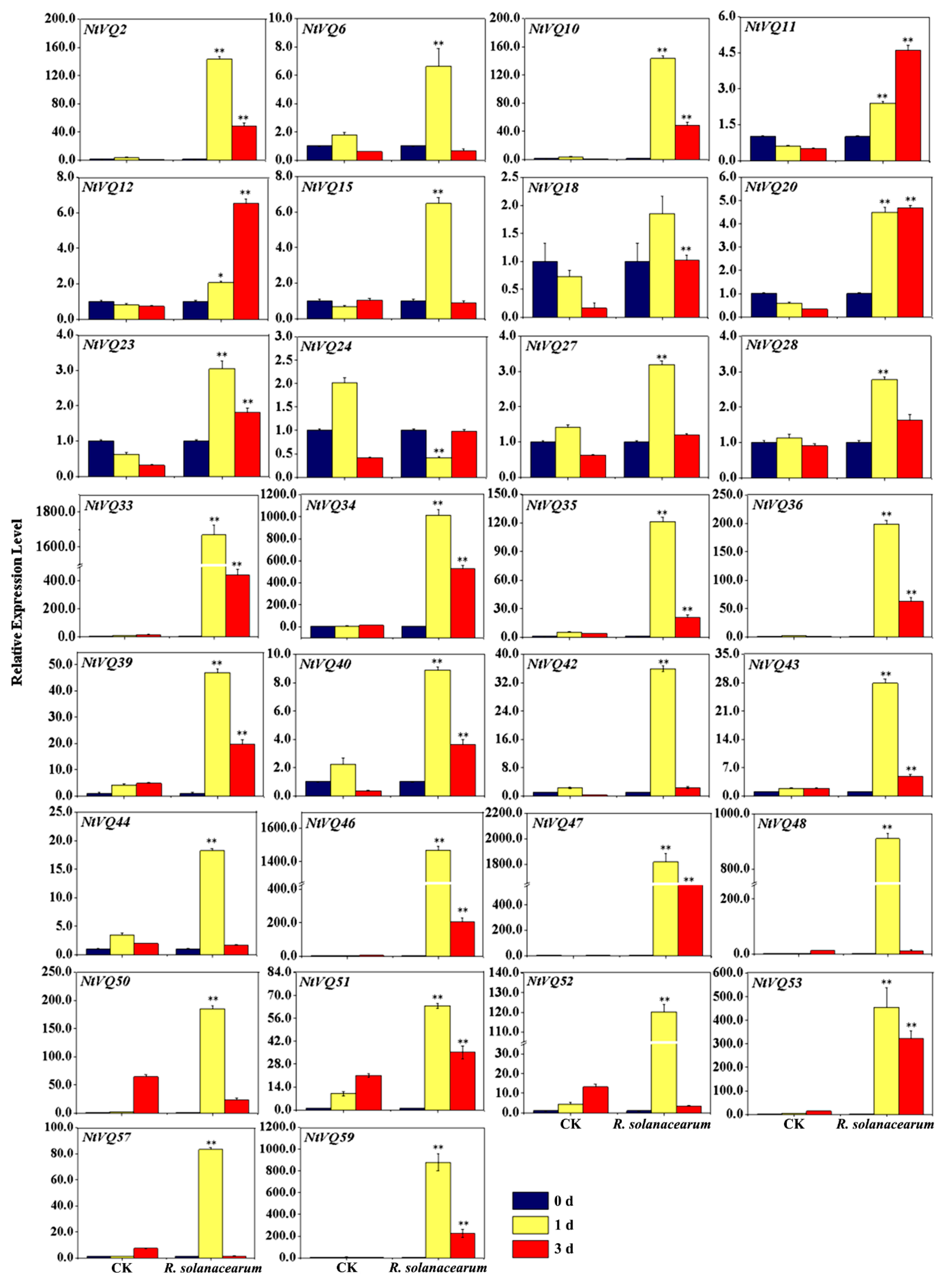
3.5. NtVQ Gene Expression Following Pathogen Infection
3.6. Analysis of Putative cis-Regulatory Elements in the Promoters of the NtVQ Gene Family Members
4. Discussion
4.1. In-Silico Analysis of NtVQ Genes
4.2. Expression Analysis of NtVQ Genes in Response to Biotic and Abiotic Stresses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oksman-Caldentey, K.M. Tropane and nicotine alkaloid biosynthesis-novel approaches towards biotechnological production of plant-derived pharmaceuticals. Curr. Pharm. Biotechno. 2007, 8, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Bokowiec, M.T.; Han, S.C.; Zhang, H.B.; Brannock, J.F.; Chen, X.F.; Laudeman, T.W.; Timko, M.P. Tobacco transcription factors: Novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008, 147, 280–295. [Google Scholar] [CrossRef] [Green Version]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Willig, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscaill, P.; Rivas, S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Liu, S.; Somssich, I.E. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Van Ruyskensvelde, V.; Van Breusegem, F.; Van der Kelen, K. Post-transcriptional regulation of the oxidative stress response in plants. Free Radic. Biol. Med. 2018, 122, 181–192. [Google Scholar] [CrossRef]
- Jing, Y.J.; Lin, R.C. The VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol. 2015, 169, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhou, Y.; Yang, Y.; Chi, Y.J.; Zhou, J.; Chen, J.Y.; Wang, F.; Fan, B.F.; Shi, K.; Zhou, Y.H.; et al. Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol. 2012, 159, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Kwon, S.I.; Choi, C.; Lee, H.; Ahn, L.; Park, S.R.; Bae, S.C.; Lee, S.C.; Hwang, D.J. Expression analysis of rice VQ genes in response to biotic and abiotic stresses. Gene 2013, 529, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Vannozzi, A.; Wang, G.; Zhong, V.; Corso, M.; Cavallini, E.; Cheng, Z.M. A comprehensive survey of the grapevine VQ gene family and its transcriptional correlation with WRKY proteins. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.Y.; Wang, F.D.; Li, J.J.; Ding, Q.; Zhang, Y.H.; Li, H.Y.; Zhang, J.N.; Gao, J.W. Genome-wide identification and analysis of the VQ motif-containing protein family in Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Int. J. Mol. Sci. 2015, 16, 28683–28704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.Y.; Liu, B.; Wang, Y.J.; Pan, F.; Chen, Z.; Yan, H.W.; Xiang, Y. Genome-wide analysis of poplar VQ gene family and expression profiling under PEG, NaCl, and SA treatments. Tree Genet. Genomes 2016, 12. [Google Scholar] [CrossRef]
- Song, W.B.; Zhao, H.M.; Zhang, X.B.; Lei, L.; Lai, J.S. Genome-wide identification of VQ motif-containing proteins and their expression profiles under abiotic stresses in maize. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, Y.; Zhou, X.J.; Chi, Y.J.; Fan, B.F.; Chen, Z.X. Structural and functional characterization of the VQ protein family and VQ protein variants from soybean. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Liu, H.L.; Zhu, D.Y.; Gao, Y.M.; Yan, H.W.; Xiang, Y. Genome-wide analysis of VQ motif-containing proteins in Moso bamboo (Phyllostachys edulis). Planta 2017, 246, 165–181. [Google Scholar] [CrossRef]
- Cao, Y.P.; Meng, D.D.; Abdullah, M.; Jin, Q.; Lin, Y.; Cai, Y.P. Genome wide identification, evolutionary, and expression analysis of VQ genes from two Pyrus species. Genes 2018, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.L.; Zhao, S.; Duan, D.Y.; Tian, Y.; Wang, Y.P.; Mao, K.; Zhou, Z.S.; Ma, F.W. Structural and functional analyses of genes encoding VQ proteins in apple. Plant Sci. 2018, 272, 208–219. [Google Scholar] [CrossRef]
- Zhong, Y.; Guo, C.; Chu, J.J.; Liu, H.; Cheng, Z.M. Microevolution of the VQ gene family in six species of Fragaria. Genome 2018, 61, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Z.; Li, Z.; Zhao, Y.; Tan, W.; Liu, Z.; Cui, S.; Yu, X.; Ma, J.; Wang, G.; et al. Genome-wide identification and expression analysis of the VQ gene family in soybean (Glycine max). Peerj 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Chen, J.; Yang, J.; Yu, Y.; Yang, Y.; Wang, W. Identification, characterization and expression analysis of the VQ motif-containing gene family in tea plant (Camellia sinensis). BMC Genom. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Yuan, G.; Mo, S.; Qian, Y.; Wu, Y.; Chen, Q.; Xu, X.; Wu, X.; Ge, C. Genome-wide analysis of the plant-specific VQ motif-containing proteins in tomato (Solanum lycopersicum) and characterization of SlVQ6 in thermotolerance. Plant Physiol. Biochem. 2019, 143, 29–39. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Xiao, Y.-Y.; Han, Y.-C.; Shan, W.; Fan, Z.-Q.; Xu, Q.-G.; Kuang, J.-F.; Lu, W.-J.; Lakshmanan, P.; Chen, J.-Y. Banana fruit VQ motif-containing protein5 represses cold-responsive transcription factor MaWRKY26 involved in the regulation of JA biosynthetic genes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.Y.; Sevugan, M.; Ramachandran, S. Valine-glutamine (VQ) motif coding genes are ancient and non-plant-specific with comprehensive expression regulation by various biotic and abiotic stresses. BMC Genom. 2018, 19. [Google Scholar] [CrossRef]
- Pecher, P.; Eschen-Lippold, L.; Herklotz, S.; Kuhle, K.; Naumann, K.; Bethke, G.; Uhrig, J.; Weyhe, M.; Scheel, D.; Lee, J. The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of “VQ-motif’-containing proteins to regulate immune responses. New Phytol. 2014, 203, 592–606. [Google Scholar] [CrossRef]
- Hu, Y.R.; Chen, L.G.; Wang, H.P.; Zhang, L.P.; Wang, F.; Yu, D.Q. Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013, 74, 730–745. [Google Scholar] [CrossRef]
- Wang, A.H.; Garcia, D.; Zhang, H.Y.; Feng, K.; Chaudhury, A.; Berger, F.; Peacock, W.J.; Dennis, E.S.; Luo, M. The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. 2010, 63, 670–679. [Google Scholar] [CrossRef]
- Perruc, E.; Charpenteau, M.; Ramirez, B.C.; Jauneau, A.; Galaud, J.P.; Ranjeva, R.; Ranty, B. A novel calmodulin-binding protein functions as a negative regulator of osmotic stress tolerance in Arabidopsis thaliana seedlings. Plant J. 2004, 38, 410–420. [Google Scholar] [CrossRef]
- Lei, R.; Li, X.; Ma, Z.; Lv, Y.; Hu, Y.; Yu, D. Arabidopsis WRKY2 and WRKY34 transcription factors interact with VQ20 protein to modulate pollen development and function. Plant J. 2017, 91, 962–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreasson, E.; Jenkins, T.; Brodersen, P.; Thorgrimsen, S.; Petersen, N.H.T.; Zhu, S.J.; Qiu, J.L.; Micheelsen, P.; Rocher, A.; Petersen, M.; et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005, 24, 2579–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.L.; Fiil, B.K.; Petersen, K.; Nielsen, H.B.; Botanga, C.J.; Thorgrimsen, S.; Palma, K.; Suarez-Rodriguez, M.C.; Sandbech-Clausen, S.; Lichota, J.; et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008, 27, 2214–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargul, J.M.; Mibus, H.; Serek, M. Manipulation of MKS1 gene expression affects Kalanchoë blossfeldiana and Petunia hybrida phenotypes. Plant Biotechnol. J. 2015, 13, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Zhou, W.; Cheng, Z.W.; Fan, M.; Wang, L.; Xie, D.X. JAV1 controls jasmonate-regulated plant defense. Mol. Cell 2013, 50, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.R.M.; Uemura, T.; Ramadan, A.; Adachi, K.; Nemoto, K.; Nozawa, A.; Hoshino, R.; Abe, H.; Sawasaki, T.; Arimura, G.I. The ring-type E3 ubiquitin ligase JUL1 targets the VQ-motif protein JAV1 to coordinate jasmonate signaling. Plant Physiol. 2019, 179, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.B.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.F.; Yu, J.Q.; Chen, Z.X. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Wang, H.; Hu, Y.; Yu, D. Arabidopsis VQ18 and VQ26 proteins interact with ABI5 transcription factor to negatively modulate ABA response during seed germination. Plant J. 2018, 95, 529–544. [Google Scholar] [CrossRef]
- Li, Y.L.; Jing, Y.J.; Li, J.J.; Xu, G.; Lin, R.C. Arabidopsis VQ MOTIF-CONTAINING PROTEIN29 represses seedling deetiolation by interacting with PHYTOCHROME-INTERACTING FACTOR1. Plant Physiol. 2014, 164, 2068–2080. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.Y.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating γ-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Gaut, B.S. Molecular clocks and nucleotide substitution rates in higher plants. Evol. Biol. 1998, 30, 93–120. [Google Scholar]
- Kim, M.Y.; Kang, Y.J.; Lee, T.; Lee, S.H. Divergence of flowering-related genes in three legume species. Plant Genome-Us 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Delaney, S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genom. 2010, 283, 233–241. [Google Scholar] [CrossRef]
- Kaul, S.; Koo, H.L.; Jenkins, J.; Rizzo, M.; Rooney, T.; Tallon, L.J.; Feldblyum, T.; Nierman, W.; Benito, M.I.; Lin, X.Y.; et al. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ni, P.X.; Wong, G.K.S. Comparing the whole-genome-shotgun and map-based sequences of the rice genome. Trends Plant Sci. 2006, 11, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099. [Google Scholar] [CrossRef] [PubMed]
- Leitch, I.J.; Hanson, L.; Lim, K.Y.; Kovarik, A.; Chase, M.W.; Clarkson, J.J.; Leitch, A.R. The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Ann. Bot. 2008, 101, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renny-Byfield, S.; Chester, M.; Kovarik, A.; Le Comber, S.C.; Grandbastien, M.A.; Deloger, M.; Nichols, R.A.; Macas, J.; Novak, P.; Chase, M.W.; et al. Next generation sequencing reveals genome downsizing in allotetraploid Nicotiana tabacum, predominantly through the elimination of paternally derived repetitive DNAs. Mol. Biol. Evol. 2011, 28, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, X.H.; Xiao, J.H.; Wang, S.P. Comprehensive analysis of VQ motif-containing gene expression in rice defense responses to three pathogens. Plant Cell Rep. 2014, 33, 1493–1505. [Google Scholar] [CrossRef]
- Garrido-Gala, J.; Higuera, J.J.; Munoz-Blanco, J.; Amil-Ruiz, F.; Caballero, J.L. The VQ motif-containing proteins in the diploid and octoploid strawberry. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- DebRoy, S.; Thilmony, R.; Kwack, Y.B.; Nomura, K.; He, S.Y. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA 2004, 101, 9927–9932. [Google Scholar] [CrossRef] [Green Version]
- Ohtsubo, N.; Mitsuhara, I.; Koga, M.; Seo, S.; Ohashi, Y. Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol. 1999, 40, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Song, S.S.; Qi, T.C.; Wasternack, C.; Xie, D.X. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014, 21, 112–119. [Google Scholar] [CrossRef]
- Yan, H.F.; Wang, Y.J.; Hu, B.; Qiu, Z.F.; Zeng, B.S.; Fan, C.J. Genome-wide characterization, evolution, and expression profiling of VQ gene family in response to phytohormone treatments and abiotic stress in Eucalyptus grandis. Int. J. Mol. Sci. 2019, 20, 1765. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.X.; Chen, C.H.; Chen, Z.X. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.J.; Yang, Y.; Zhou, Y.; Zhou, J.; Fan, B.F.; Yu, J.Q.; Chen, Z.X. Protein-protein interactions in the regulation of WRKY transcription factors. Mol. Plant 2013, 6, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyhe, M.; Eschen-Lippold, L.; Pecher, P.; Scheel, D.; Lee, J. Menage a trois: The complex relationships between mitogen-activated protein kinases, WRKY transcription factors, and VQ-motif-containing proteins. Plant Signal. Behav. 2014, 9, e29519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.A.; Liu, Y.; Shen, Q.X.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.X.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.P.; Zhang, W.H.; Su, Y.; Xiao, L.T.; Deng, H.T.; Xie, D.X. Injury activates Ca2+/calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol. Cell 2018, 70, 136. [Google Scholar] [CrossRef] [Green Version]


| Nt-Nt | Nt-Nt | Nt-At |
|---|---|---|
| NtVQ1/NtVQ2 | NtVQ48/NtVQ51 | NtVQ2/AtVQ1 |
| NtVQ2/NtVQ1 | NtVQ48/NtVQ52 | NtVQ6/AtVQ2 |
| NtVQ3/NtVQ13 | NtVQ48/NtVQ56 | NtVQ6/AtVQ3 |
| NtVQ4/NtVQ5 | NtVQ48/NtVQ57 | NtVQ27/AtVQ4 |
| NtVQ5/NtVQ4 | NtVQ49/NtVQ45 | NtVQ31/AtVQ5 |
| NtVQ6/NtVQ17 | NtVQ50/NtVQ48 | NtVQ30/AtVQ6 |
| NtVQ9/NtVQ10 | NtVQ50/NtVQ51 | NtVQ41/AtVQ7 |
| NtVQ10/NtVQ9 | NtVQ50/NtVQ52 | NtVQ40/AtVQ8 |
| NtVQ11/NtVQ12 | NtVQ50/NtVQ56 | NtVQ38/AtVQ9 |
| NtVQ12/NtVQ11 | NtVQ50/NtVQ57 | NtVQ2/AtVQ10 |
| NtVQ13/NtVQ3 | NtVQ51/NtVQ48 | NtVQ4/AtVQ11 |
| NtVQ15/NtVQ16 | NtVQ51/NtVQ50 | NtVQ56/AtVQ12 |
| NtVQ16/NtVQ15 | NtVQ51/NtVQ52 | NtVQ27/AtVQ13 |
| NtVQ17/NtVQ6 | NtVQ51/NtVQ56 | NtVQ30/AtVQ14 |
| NtVQ18/NtVQ19 | NtVQ51/NtVQ57 | NtVQ45/AtVQ15 |
| NtVQ19/NtVQ18 | NtVQ52/NtVQ48 | NtVQ14/AtVQ16 |
| NtVQ20/NtVQ23 | NtVQ52/NtVQ50 | NtVQ7/AtVQ17 |
| NtVQ21/NtVQ22 | NtVQ52/NtVQ51 | NtVQ7/AtVQ18 |
| NtVQ22/NtVQ21 | NtVQ52/NtVQ56 | NtVQ26/AtVQ19 |
| NtVQ23/NtVQ20 | NtVQ52/NtVQ57 | NtVQ18/AtVQ20 |
| NtVQ24/NtVQ41 | NtVQ53/NtVQ55 | NtVQ6/AtVQ21 |
| NtVQ25/NtVQ26 | NtVQ55/NtVQ53 | NtVQ53/AtVQ22 |
| NtVQ26/NtVQ25 | NtVQ56/NtVQ48 | NtVQ14/AtVQ23 |
| NtVQ27/NtVQ28 | NtVQ56/NtVQ50 | NtVQ49/AtVQ24 |
| NtVQ28/NtVQ27 | NtVQ56/NtVQ51 | NtVQ13/AtVQ25 |
| NtVQ29/NtVQ36 | NtVQ56/NtVQ52 | NtVQ7/AtVQ26 |
| NtVQ30/NtVQ31 | NtVQ56/NtVQ57 | NtVQ53/AtVQ27 |
| NtVQ31/NtVQ30 | NtVQ57/NtVQ48 | NtVQ53/AtVQ28 |
| NtVQ33/NtVQ34 | NtVQ57/NtVQ50 | NtVQ57/AtVQ29 |
| NtVQ34/NtVQ33 | NtVQ57/NtVQ51 | NtVQ26/AtVQ31 |
| NtVQ35/NtVQ39 | NtVQ57/NtVQ52 | NtVQ30/AtVQ32 |
| NtVQ36/NtVQ29 | NtVQ57/NtVQ56 | NtVQ25/AtVQ33 |
| NtVQ37/NtVQ38 | NtVQ58/NtVQ46 | NtVQ11/AtVQ34 |
| NtVQ38/NtVQ37 | ||
| NtVQ39/NtVQ35 | ||
| NtVQ41/NtVQ24 | ||
| NtVQ43/NtVQ44 | ||
| NtVQ44/NtVQ43 | ||
| NtVQ45/NtVQ49 | ||
| NtVQ46/NtVQ58 | ||
| NtVQ48/NtVQ50 |
| Gene 1 | Gene 2 | Ka | Ks | Ka/Ks |
|---|---|---|---|---|
| NtVQ10 | NtVQ9 | 0.0236959 | 0.0854132 | 0.277427 |
| NtVQ11 | NtVQ12 | 0.0336896 | 0.142241 | 0.236849 |
| NtVQ15 | NtVQ16 | 0.0247142 | 0.119972 | 0.206 |
| NtVQ17 | NtVQ6 | 0.0237371 | 0.0870851 | 0.272573 |
| NtVQ18 | NtVQ19 | 0.0288426 | 0.10777 | 0.267632 |
| NtVQ20 | NtVQ23 | 0.0699917 | 0.209588 | 0.333949 |
| NtVQ21 | NtVQ22 | 0.0353919 | 0.108026 | 0.327624 |
| NtVQ24 | NtVQ41 | 0.0322853 | 0.0903173 | 0.357465 |
| NtVQ25 | NtVQ26 | 0.00768491 | 0.128751 | 0.059688 |
| NtVQ27 | NtVQ28 | 0.0129349 | 0.0794967 | 0.162709 |
| NtVQ29 | NtVQ36 | 0.0660472 | 0.17302 | 0.381732 |
| NtVQ30 | NtVQ31 | 0.0151727 | 0.157758 | 0.096177 |
| NtVQ33 | NtVQ34 | 0.0446333 | 0.203136 | 0.219722 |
| NtVQ37 | NtVQ38 | 0.0175787 | 0.061387 | 0.286358 |
| NtVQ43 | NtVQ44 | 0.0217306 | 0.0879767 | 0.247004 |
| NtVQ45 | NtVQ49 | 0.0300847 | 0.15376 | 0.19566 |
| NtVQ48 | NtVQ50 | 0.0729002 | 0.32705 | 0.222902 |
| NtVQ48 | NtVQ51 | 0.0709646 | 0.408619 | 0.173669 |
| NtVQ48 | NtVQ52 | 0.0681005 | 0.386549 | 0.176176 |
| NtVQ48 | NtVQ56 | 0.079978 | 0.378101 | 0.211525 |
| NtVQ48 | NtVQ57 | 0.0695372 | 0.254073 | 0.273689 |
| NtVQ4 | NtVQ5 | 0.0233695 | 0.132132 | 0.176864 |
| NtVQ50 | NtVQ51 | 0.105789 | 0.36515 | 0.289714 |
| NtVQ50 | NtVQ52 | 0.100707 | 0.571344 | 0.176263 |
| NtVQ50 | NtVQ56 | 0.10983 | 0.306966 | 0.357792 |
| NtVQ50 | NtVQ57 | 0.104101 | 0.370383 | 0.281064 |
| NtVQ51 | NtVQ52 | 0.041644 | 0.142729 | 0.29177 |
| NtVQ51 | NtVQ56 | 0.119672 | 0.258776 | 0.462456 |
| NtVQ51 | NtVQ57 | 0.13037 | 0.264411 | 0.493056 |
| NtVQ52 | NtVQ56 | 0.0932197 | 0.292244 | 0.318979 |
| NtVQ52 | NtVQ57 | 0.0769438 | 0.236928 | 0.324756 |
| NtVQ53 | NtVQ55 | 0.025791 | 0.099437 | 0.25937 |
| NtVQ56 | NtVQ57 | 0.0442237 | 0.206447 | 0.214214 |
| NtVQ5 | NtVQ4 | 0.0233695 | 0.132132 | 0.176864 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, H.; Zhou, C.; Timko, M.P. Genome-Wide Identification of the VQ Protein Gene Family of Tobacco (Nicotiana tabacum L.) and Analysis of Its Expression in Response to Phytohormones and Abiotic and Biotic Stresses. Genes 2020, 11, 284. https://doi.org/10.3390/genes11030284
Liu C, Liu H, Zhou C, Timko MP. Genome-Wide Identification of the VQ Protein Gene Family of Tobacco (Nicotiana tabacum L.) and Analysis of Its Expression in Response to Phytohormones and Abiotic and Biotic Stresses. Genes. 2020; 11(3):284. https://doi.org/10.3390/genes11030284
Chicago/Turabian StyleLiu, Cuihua, Hai Liu, Changyong Zhou, and Michael P. Timko. 2020. "Genome-Wide Identification of the VQ Protein Gene Family of Tobacco (Nicotiana tabacum L.) and Analysis of Its Expression in Response to Phytohormones and Abiotic and Biotic Stresses" Genes 11, no. 3: 284. https://doi.org/10.3390/genes11030284





