Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats
Abstract
1. Introduction
2. Results and Discussion
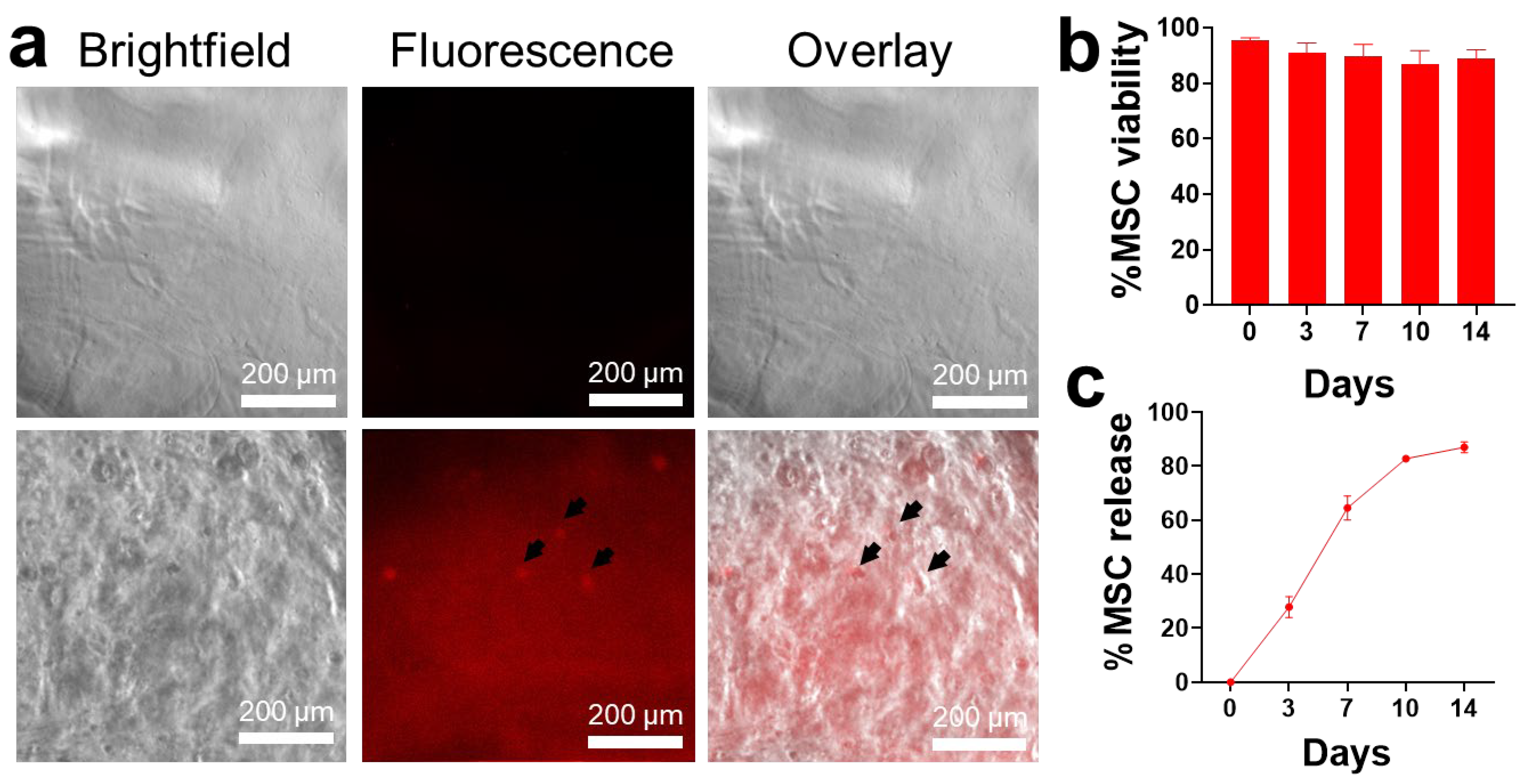
2.1. MSC Loading in Alginate Hydrogels
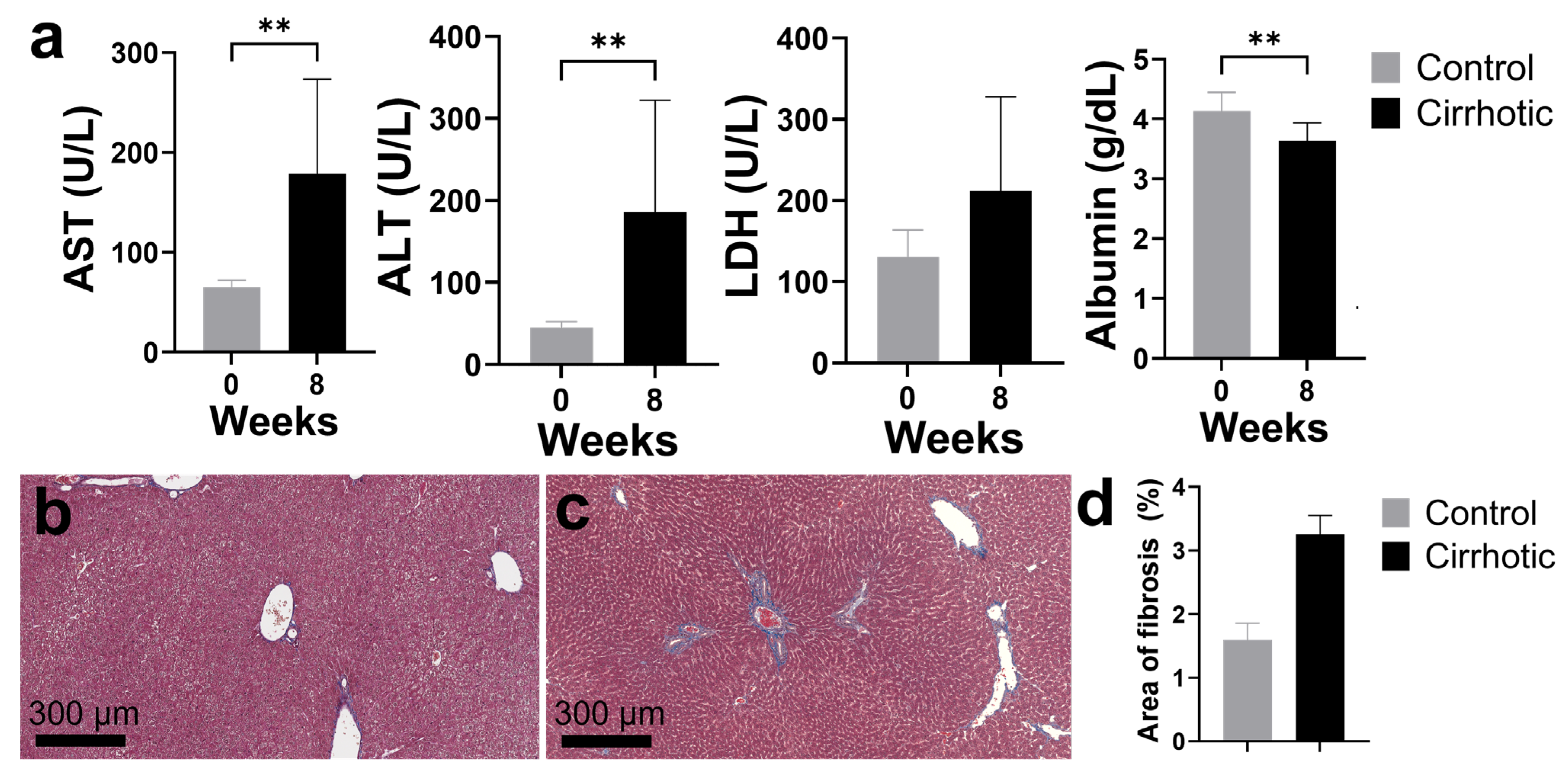
2.2. Establishing a Rat Model for Cirrhosis and Portal Vein Ligation
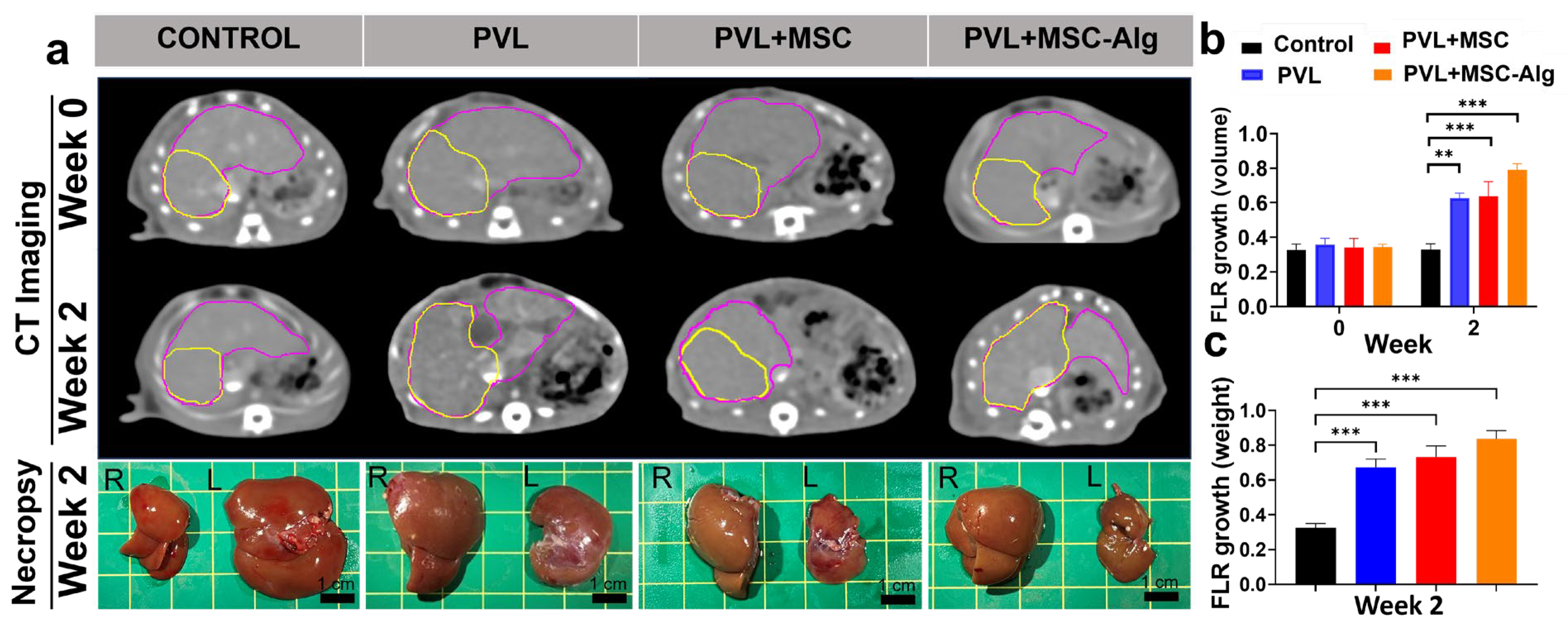
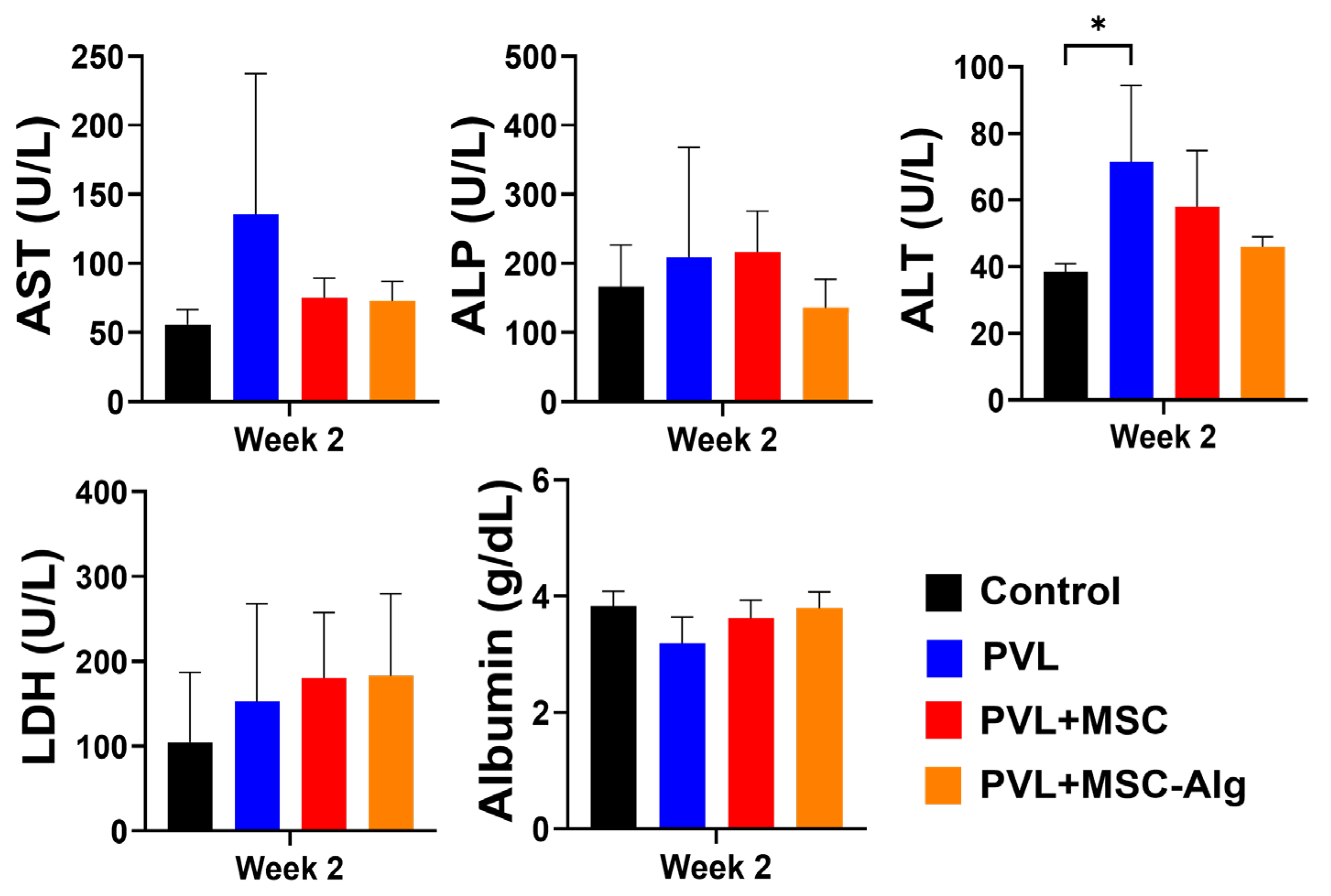
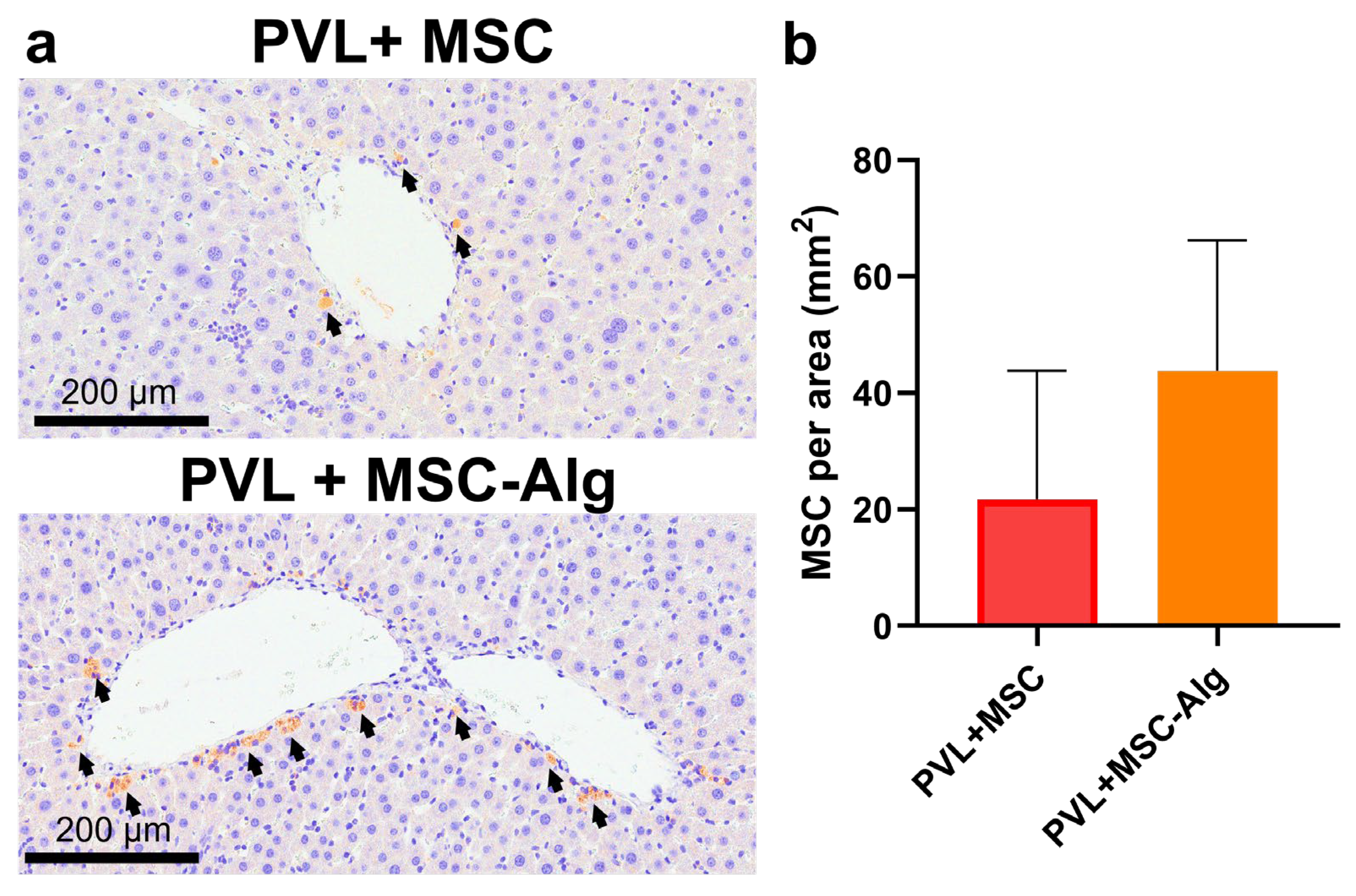
2.3. Histopathological Analysis of Liver Samples After Treatment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Animals
4.3. Preparation of MSC-Loaded Hydrogels
4.4. In Vitro MSC Survival and Release in Hydrogels
4.5. Cirrhosis Induction and Portal Vein Ligation in Rats
4.6. Monitoring of Liver Volume, Weight, and Function
4.7. Histopathological Examination
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Tarao, K.; Nozaki, A.; Ikeda, T.; Sato, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019, 8, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Franssen, B.; Jibara, G.; Tabrizian, P.; Schwartz, M.E.; Roayaie, S. Actual 10-year survival following hepatectomy for hepatocellular carcinoma. HPB 2014, 16, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Goumard, C.; Lim, C.; Le Cleach, A.; Wagner, M.; Scatton, O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020, 2, 100134. [Google Scholar] [CrossRef]
- Rodimova, S.; Mozherov, A.; Elagin, V.; Karabut, M.; Shchechkin, I.; Kozlov, D.; Krylov, D.; Gavrina, A.; Bobrov, N.; Zagainov, V.; et al. Effect of Hepatic Pathology on Liver Regeneration: The Main Metabolic Mechanisms Causing Impaired Hepatic Regeneration. Int. J. Mol. Sci. 2023, 24, 9112. [Google Scholar] [CrossRef]
- Lim, C.; Farges, O. Portal vein occlusion before major hepatectomy in patients with colorectal liver metastases: Rationale, indications, technical aspects, complications and outcome. J. Visc. Surg. 2012, 149, e86–e96. [Google Scholar] [CrossRef]
- Alvarez, F.A.; Castaing, D.; Figueroa, R.; Allard, M.A.; Golse, N.; Pittau, G.; Ciacio, O.; Sa Cunha, A.; Cherqui, D.; Azoulay, D.; et al. Natural history of portal vein embolization before liver resection: A 23-year analysis of intention-to-treat results. Surgery 2018, 163, 1257–1263. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.G.; Ko, G.Y.; Kim, B.S.; Sung, K.B.; Kim, M.H.; Lee, S.K.; Hong, H.N. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann. Surg. 2009, 249, 608–616. [Google Scholar] [CrossRef]
- Wang, J.L.; Ding, H.R.; Pan, C.Y.; Shi, X.L.; Ren, H.Z. Mesenchymal stem cells ameliorate lipid metabolism through reducing mitochondrial damage of hepatocytes in the treatment of post-hepatectomy liver failure. Cell Death Dis. 2021, 12, 111. [Google Scholar] [CrossRef]
- Eom, Y.W.; Kang, S.H.; Kim, M.Y.; Lee, J.I.; Baik, S.K. Mesenchymal stem cells to treat liver diseases. Ann. Transl. Med. 2020, 8, 563. [Google Scholar] [CrossRef]
- Itaba, N.; Matsumi, Y.; Okinaka, K.; Ashla, A.A.; Kono, Y.; Osaki, M.; Morimoto, M.; Sugiyama, N.; Ohashi, K.; Okano, T.; et al. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci. Rep. 2015, 5, 16169. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Perez, J.V.D.; Bernardino, M.R.; Damasco, J.A.; San Valentin, E.M.D.; Klusman, C.; Martin, B.; Canlas, G.M.; Heralde, F.M.; Fowlkes, N.; et al. Bismuth-infused perivascular wrap facilitates delivery of mesenchymal stem cells and attenuation of neointimal hyperplasia in rat arteriovenous fistulas. Biomater. Adv. 2025, 166, 214052. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Owens, T.C.; Melancon, S.; Workeneh, I.; Tran Cao, H.S.; Vauthey, J.N.; Huang, S.Y. Current Perspectives and Progress in Preoperative Portal Vein Embolization with Stem Cell Augmentation (PVESA). Stem Cell Rev. Rep. 2024, 20, 1236–1251. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Nevi, L.; Safarikia, S.; Di Matteo, S.; Biancaniello, F.; Chiappetta, M.F.; Cardinale, V. Hyaluronan-Based Grafting Strategies for Liver Stem Cell Therapy and Tracking Methods. Stem Cells Int. 2019, 2019, 3620546. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pandey, V.K.; Shankar, B.S.; Melo, J.S. Spray drying as an efficient route for synthesis of silica nanoparticles-sodium alginate biohybrid drug carrier of doxorubicin. Colloids Surf. B Biointerfaces 2021, 197, 111445. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. [Google Scholar] [CrossRef]
- Reyes, M.S.S.; Medina, P.M.B. Leachates from plastics and bioplastics reduce lifespan, decrease locomotion, and induce neurotoxicity in Caenorhabditis elegans. Environ. Pollut. 2024, 357, 124428. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Kumar, S.; Kabat, M.; Basak, S.; Babiarz, J.; Berthiaume, F.; Grumet, M. Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation. Biomolecules 2022, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of Electrospun Scaffolds and Biological Polymers for Enhancing the Delivery and Efficacy of Mesenchymal Stem/Stromal Cell Therapies. Front. Biosci. 2024, 29, 228. [Google Scholar] [CrossRef]
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, R.K.; Aghlara-Fotovat, S.; Pugazenthi, A.; Ryan, C.T.; Singh, V.P.; Mathison, M.; Jarvis, M.I.; Mukherjee, S.; Hernandez, A.; Veiseh, O. Immune-modulatory alginate protects mesenchymal stem cells for sustained delivery of reparative factors to ischemic myocardium. Biomater. Sci. 2020, 8, 5061–5070. [Google Scholar] [CrossRef]
- Joddar, B.; Tasnim, N.; Thakur, V.; Kumar, A.; McCallum, R.W.; Chattopadhyay, M. Delivery of Mesenchymal Stem Cells from Gelatin-Alginate Hydrogels to Stomach Lumen for Treatment of Gastroparesis. Bioengineering 2018, 5, 12. [Google Scholar] [CrossRef]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef]
- Mao, A.S.; Özkale, B.; Shah, N.J.; Vining, K.H.; Descombes, T.; Zhang, L.; Tringides, C.M.; Wong, S.-W.; Shin, J.-W.; Scadden, D.T.; et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl. Acad. Sci. USA 2019, 116, 15392–15397. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.; Mahou, R.; Morel, P.; Meyer, J.; Montanari, E.; Muller, Y.D.; Christofilopoulos, P.; Wandrey, C.; Gonelle-Gispert, C.; Bühler, L.H. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015, 62, 634–641. [Google Scholar] [CrossRef]
- Enoch, K.; Rakavi, C.S.; Somasundaram, A.A. Tuning the rheological properties of chitosan/alginate hydrogels for tissue engineering application. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134434. [Google Scholar] [CrossRef]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D culture of alginate-hyaluronic acid hydrogel supports the stemness of human mesenchymal stem cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Rezaei, S.; Shakibaie, M.; Kabir-Salmani, M.; Soltani Moghaddam, M.; Rezvani, M.; Shahali, M.; Naseri, M. Improving the Growth Rate of Human Adipose-Derived Mesenchymal Stem Cells in Alginate/Gelatin Versus Alginate Hydrogels. Iran. J. Biotechnol. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Rosa, G.D.S.; Rossi, M.C.; Stievani, F.D.C.; Pfeifer, J.P.H.; Krieck, A.M.T.; Bovolato, A.L.D.C.; Fonseca-Alves, C.E.; Borrás, V.A.; Alves, A.L.G. In Vitro Biological Performance of Alginate Hydrogel Capsules for Stem Cell Delivery. Front. Bioeng. Biotechnol. 2021, 9, 674581. [Google Scholar] [CrossRef]
- Hunt, N.C.; Shelton, R.M.; Henderson, D.J.; Grover, L.M. Calcium-alginate hydrogel-encapsulated fibroblasts provide sustained release of vascular endothelial growth factor. Tissue Eng. Part A 2013, 19, 905–914. [Google Scholar] [CrossRef]
- Young, S.A.; Sherman, S.E.; Cooper, T.T.; Brown, C.; Anjum, F.; Hess, D.A.; Flynn, L.E.; Amsden, B.G. Mechanically resilient injectable scaffolds for intramuscular stem cell delivery and cytokine release. Biomaterials 2018, 159, 146–160. [Google Scholar] [CrossRef]
- Babu, S.; Albertino, F.; Omidinia Anarkoli, A.; De Laporte, L. Controlling Structure with Injectable Biomaterials to Better Mimic Tissue Heterogeneity and Anisotropy. Adv. Healthc. Mater. 2021, 10, 2002221. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 2015, 49, 4–11. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Animal models of drug-induced liver injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1031–1039. [Google Scholar] [CrossRef]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 2015, 94, 52438. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.D.; Yin, R.; Liu, Y.; Yang, Y.; Mitchell-Richards, K.A.; Nam, J.H.; Li, R.; Wang, L.; Iwakiri, Y.; et al. O-GlcNAc transferase suppresses necroptosis and liver fibrosis. JCI Insight 2019, 4, e127709. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Q.L.; Song, Y.N.; Sun, Y.; Wei, B.; Li, X.Y.; Hu, Y.Y.; Liu, P.; Su, S.B. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci. 2016, 41, 561–572. [Google Scholar] [CrossRef]
- Boyd, J.W. The Comparative Activity of Some Enzymes in Sheep, Cattle and Rats—Normal Serum and Tissue Levels and Changes During Experimental Liver Necrosis. Res. Vet. Sci. 1962, 3, 256–270. [Google Scholar] [CrossRef]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet. Med. Int. 2020, 2020, 5346483. [Google Scholar] [CrossRef]
- Fortea, J.I.; Fernández-Mena, C.; Puerto, M.; Ripoll, C.; Almagro, J.; Bañares, J.; Bellón, J.M.; Bañares, R.; Vaquero, J. Comparison of Two Protocols of Carbon Tetrachloride-Induced Cirrhosis in Rats—Improving Yield and Reproducibility. Sci. Rep. 2018, 8, 9163. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal models of chronic liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef]
- Nakayama, Y.; Li, Q.; Katsuragawa, S.; Ikeda, R.; Hiai, Y.; Awai, K.; Kusunoki, S.; Yamashita, Y.; Okajima, H.; Inomata, Y.; et al. Automated Hepatic Volumetry for Living Related Liver Transplantation At Multisection CT. Radiology 2006, 240, 743–748. [Google Scholar] [CrossRef]
- Liška, V.; Třeška, V.; Mírka, H.; Vyčítal, O.; Brůha, J.; Haidingerová, L.; Beneš, J.; Tonar, Z.; Pálek, R.; Rosendorf, J. Experimental promotion of liver regeneration after portal vein branch ligation. Rozhl. Chir. 2018, 97, 239–245. [Google Scholar]
- Seo, K.-W.; Sohn, S.-Y.; Bhang, D.-H.; Nam, M.-J.; Lee, H.-W.; Youn, H.-Y. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol. Int. 2014, 38, 106–116. [Google Scholar] [CrossRef]
- Elzainy, A.; El Sadik, A.; Altowayan, W.M. Comparison between the Regenerative and Therapeutic Impacts of Bone Marrow Mesenchymal Stem Cells and Adipose Mesenchymal Stem Cells Pre-Treated with Melatonin on Liver Fibrosis. Biomolecules 2024, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Rymar, S.; Pikus, P.; Buchek, P.; Shuvalova, N.; Pokholenko, I.; Irodov, D.; Kordium, V. Comparison of the therapeutic effects of hUC-MSC intravenous delivery and intraperitoneal administration of MSCs encapsulated in alginate capsules for the treatment of rat liver cirrhosis. Biointerface Res. Appl. Chem. 2021, 12, 5054–5070. [Google Scholar] [CrossRef]
- Sa’dyah, N.A.C.; Putra, A.; Dirja, B.T.; Hidayah, N.; Azzahara, S.Y.; Irawan, R.C.S. Suppression of transforming growth factor-β by mesenchymal stem-cells accelerates liver regeneration in liver fibrosis animal model. Universa Med. 2021, 40, 29–35. [Google Scholar] [CrossRef]
- Hermansyah, D.; Putra, A.; Muhar, A.M.; Retnaningsih; Wirastuti, K.; Dirja, B.T. Mesenchymal Stem Cells Suppress TGF-β Release to Decrease α-SMA Expression in Ameliorating CCl4-Induced Liver Fibrosis. Med. Arch. 2021, 75, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, K.; Qi, Q.; Zhou, W.; Yu, F.; Zhang, Y. Human umbilical cord-derived mesenchymal stem cells attenuate hepatic stellate cells activation and liver fibrosis. Mol. Biol. Rep. 2024, 51, 734. [Google Scholar] [CrossRef]
- Ezquer, F.; Bahamonde, J.; Huang, Y.L.; Ezquer, M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res. Ther. 2017, 8, 20. [Google Scholar] [CrossRef]
- Wang, W.; Du, Z.; Yan, J.; Ma, D.; Shi, M.; Zhang, M.; Peng, C.; Li, H. Mesenchymal Stem Cells Promote Liver Regeneration and Prolong Survival in Small-For-Size Liver Grafts: Involvement of C-Jun N-Terminal Kinase, Cyclin D1, and NF-κB. PLoS ONE 2014, 9, e112532. [Google Scholar] [CrossRef]
- Jain, E.; Damania, A.; Kumar, A. Biomaterials for liver tissue engineering. Hepatol. Int. 2014, 8, 185–197. [Google Scholar] [CrossRef]
- Khatab, S.; Leijs, M.J.; van Buul, G.; Haeck, J.; Kops, N.; Nieboer, M.; Bos, P.K.; Verhaar, J.A.N.; Bernsen, M.; van Osch, G. MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell Biol. Toxicol. 2020, 36, 553–570. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen–hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Melo, J.S.; Bhainsa, K.C. Application of conventional spray dryer to prepare nanosilica-alginate based rhodamine loaded microstructures and nanobeads. J. Mater. Res. 2023, 38, 3362–3371. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Lee, S.J.; Guliyeva, N.; Lee, J.; Alsberg, E. Stem cell-laden hydrogel bioink for generation of high resolution and fidelity engineered tissues with complex geometries. Bioact. Mater. 2022, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Remes, A.; Basha, D.I.; Puehler, T.; Borowski, C.; Hille, S.; Kummer, L.; Wagner, A.H.; Hecker, M.; Soethoff, J.; Lutter, G.; et al. Alginate hydrogel polymers enable efficient delivery of a vascular-targeted AAV vector into aortic tissue. Mol. Ther. Methods Clin. Dev. 2021, 21, 83–93. [Google Scholar] [CrossRef]
- Farjadian, F.; Akbarizadeh, A.R.; Tayebi, L. Synthesis of novel reducing agent for formation of metronidazole-capped silver nanoparticle and evaluating antibacterial efficiency in gram-positive and gram-negative bacteria. Heliyon 2020, 6, e04747. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolinas, D.K.M.; Barcena, A.J.R.; Mishra, A.; Bernardino, M.R.; Lin, V.; Heralde, F.M., III; Chintalapani, G.; Fowlkes, N.W.; Huang, S.Y.; Melancon, M.P. Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats. Gels 2025, 11, 250. https://doi.org/10.3390/gels11040250
Bolinas DKM, Barcena AJR, Mishra A, Bernardino MR, Lin V, Heralde FM III, Chintalapani G, Fowlkes NW, Huang SY, Melancon MP. Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats. Gels. 2025; 11(4):250. https://doi.org/10.3390/gels11040250
Chicago/Turabian StyleBolinas, Dominic Karl M., Allan John R. Barcena, Archana Mishra, Marvin R. Bernardino, Vincent Lin, Francisco M. Heralde, III, Gouthami Chintalapani, Natalie W. Fowlkes, Steven Y. Huang, and Marites P. Melancon. 2025. "Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats" Gels 11, no. 4: 250. https://doi.org/10.3390/gels11040250
APA StyleBolinas, D. K. M., Barcena, A. J. R., Mishra, A., Bernardino, M. R., Lin, V., Heralde, F. M., III, Chintalapani, G., Fowlkes, N. W., Huang, S. Y., & Melancon, M. P. (2025). Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats. Gels, 11(4), 250. https://doi.org/10.3390/gels11040250





