Multifunctional Janus Hydrogels: Surface Design Strategies for Next-Generation Clinical Solutions
Abstract
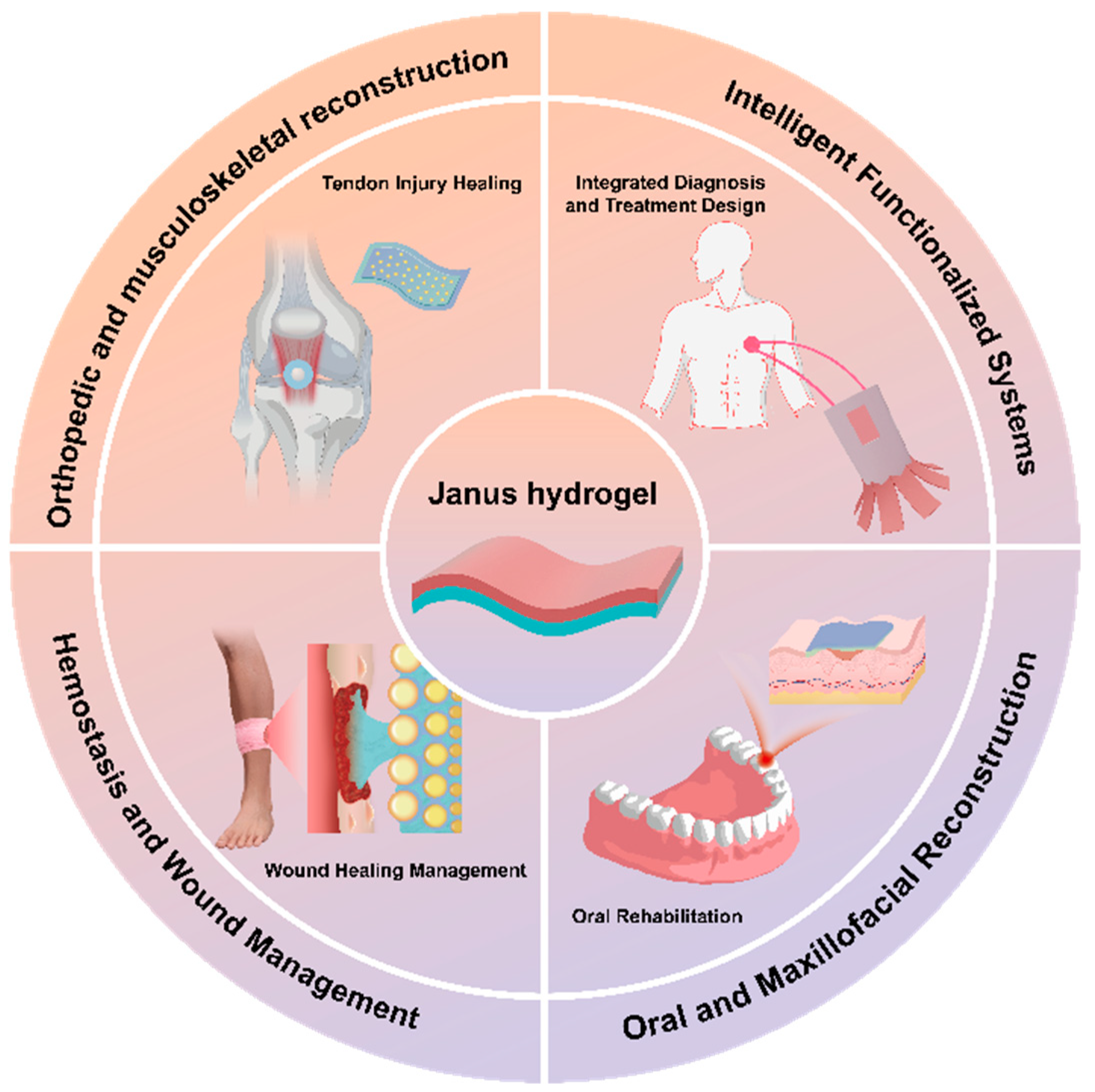
:1. Introduction
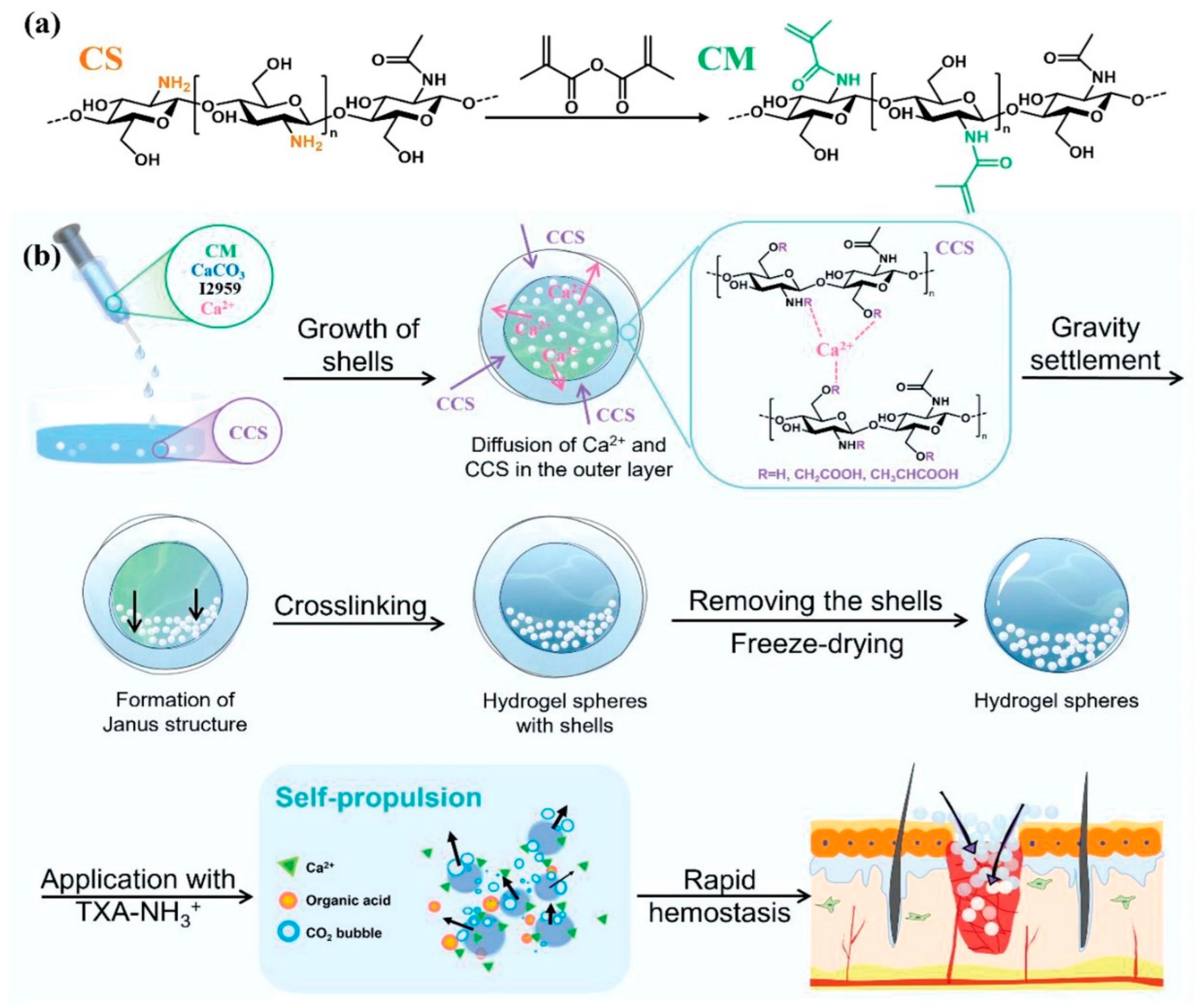
2. Hemostasis and Wound Management
2.1. Hemostatic Mechanisms and Interface Design Advantages
2.2. Expansion of Postoperative Anti-Adhesion Functionality
3. Oral and Maxillofacial Reconstruction
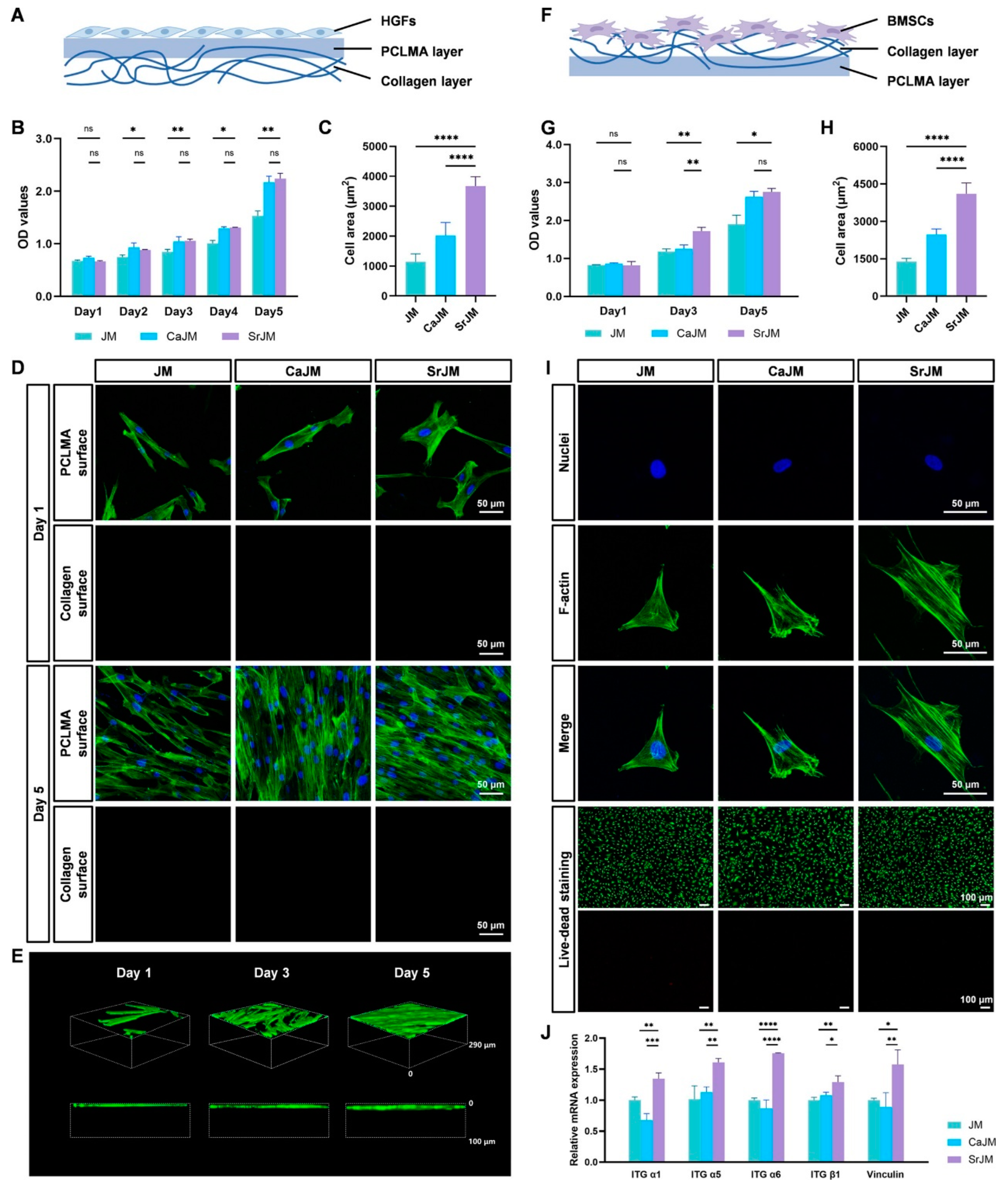
4. Orthopedic and Musculoskeletal Reconstruction
5. Intelligent Functionalized Systems
5.1. Integrated Diagnosis and Treatment Design
5.2. Targeted Delivery and Barrier Penetration
6. Conclusions and Future Work and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Yuk, H.; Wu, J.; Nabzdyk, C.S.; Zhao, X. A Multifunctional Origami Patch for Minimally Invasive Tissue Sealing. Adv. Mater. 2021, 33, e2007667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kim, J.; Hassan, S.; Suo, Z. Self-assembled nanocomposites of high water content and load-bearing capacity. Proc. Natl. Acad. Sci. USA 2022, 119, e2203962119. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Cheng, X.; Kong, Y.; Gao, Y.; Dan, H.; Wei, Y.; Yin, W.; Gao, B.; Yue, Q. One-step construction of P(AM-DMDAAC)/GO aerogel evaporator with Janus wettability for stable solar-driven desalination. Sep. Purif. Technol. 2022, 303, 122285. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, D.; Zhang, J.; Yu, Z.; Liu, M.; Fu, S. Biomass eggplant-derived photothermal aerogels with Janus wettability for cost-effective seawater desalination. Desalination 2022, 527, 115585. [Google Scholar] [CrossRef]
- You, I.; Mackanic, D.G.; Matsuhisa, N.; Kang, J.; Kwon, J.; Beker, L.; Mun, J.; Suh, W.; Kim, T.Y.; Tok, J.B.-H.; et al. Artificial multimodal receptors based on ion relaxation dynamics. Science 2020, 370, 961–965. [Google Scholar] [CrossRef]
- Liu, X. The more and less of electronic-skin sensors. Science 2020, 370, 910–911. [Google Scholar] [CrossRef]
- Wang, H.; Yi, X.; Liu, T.; Liu, J.; Wu, Q.; Ding, Y.; Liu, Z.; Wang, Q. An integrally formed janus hydrogel for robust wet-tissue adhesive and anti-postoperative adhesion. Adv. Mater. 2023, 35, 2300394. [Google Scholar] [CrossRef]
- Priemel, T.; Palia, G.; Förste, F.; Jehle, F.; Sviben, S.; Mantouvalou, I.; Zaslansky, P.; Bertinetti, L.; Harrington, M.J. Microfluidic-like fabrication of metal ion-cured bioadhesives by mussels. Science 2021, 374, 206–211. [Google Scholar] [CrossRef]
- Pan, M.; Shui, T.; Zhao, Z.; Xiang, L.; Yan, B.; Gu, N.; Zeng, H. Engineered Janus hydrogels: Biomimetic surface engineering and biomedical applications. Natl. Sci. Rev. 2024, 11, nwae316. [Google Scholar] [CrossRef]
- Zhang, J.; Zha, X.; Liu, G.; Zhao, H.; Liu, X.; Zha, L. Injectable extracellular matrix-mimetic hydrogel based on electrospun Janus fibers. Mater. Horiz. 2024, 11, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, Z.; Huang, H.; Fang, Y.; Weng, Y.; Wang, Z.; Zhao, H.; Liu, H. A tough Janus poly(vinyl alcohol)-based hydrogel for wound closure and anti postoperative adhesion. Acta Biomater. 2024, 188, 103–116. [Google Scholar] [CrossRef]
- You, J.B.; Choi, A.Y.; Baek, J.; Oh, M.S.; Im, S.G.; Lee, K.E.; Gwak, H.S. Application of monodirectional Janus patch to oromucosal delivery system. Adv. Healthc. Mater. 2015, 4, 2229–2236. [Google Scholar] [CrossRef]
- Cao, L.; Tian, D.; Lin, B.; Wang, W.; Bai, L.; Chen, H.; Yang, L.; Yang, H.; Wei, D. Fabrication of self-healing nanocomposite hydrogels with the cellulose nanocrystals-based Janus hybrid nanomaterials. Int. J. Biol. Macromol. 2021, 184, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhou, Y.; Hao, K.; Liu, C.; Yu, R.; Huang, A.; Wu, C.; Yang, Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, e2306360. [Google Scholar] [CrossRef]
- Qi, H.; Jing, X.; Hu, Y.; Wu, P.; Zhang, X.; Li, Y.; Zhao, H.; Ma, Q.; Dong, X.; Mahadevan, C. Electrospun green fluorescent-highly anisotropic conductive Janus-type nanoribbon hydrogel array film for multiple stimulus response sensors. Compos. Part B Eng. 2025, 288, 111933. [Google Scholar] [CrossRef]
- Zhong, S.; Xin, Z.; Hou, Y.; Li, Y.; Huang, H.W.; Sun, T.; Shi, Q.; Wang, H. Double-Modal Locomotion of a Hydrogel Ultra-Soft Magnetic Miniature Robot with Switchable Forms. Cyborg Bionic Syst. 2024, 6, 0077. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, J.; Song, J.; Cao, X.; Zhou, B.; Yang, L.; Li, C.; Wang, Y. A motion-responsive injectable lubricative hydrogel for efficient Achilles tendon adhesion prevention. Mater. Today Bio 2025, 30, 101458. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Dąbczyński, P.; Kostruba, A.; Budkowski, A. Temperature- and pH-Responsive Schizophrenic Copolymer Brush Coatings with Enhanced Temperature Response in Pure Water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive Smart Copolymer Coatings Based on P(NIPAM-co-HEMA) and P(OEGMA-co-HEMA) Brushes for Regenerative Medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Sun, S.; Lu, M.; Zhang, Y.; Liu, L.; Guo, Z.; Zhang, Z.; Ye, Z.; Zhang, J.; Chen, B.; et al. Super-hydrophilic and super-lubricating Zwitterionic hydrogel coatings coupled with polyurethane to reduce postoperative dura mater adhesions and infections. Acta Biomater. 2025, 192, 206–217. [Google Scholar] [CrossRef]
- Ju, Y.; Ma, C.; Ding, L.; Shi, M.; Wang, X.; Wu, D.; Wu, Q.; Qin, X.; Wang, Q. Surface enzyme-polymerization endows Janus hydrogel tough adhesion and regenerative repair in penetrating orocutaneous fistulas. Nat. Commun. 2024, 15, 10903. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, J.; Lai, J.; Zheng, X.; Wang, H.; Lu, B.; Huang, R.; Zhang, L. Novel Natural Polymer-Based Hydrogel Patches with Janus Asymmetric-Adhesion for Emergency Hemostasis and Wound Healing. Adv. Funct. Mater. 2024, 34, 2401030. [Google Scholar] [CrossRef]
- Zhao, Z.; Yuan, H.; Wang, J.; Tian, J.; Gao, H.; Nie, Y. “Release-Occupy” strategy to fabricate an integrally formed Janus adhesive hydrogel for wearable sensor. Chem. Eng. J. 2025, 505, 159540. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, W.; Cao, P.; Wang, Y.; Xie, A.-Q.; Niu, S.; Xu, Y.; Li, L.; Zhang, K.-Q.; Wang, X.-Q. Bioinspired Programmable and Ultrastretchable Janus Helical Hydrogel Fibers for Strain-Invariant Thermoelectric Body Heat Harvesting and Sensation. Nano Lett. 2025, 25, 2509–2518. [Google Scholar] [CrossRef]
- Luo, C.; Guo, A.; Li, J.; Tang, Z.; Luo, F. Janus Hydrogel to Mimic the Structure and Property of Articular Cartilage. ACS Appl. Mater. Interfaces 2022, 14, 35434–35443. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Mao, C.; Wang, C.; Liu, H.; Li, Z.; Zhu, S.; Jiang, H.; Cui, Z.; Zheng, Y.; et al. A Customized Janus Hydrogel with Robust Bio-Adhesion and Multi-Mode Disinfection for Rapid Recovery of Multi-Drug-Resistant Staphylococcus aureus-Infected Open Wounds. Adv. Funct. Mater. 2025, 2420443. [Google Scholar] [CrossRef]
- Zhuo, S.; Deng, Z.; Wu, Z.; Guo, Y.; Wu, Y.; Zhao, X.; Han, Y.; Guo, B. Janus gels for biomedical applications: Progress and future prospective. Prog. Polym. Sci. 2024, 155, 101856. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, W.; Wen, C.; Ji, X.; Diao, H.; Hou, Y.; Fan, J.; Liu, Z.; Ji, T.; Sun, F.; et al. Cellulose-Based Cryogel Microspheres with Nanoporous and Controllable Wrinkled Morphologies for Rapid Hemostasis. Nano Lett. 2022, 22, 6350–6358. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Sun, F.; Dou, X.; Liu, J.; Wang, Y.; Li, M.; Gao, J.; Liu, X.; Wang, X.; et al. A Super Tough, Rapidly Biodegradable, Ultrafast Hemostatic Bioglue. Adv. Mater. 2023, 35, e2208622. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Ke, X.; Zhang, M.; Ding, Y.; Wang, H.; Chen, H.; Xie, J.; Li, J. A janus adhesive hydrogel with integrated attack and defense for bacteria killing and antifouling. BME Front. 2024, 5, 0059. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, A.; Sheng, N.; He, Y.; Liu, W.; Li, Z.; Luo, F.; Li, J.; Tan, H. Janus Polyurethane Adhesive Patch with Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 15970–15980. [Google Scholar] [CrossRef]
- Fang, Y.; Zheng, Y.; Chi, C.; Jiang, S.; Qin, W.; Zhang, Y.; Liu, H.; Chen, Q. PAA-PU Janus Hydrogels Stabilized by Janus Particles and its Interfacial Performance During Hemostatic Processing. Adv. Heal. Mater. 2024, 13, e2303802. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, J.; Li, Q.; Chen, Q. Chitosan based Janus cryogel with anisotropic wettability, antibacterial activity, and rapid shape memory for effective hemostasis. Int. J. Biol. Macromol. 2024, 254 Pt 2, 127821. [Google Scholar] [CrossRef]
- Yu, Q.; Su, B.; Zhao, W.; Zhao, C. Janus Self-Propelled Chitosan-Based Hydrogel Spheres for Rapid Bleeding Control. Adv. Sci. 2023, 10, 2205989. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Gao, Y.; Yan, B.-Y.; Ding, L.-Q.; Sun, T.-C.; Liu, Z.; Ramakrishna, S.; Long, Y.-Z.; Zhang, J. Collocalia birds inspired Janus-structured bandage with strong wet tissue adhesion for rapid hemostasis and wound healing. Chem. Eng. J. 2023, 464, 142458. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Wu, X.; Han, M.; Peng, R.; Zhao, R.; Qin, M.; Li, T.; Yin, J.; Yu, L.; et al. A Sprayable Janus Hydrogel as an Effective Bioadhesive for Gastrointestinal Perforation Repair. Adv. Funct. Mater. 2024, 34, 2408479. [Google Scholar] [CrossRef]
- Gong, Y.; Zhu, B.; Chen, Y.; Li, F.; Duan, W.; Martin-Saldaña, S.; Yang, R.; Gao, X.; Zhang, B.; Luo, L.; et al. Organism-Inspired Antioxidant Bioadhesive with Strong Sealing Ability to Prevent Epidural Adhesion. ACS Nano 2024, 18, 21411–21432. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Duan, W.; Martin-Saldaña, S.; Li, C.; Yu, H.; Feng, L.; Zhang, X.; Du, B.; Li, G.; et al. Succinic Ester-Based Shape Memory Gelatin Sponge for Noncompressible Hemorrhage without Hindering Tissue Regeneration. Adv. Heal. Mater. 2023, 12, e2202122. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Lai, Y.; Wang, Y.; Liu, P.; Shen, J. An Adhesive/Anti-Adhesive Janus Tissue Patch for Efficient Closure of Bleeding Tissue with Inhibited Postoperative Adhesion. Adv. Sci. 2023, 10, e2301427. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Xu, J.; Yu, L.; Qin, M.; Li, J.; Liu, S.; Zheng, W.; Li, Z.; Ouyang, J.; et al. Photocurable injectable Janus hydrogel with minimally invasive delivery for all-in-one treatment of gastric perforations and postoperative adhesions. Theranostics 2023, 13, 5365–5385. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Feng, J.; Feng, Z.; Liu, J.; Yang, Y.; Li, X.; Lei, M.; Guo, H.; Wei, Z.; Lv, Y.; et al. An endoscopically compatible fast-gelation powder forms Janus-adhesive hydrogel barrier to prevent postoperative adhesions. Proc. Natl. Acad. Sci. USA 2023, 120, e2219024120. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, M.; Zhao, S.; Li, H.; Wang, W.; Cheng, J.; Jin, L.; Wang, Y. Janus amphiphilic nanofiber membranes synergistically drive antibacterial and anti-inflammatory strategies for skin wound healing. Mater. Des. 2023, 227, 111778. [Google Scholar] [CrossRef]
- He, Y.; Li, Q.; Chen, P.; Duan, Q.; Zhan, J.; Cai, X.; Wang, L.; Hou, H.; Qiu, X. A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention. Nat. Commun. 2022, 13, 7666. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Qiao, F.; Ma, J.; Miao, H.; Gao, S.; Ma, Y.; Yu, X.; Liu, S.; Yuan, H.; et al. Bioinspired Asymmetric-Adhesion Janus Hydrogel Patch Regulating by Zwitterionic Polymers for Wet Tissues Adhesion and Postoperative Adhesion Prevention. Adv. Healthc. Mater. 2024, 13, e2402268. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.; Yang, Q.; Chen, S.; Yan, Z.; Li, X.; Liang, L.; Guo, B.; Wang, B.; Zhang, H.; et al. A Single-Component Janus Zwitterionic Hydrogel Patch with a Bionic Microstructure for Postoperative Adhesion Prevention. ACS Appl Mater Interfaces 2024, 16, 22900–22913. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Wang, Y.; Cai, H.; Zhang, D.; Tan, H.; Li, J. Fabrication of a multifunctional hydrogel with a robust interface bioinspired by the structure of the dentogingival junction. Chem. Commun. 2020, 56, 3633–3636. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; He, Y.; Zhao, X.; Lin, J.; Feng, Y.; Chen, J.; Luo, F.; Li, Z.; Li, J.; et al. A bioinspired Janus polyurethane membrane for potential periodontal tissue regeneration. J. Mater. Chem. B 2022, 10, 2602–2616. [Google Scholar] [CrossRef]
- Shi, T.; Xiong, Y.-H.; Song, W.; Sun, M.; Wu, R.; Li, Y.; Sun, Q.; Duan, S.; Xu, F.-J. Polysaccharide-based antibacterial nanocomposite hydrogels with Janus structure for treatment of infected extraction socket. Sci. China Mater. 2024, 67, 2550–2557. [Google Scholar] [CrossRef]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, G.; Ma, L.; Yang, G.; Lin, S.; Sun, N.; Wang, J.; Ma, H.; Jiang, X.; Zhang, W. An axolotl limb regeneration-inspired strategy to enhance alveolar bone regeneration. Bioact. Mater. 2025, 48, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Agnes, C.J.; Li, L.; Bertrand, D.; Murshed, M.; Willie, B.M.; Tabrizian, M. Assessment of bone regeneration potential for a 6-bromoindirubin-3′-oxime (BIO) encapsulated chitosan based scaffold in a mouse critical sized bone defect model. Int. J. Biol. Macromol. 2025, 304 Pt 2, 140995. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, L.; Guo, Y.; Zhao, S.; Yang, Y.; Dong, D.; Ding, W.; Dai, K.; Gong, W.; Yuan, G.; et al. Effectiveness and safety of biodegradable Mg-Nd-Zn-Zr alloy screws for the treatment of medial malleolar fractures. J. Orthop. Transl. 2021, 27, 96–100. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Pei, J.; Tian, Y.; Zhang, J.; Jiang, C.; Huang, J.; Pang, Z.; Cao, Y.; Wang, X.; et al. Degradation and osteogenic induction of a SrHPO4-coated Mg–Nd–Zn–Zr alloy intramedullary nail in a rat femoral shaft fracture model. Biomaterials 2020, 247, 119962. [Google Scholar] [CrossRef]
- Wang, X.; Shen, P.; Gu, N.; Shao, Y.; Lu, M.; Tang, C.; Wang, C.; Chu, C.; Xue, F.; Bai, J. Dual Mg-Reinforced PCL Membrane with a Janus Structure for Vascularized Bone Regeneration and Bacterial Elimination. ACS Biomater. Sci. Eng. 2024, 10, 537–549. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, W.; Wu, X.; Gao, X.; Song, F.; Duan, B.; Lu, A.; Yang, H.; Huang, C. Janus Membrane with Intrafibrillarly Strontium-Apatite-Mineralized Collagen for Guided Bone Regeneration. ACS Nano 2024, 18, 7204–7222. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Lee, H.L.; Wen, B.; Daubert, D.; Darveau, R. Gingival crevicular fluid during experimental gingivitis: A review of immune and tissue regulation. J. Periodontol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Danz, J.C.; Degen, M. Selective modulation of the bone remodeling regulatory system through orthodontic tooth movement-a review. Front. Oral Health 2025, 6, 1472711. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, W.; Yu, H.; He, C.; Yu, M.; Zhou, Y.; Jiang, Y.; Bian, L.; Peng, X. Solvent volatilization annealing-prepared Janus film with asymmetric bioadhesion and inherent biological functions to expedite oral ulcer healing. Biomaterials 2025, 318, 123131. [Google Scholar] [CrossRef]
- Huang, L.; Wu, T.; Sun, J.; Lin, X.; Peng, Y.; Zhang, R.; Gao, Y.; Xu, S.; Sun, Y.; Zhou, Y.; et al. Biocompatible chitin-based Janus hydrogel membranes for periodontal repair. Acta Biomater. 2024, 190, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.M.; Trigo-Navarro, L.; Diestre-Morcillo, E.; Pastor-Ramon, E.; Puerto-Parejo, L.M. Nutritional Interventions for Pressure Ulcer Prevention in Hip Fracture Patients: A Systematic Review and Meta-Analysis of Controlled Trials. Nutrients 2025, 17, 644. [Google Scholar] [CrossRef]
- Parra-Rojas, S.; Velázquez-Cayón, R.T.; Ciortan-Pop, M.E.; Martins, M.D.; Spanemberg, J.C. Preventive Photobiomodulation for Chemotherapy-Induced Oral Mucositis: A Systematic Review of Randomized Clinical Trials. Biomedicines 2025, 13, 268. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yu, X.; Rong, H.; Chen, W.; Zhang, J.; Dong, A.; Feng, Z.; Li, S. A bio-inspired Janus hydrogel patch facilitates oral ulcer repair by combining prolonged wet adhesion and lubrication. Acta Biomater. 2024, 190, 120–132. [Google Scholar] [CrossRef]
- Kwon, D.; Genden, E.M.; de Bree, R.; Rodrigo, J.P.; Rinaldo, A.; Sanabria, A.; Rapidis, A.D.; Takes, R.P.; Ferlito, A. Overcoming wound complications in head and neck salvage surgery. Auris Nasus Larynx 2018, 45, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Yan, X.; Yuan, F.Z.; Lin, L.; Wang, S.J.; Ye, J.; Zhang, J.Y.; Yang, M.; Wu, D.C.; Wang, X.; et al. Kartogenin-Conjugated Double-Network Hydrogel Combined with Stem Cell Transplantation and Tracing for Cartilage Repair. Adv. Sci. 2022, 9, e2105571. [Google Scholar] [CrossRef]
- Ouyang, C.; Tu, T.; Yu, H.; Wang, L.; Ni, Z.; Yang, J.; Dong, Y.; Zou, X.; Zhou, W.; Liu, J. One-Step Formed Janus Hydrogel with Time-Space Regulating Properties for Suture-Free and High-Quality Tendon Healing. Adv. Sci. 2025, 12, 2411400. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, X.; Li, X.; An, Q.; Zhang, Y.; Guo, C.; Zhao, Y.; Zhang, Y. Shear Flow-Assembled Janus Membrane with Bifunctional Osteogenic and Antibacterial Effects for Guided Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 3984–3993. [Google Scholar] [CrossRef]
- Prajatelistia, E.; Sanandiya, N.D.; Nurrochman, A.; Marseli, F.; Choy, S.; Hwang, D.S. Biomimetic Janus chitin nanofiber membrane for potential guided bone regeneration application. Carbohydr. Polym. 2021, 251, 117032. [Google Scholar] [CrossRef]
- Lv, S.; Yuan, X.; Xiao, J.; Jiang, X. Hemostasis-osteogenesis integrated Janus carboxymethyl chitin/hydroxyapatite porous membrane for bone defect repair. Carbohydr. Polym. 2023, 313, 120888. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Mao, Z.; Li, L.; Zhang, Y.; Yang, L.; Hou, S.; Guan, J.; Zheng, Y.; Li, X.; Fan, Y. Janus decellularized membrane with anisotropic cell guidance and anti-adhesion silk-based coatings for spinal dural repair. Nat. Commun. 2025, 16, 1674. [Google Scholar] [CrossRef]
- Pan, P.; Wang, J.; Wang, X.; Yu, X.; Chen, T.; Jiang, C.; Liu, W. Barrier Membrane with Janus Function and Structure for Guided Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 47178–47191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Y.; Suo, D.; Zhao, S.; Cheung, J.C.-W.; Leung, P.H.-M.; Zhao, X. A Biomimetic Adhesive and Robust Janus Patch with Anti-Oxidative, Anti-Inflammatory, and Anti-Bacterial Activities for Tendon Repair. ACS Nano 2023, 17, 16798–16816. [Google Scholar] [CrossRef]
- Freedman, B.R.; Kuttler, A.; Beckmann, N.; Nam, S.; Kent, D.; Schuleit, M.; Ramazani, F.; Accart, N.; Rock, A.; Li, J.; et al. Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity. Nat. Biomed. Eng. 2022, 6, 1167–1179. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xiao, L.; Nie, Y.; Wang, W.; Bai, L.; Chen, H.; Yang, L.; Yang, H.; Wei, D. Fabrication of Janus-type nanocomposites from cellulose nanocrystals for self-healing hydrogels’ flexible sensors. Colloids Surf. B Biointerfaces 2022, 216, 112554. [Google Scholar] [CrossRef]
- Wang, Q.; Du, J.; Meng, J.; Yang, J.; Cao, Y.; Xiang, J.; Yu, J.; Li, X.; Ding, B. Janus Nanofibrous Patch with In Situ Grown Superlubricated Skin for Soft Tissue Repair with Inhibited Postoperative Adhesion. ACS Nano 2024, 18, 12341–12354. [Google Scholar] [CrossRef]
- Luo, J.; Jin, Y.; Li, L.; Chang, B.; Zhang, B.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Wang, J.; et al. A selective frequency damping and Janus adhesive hydrogel as bioelectronic interfaces for clinical trials. Nat. Commun. 2024, 15, 8478. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, W.; Xia, Y.; Zhang, L.; Cao, Y.; Li, S.; Lu, W.; Liu, C.; Fu, S. Implantable Resistive Strain Sensor-Decorated Colloidal Crystal Hydrogel Catheter for Intestinal Tract Pressure Sensing. ACS Appl. Mater. Interfaces 2024, 16, 21736–21745. [Google Scholar] [CrossRef]
- Wang, W.; Harimurti, S.; Inoue, D.; Nayeem, O.G.; Wang, J.; Okuda, C.; Hashizume, D.; Lee, S.; Fukuda, K.; Yokota, T.; et al. Janus Membrane-Based Wearable pH Sensor with Sweat Absorption, Gas Permeability, and Self-Adhesiveness. ACS Appl. Mater. Interfaces 2024, 16, 27065–27074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.; Zhang, L.; Cao, Y.; Li, S.; Lu, W.; Mao, Z.; Jiang, Z.; Wang, Y.; Liu, C.; et al. Bioinspired colloidal crystal hydrogel pressure sensors with Janus wettability for uterus cervical canal tension perception. J. Mater. Chem. B 2024, 12, 8941–8951. [Google Scholar] [CrossRef]
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Lin, Q.; Boo, Y.J.; Ow, V.; Zhao, X.; Wong, D.S.L.; Lim, J.Y.C.; Xue, K.; Su, X.; et al. Injectable PTHF-based thermogelling polyurethane implants for long-term intraocular application. Biomater. Res. 2022, 26, 70. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Huang, E.; Lui, K.-H. Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs. Asian J. Pharm. Sci. 2021, 16, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, X.; Li, Y.; Wu, Z.; Xu, C.; Chen, Z.; He, W. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm. Sin. B 2021, 11, 941–960. [Google Scholar] [CrossRef]
- Zhu, Z.-R.; Huang, J.-N.; Li, J.-Z.; Cao, H.; Lin, Z.-Y.; Li, Y. Janus hydrogel/electrospun-membrane dressing enhancing wound healing in rats. Biomed. Eng. Commun. 2024, 3, 10. [Google Scholar] [CrossRef]
- Schadendorf, D.; Van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Y.; He, Y.; Li, M.; Liu, Y.; Zhou, X.; Hou, J.; Zhou, S. In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing. Nat. Commun. 2024, 15, 814. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Song, Y.; He, X.; Xu, T.; Zhang, X. Hydrogel-Functionalized Bandages with Janus Wettability for Efficient Unidirectional Drug Delivery and Wound Care. ACS Nano 2024, 18, 3468–3479. [Google Scholar] [CrossRef]
- Nummelin, S.; Liljestrom, V.; Saarikoski, E.; Ropponen, J.; Nykanen, A.; Linko, V.; Seppala, J.; Hirvonen, J.; Ikkala, O.; Bimbo, L.M.; et al. Self-assembly of amphiphilic Janus dendrimers into mechanically robust supramolecular hydrogels for sustained drug release. Chemistry 2015, 21, 14433–14439. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Cheng, Y.; Chen, G.; Zhao, Y. 3D-Printed Janus Piezoelectric Patches for Sonodynamic Bacteria Elimination and Wound Healing. Research 2023, 6, 0022. [Google Scholar] [CrossRef] [PubMed]
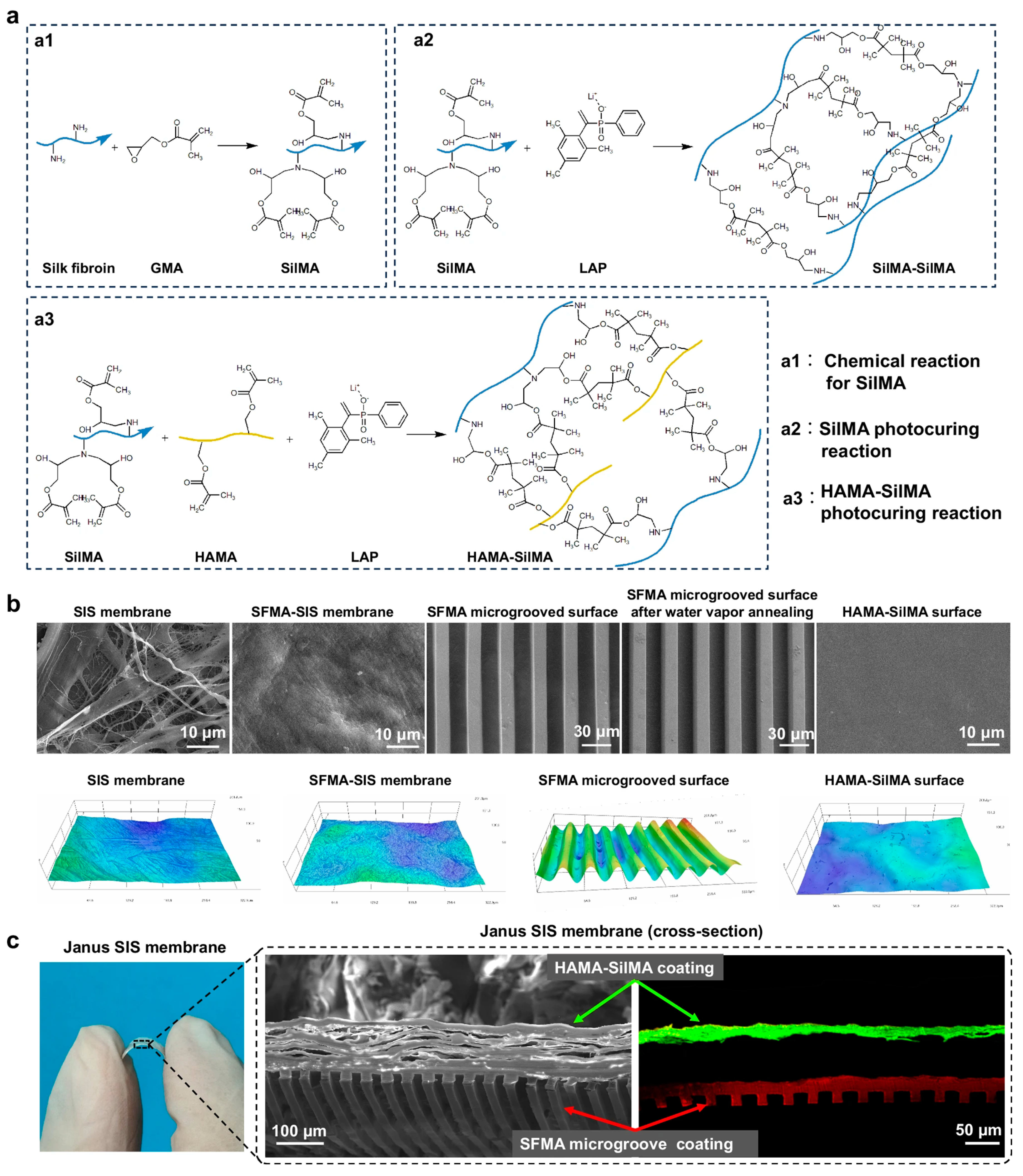
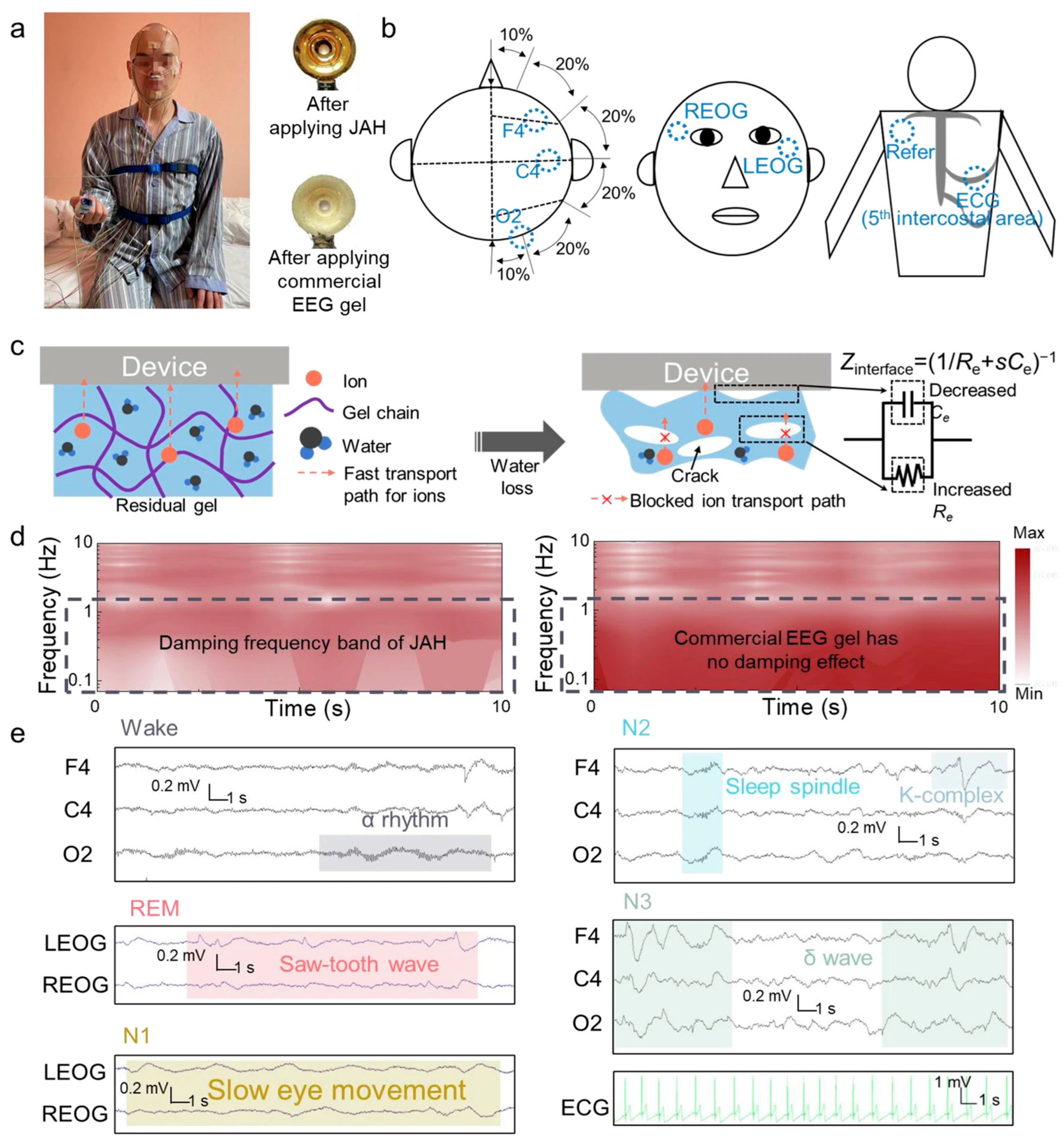
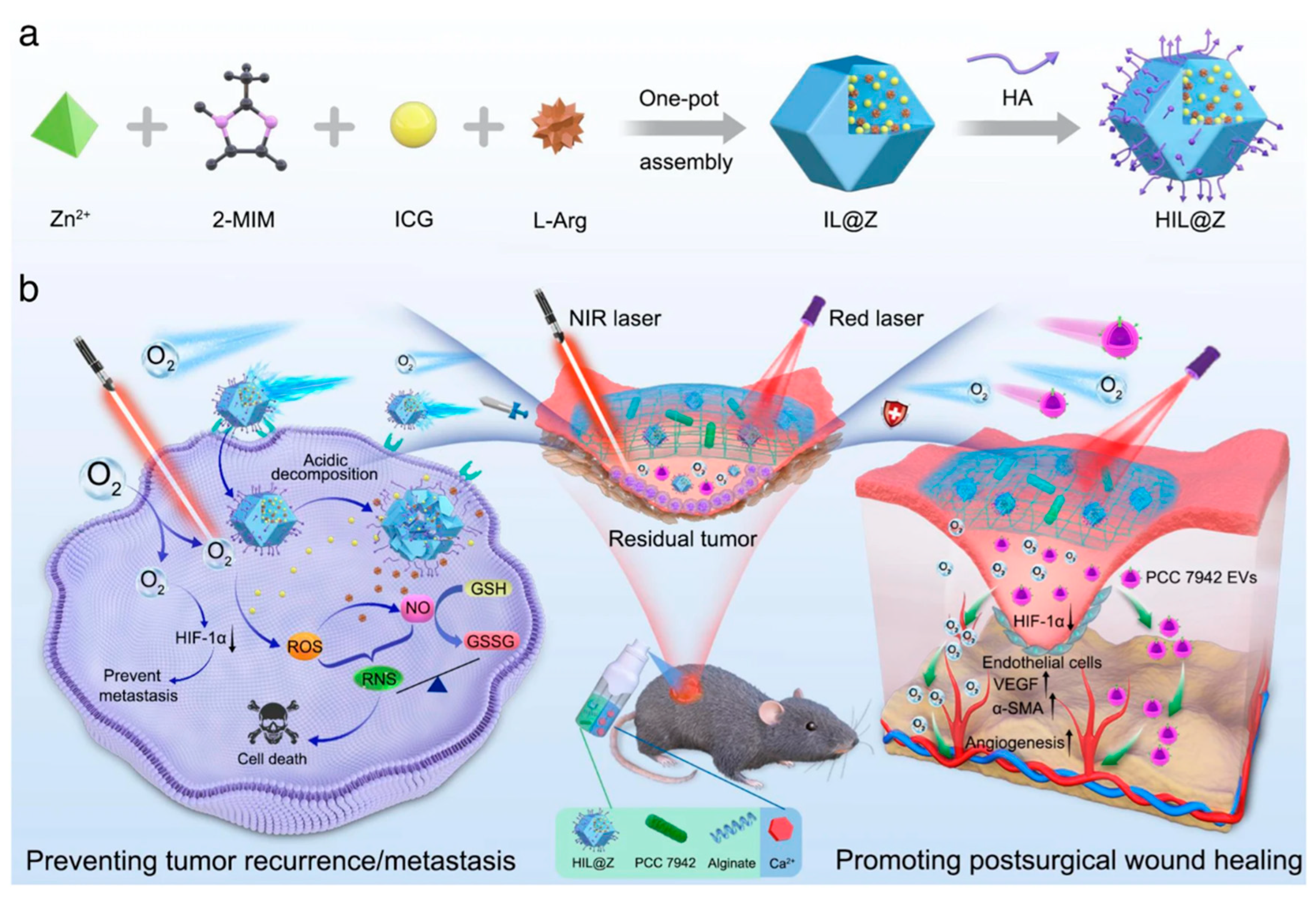
| Key Materials | Construction Strategy | Application Function | Ref. |
|---|---|---|---|
| SFMA microgroove coating, HAMA-SilMA anti-adhesion coating, SIS substrate | UV photocuring and micromolding | Spinal dura mater repair and anti-adhesion barrier | [72] |
| Alginate, polyacrylamide, chitosan, and triamcinolone acetonide delivery | Double-network formation and unilateral adhesion | Tendon adhesion enhancement and anti-inflammatory therapy | [75] |
| HAD polymer (hyaluronic acid–dopamine–methacrylate), LAP photoinitiator | Injectable UV photocrosslinking and asymmetric adhesion | Sutureless gastric perforation repair and anti-adhesion barrier | [42] |
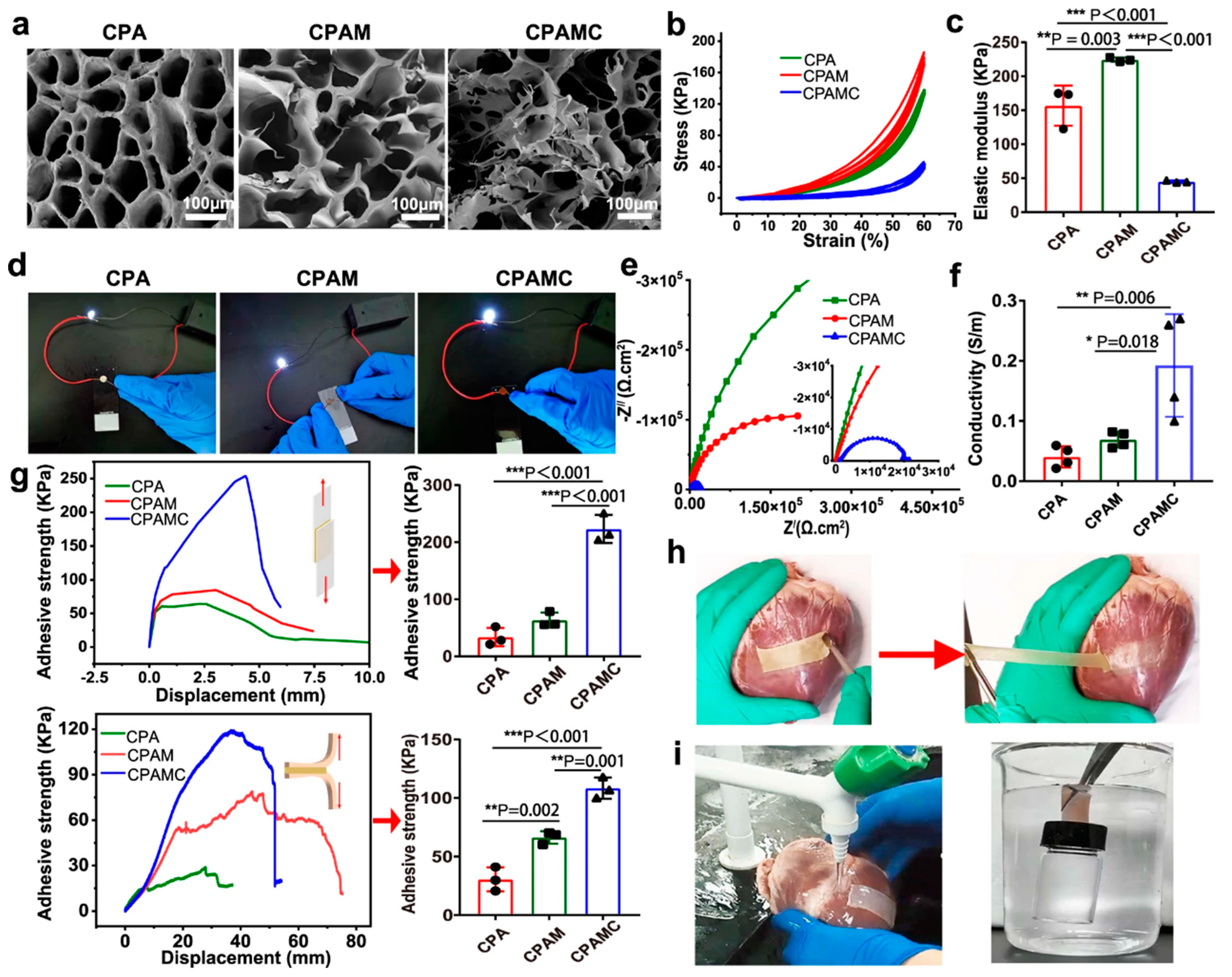
| CPAMC hydrogel and PCA hydrogel | Interfacial copolymerization of redox-responsive conductive and anti-adhesion hydrogels | Cardiac infarction repair and prevention of postoperative tissue synechia | [45] |
| Acrylamide hydrogel with silver nanoparticles | Natural sedimentation-assisted gelation with asymmetric silver nanoparticle distribution | Selective respiration noise damping and stable bioelectrical signal transmission | [79] |
| PEGDA-BTO-Au hydrogel and GelMA-VEGF hydrogel | Extrusion-based 3D printing of dual-functional hydrogel layers | Sonodynamic bacterial elimination and programmable wound healing | [92] |
| Strontium–apatite-mineralized collagen, PCLMA dense layer | Biomimetic mineralization and photocrosslinking | Guided bone regeneration and anti-soft tissue invasion | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Cheng, J.; Liu, H.; Wang, Y.; Zhang, C.; Huang, D.; Liu, J.; Wang, Z. Multifunctional Janus Hydrogels: Surface Design Strategies for Next-Generation Clinical Solutions. Gels 2025, 11, 343. https://doi.org/10.3390/gels11050343
Yan T, Cheng J, Liu H, Wang Y, Zhang C, Huang D, Liu J, Wang Z. Multifunctional Janus Hydrogels: Surface Design Strategies for Next-Generation Clinical Solutions. Gels. 2025; 11(5):343. https://doi.org/10.3390/gels11050343
Chicago/Turabian StyleYan, Taoxu, Junyao Cheng, Haoming Liu, Yifan Wang, Chuyue Zhang, Da Huang, Jianheng Liu, and Zheng Wang. 2025. "Multifunctional Janus Hydrogels: Surface Design Strategies for Next-Generation Clinical Solutions" Gels 11, no. 5: 343. https://doi.org/10.3390/gels11050343
APA StyleYan, T., Cheng, J., Liu, H., Wang, Y., Zhang, C., Huang, D., Liu, J., & Wang, Z. (2025). Multifunctional Janus Hydrogels: Surface Design Strategies for Next-Generation Clinical Solutions. Gels, 11(5), 343. https://doi.org/10.3390/gels11050343









