In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization
Abstract
1. Introduction
2. Results and Discussion
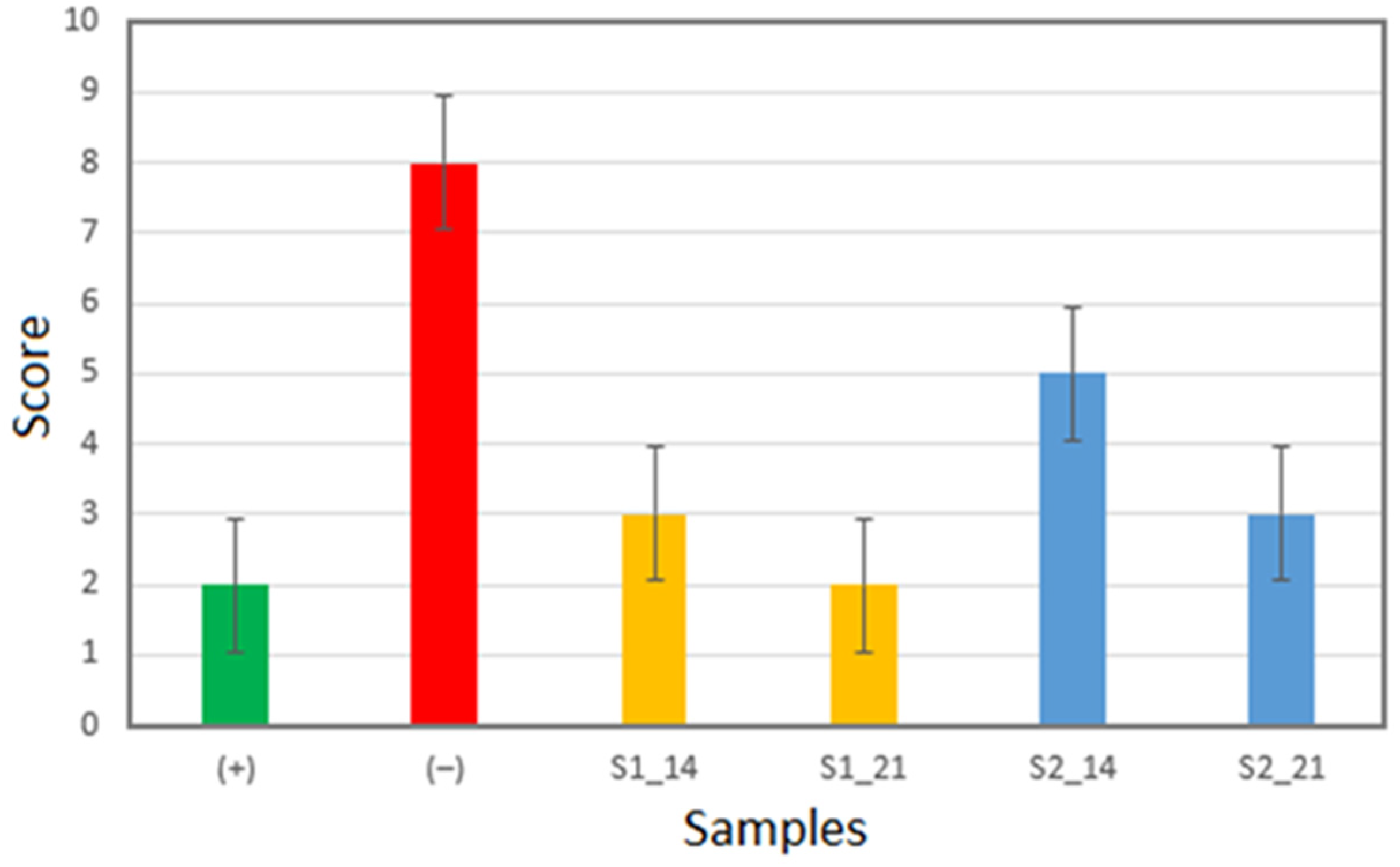
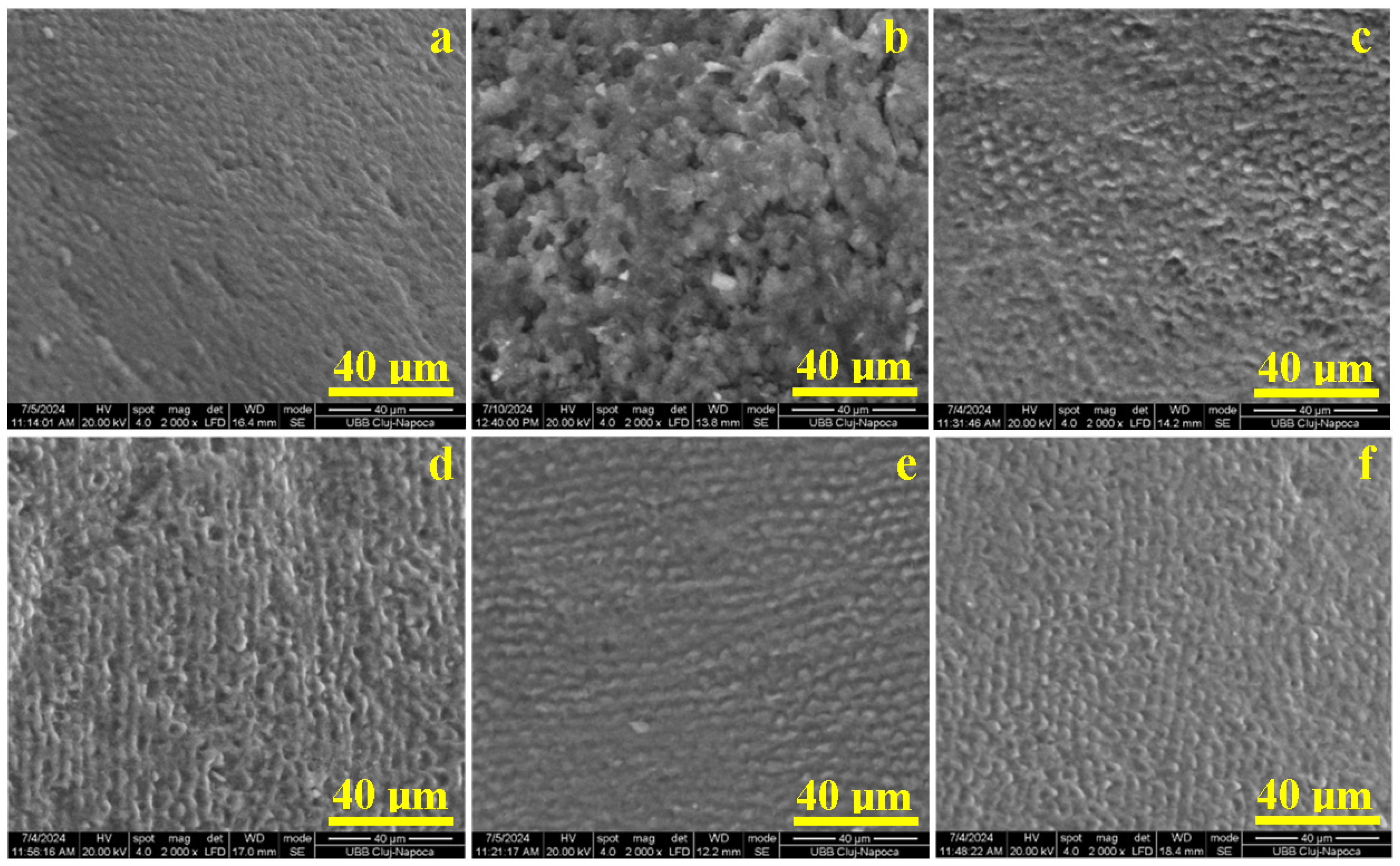
2.1. Enamel Surface Quality Assessment
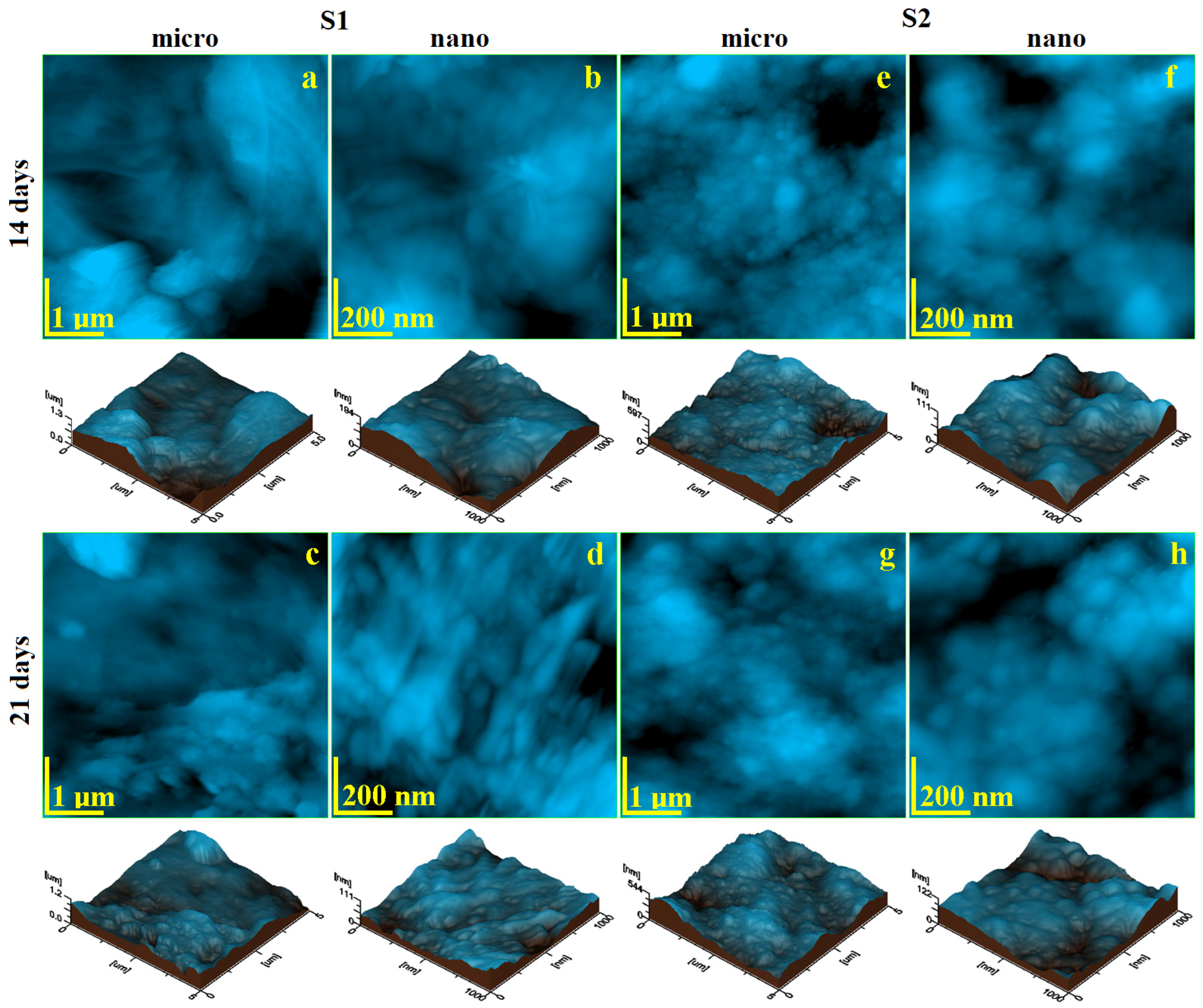
2.2. Micro- and Nanostructure Assessment
2.3. Microhardness
3. Conclusions
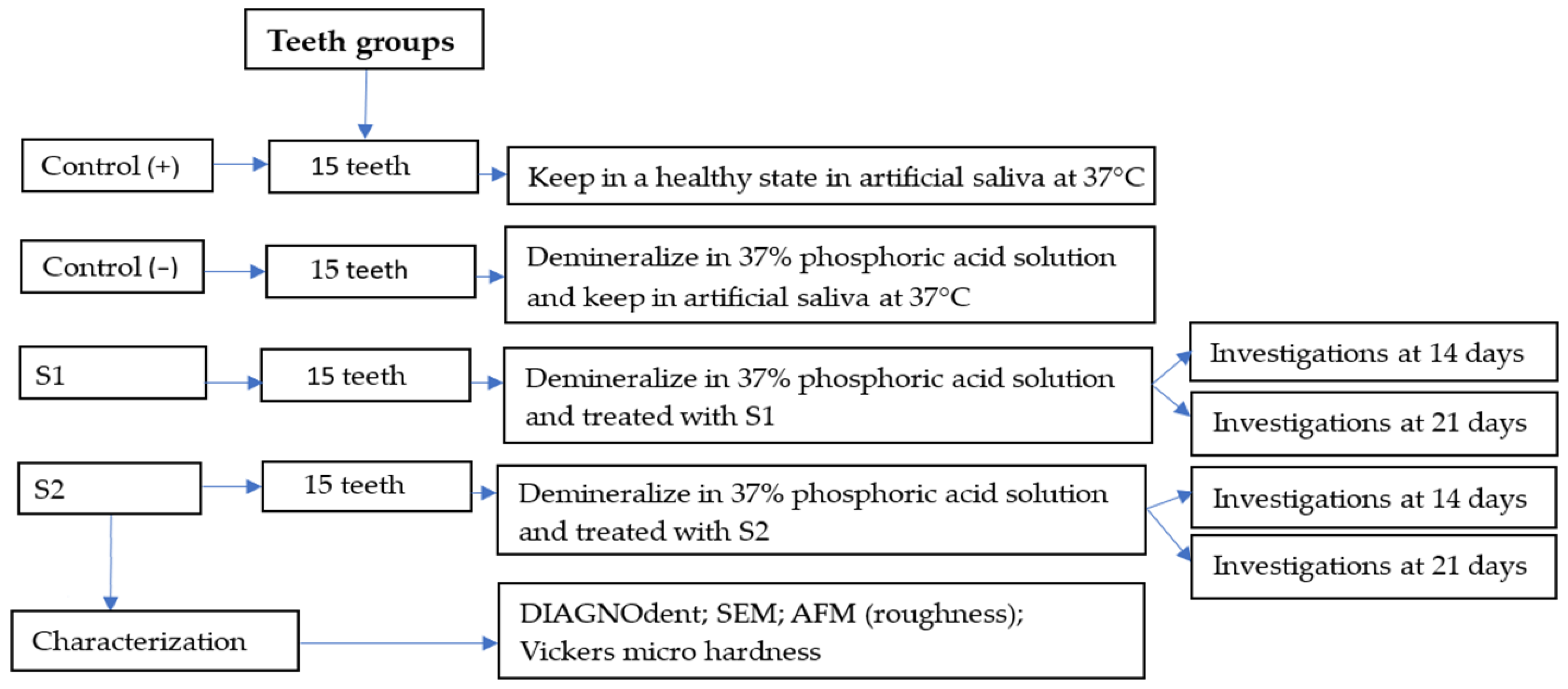
4. Materials and Methods
4.1. Materials
4.2. Protocol of Demineralization/Remineralization of Enamel Surface
4.3. Characterizations
4.3.1. Measurement of Samples with DIAGNOdent
4.3.2. The Scanning Electron Microscopy (SEM)
4.3.3. Atomic Force Microscopy (AFM)
4.3.4. The Hardness of the Enamel Surface
4.3.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, L.; Zheng, L.; Xiao, H.; Zheng, J.; Zhou, Z. Wear mechanism of human tooth enamel: The role of interfacial protein bonding between HA crystals. J. Mech. Behav. Biomed. Mater. 2020, 110, 103845. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Ceci, M.; Beltrami, R.; Lombardini, M.; Colombo, M. Atomic force microscopy study of enamel remineralization. Ann. Stomatol. 2014, 5, 98–102. [Google Scholar]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and Its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef] [PubMed]
- Miyayoshi, Y.; Hamba, H.; Nakamura, K.; Ishizuka, H.; Muramatsu, T. Remineralization effects of enamel binding peptide, WGNYAYK, on enamel subsurface demineralization in vitro. Heliyon 2024, 10, e23176. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Salwa, I.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 19, 4743–4763. [Google Scholar] [CrossRef]
- Han, S.; Fan, Y.; Zhou, Z.; Tu, H.; Li, D.; Lv, H.; Ding, L.; Zhang, L. Promotion of enamel caries remineralization by an amelogenin-derived peptide in a rat model. Arch. Oral Biol. 2017, 73, 66–71. [Google Scholar] [CrossRef]
- Geethu, P.N.; Suvedha, N.; Kurien, A.A.; Chakravarthy, Y.; Pallavi, V. Demineralization and remineralization in restorative dentistry. J. Acad. Dent. Educ. 2023, 9, 28–30. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yu, O.Y.; Mei, M.L.; Jakubovics, N.S.; Chu, C.H. Development of a Novel Peptide with Antimicrobial and Mineralising Properties for Caries Management. Pharmaceutics 2023, 15, 2560. [Google Scholar] [CrossRef]
- Alkilzy, M.; Qadri, G.; Splieth, C.H.; Santamaría, R.M. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4. Biomimetics 2023, 8, 290. [Google Scholar] [CrossRef]
- Attik, N.; Garric, X.; Bethry, A.; Subra, G.; Chevalier, C.; Bouzouma, B.; Verdié, P.; Grosgogeat, B.; Gritsch, K. Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile. J. Funct. Biomater. 2023, 14, 53. [Google Scholar] [CrossRef]
- Hardan, L.; Chedid, J.C.A.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Tosco, V.; Monjarás-Ávila, A.J.; Jabra, M.; Salloum-Yared, F.; Kharouf, N.; et al. Peptides in Dentistry: A Scoping Review. Bioengineering 2023, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ayuso, C.A.; Aranda-Herrera, B.; Guzman-Rocha, D.; Chavez-Granados, P.A.; Garcia-Contreras, R. Synthetic Proteins in Dental Applications. SynBio 2024, 2, 1–20. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Xue, M.; Kang, Y.; Liu, D.; Wen, Y.; Zhao, D.; Guan, B. Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration. Molecules 2023, 28, 6373. [Google Scholar] [CrossRef]
- Shen, P.; Fernando, J.R.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Comparative Efficacy of Novel Biomimetic Remineralising Technologies. Biomimetics 2023, 8, 17. [Google Scholar] [CrossRef]
- Kegulian, N.C.; Langen, R.; Moradian-Oldak, J. The Dynamic Interactions of a Multitargeting Domain in Ameloblastin Protein with Amelogenin and Membrane. Int. J. Mol. Sci. 2023, 24, 3484. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Y.; Liu, Y.; Zhang, J.; Liang, S.; Chen, R.; Jiang, H.; Tai, B.; Du, M.; Liu, C. Evaluation of DIAGNOdent pen for initial occlusal caries diagnosis in permanent teeth. BMC Oral Health 2024, 24, 1111. [Google Scholar] [CrossRef]
- Bahrololoomi, Z.; Musavi, S.A.; Kabudan, M. In vitro evaluation of the efficacy of laser fluorescence (DIAGNOdent) to detect demineralization and remineralization of smooth enamel lesions. J. Conserv. Dent. 2013, 16, 362–366. [Google Scholar] [CrossRef]
- Gungormus, M.; Oren, E.E.; Horst, J.A.; Fong, H.; Hnilova, M.; Somerman, M.J.; Snead, M.; Samudrala, R.; Tamerler, C.; Sarikaya, M. Cementomimetics—Constructing a cementum-like biomineralized microlayer via amelogenin-derived peptides. Int. J. Oral Sci. 2012, 4, 69–77. [Google Scholar] [CrossRef]
- Amin, D.; Olson, I.; Knowles, J.; Dard, M.; Donos, N. Interaction of enamel matrix proteins with human periodontal ligament cells. Clin. Oral Investig. 2016, 20, 339–347. [Google Scholar] [CrossRef]
- Ding, L.; Han, S.; Wanf, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 3, 283–292. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Han, S.; Ding, L.; Lu, Z.; Zhang, L. Unveiling the mechanism of an amelogenin-derived peptide in promoting enamel biomimetic remineralization. Int. J. Biol. Macromol. 2023, 253, 127322. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.; Hoz, L.; Montoya, G.; Nidome, M.; Pérez-Soria, A.; Romo, E.; Soto-Barreras, U.; Garnica-Palazuelos, J.; Aguilar-Medina, M.; Ramos-Payán, R.; et al. Bioactive Synthetic Peptides for Oral Tissues Regeneration. Front. Mater. 2021, 8, 655495. [Google Scholar] [CrossRef]
- Besnard, C.; Marie, A.; Sasidharan, S.; Harper, R.A.; Marathe, S.; Moffat, J.; Shelton, R.M.; Landini, G.; Korsunsky, A.M. Time-Lapse In Situ 3D Imaging Analysis of Human Enamel Demineralisation Using X-ray Synchrotron Tomography. Dent. J. 2023, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.A.; Santucci, N.M.; Surti, B.; Habelitz, S.; Bhattacharyya, M.; Noble, W. Evaluation of Enamel Volume Loss after Exposure to Energy Drinks. Oral 2024, 4, 101–112. [Google Scholar] [CrossRef]
- Yu, H.; Wegehaupt, F.J.; Wiegand, A.; Roos, M.; Attin, T.; Buchalla, W. Erosion and abrasion of tooth-colored restorative materials and human enamel. J. Dent. 2009, 37, 913–922. [Google Scholar] [CrossRef]
- Almansour, A.; Addison, O.; Bartlett, D. The effect of location/site on polished human enamel after mechanical and chemical wear. J. Dent. 2024, 141, 104803. [Google Scholar] [CrossRef]
- Dussa, S.; Sunitha, C.; Kumar, P.K.; Saritha, T. Qualitative and quantitative evaluation of enamel surface roughness and remineralization after interproximal reduction: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 2024, 166, 227–234. [Google Scholar] [CrossRef]
- Chabuk, M.M.G.; Al-Shamma, A.M.W. Surface roughness and microhardness of enamel white spot lesions treated with different treatment methods. Heliyon 2023, 9, e18283. [Google Scholar] [CrossRef]
- Harper, R.A.; Shelton, R.M.; James, J.D.; Salvati, E.; Besnard, C.; Korsunsky, A.M.; Landini, G. Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarised light imaging. Acta Biomater. 2021, 120, 240–248. [Google Scholar] [CrossRef]
- Danesi, A.L.; Athanasiadou, D.; Mansouri, A.; Phen, A.; Neshatian, M.; Holcroft, J.; Bonde, J.; Ganss, B.; Carneiro, K.M.M. Uniaxial Hydroxyapatite Growth on a Self-Assembled Protein Scaffold. Int. J. Mol. Sci. 2021, 22, 12343. [Google Scholar] [CrossRef]
- Muntean, A.; Sarosi, C.; Petean, I.; Cuc, S.; Carpa, R.; Chis, I.A.; Ilea, A.; Delean, A.G.; Moldovan, M. Developing Bioactive Hydrogels with Peptides for Dental Application. Biomedicines 2024, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, G.d.A.P.; Fraga, M.A.A.; de Souza Araújo, I.J.; Pacheco, R.R.; Correr, A.B.; Puppin-Rontani, R.M. Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel. J. Funct. Biomater. 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.J.C.; Fu, Y.; Li, K.C.; Loho, T.; Loch, C.; Ekambaram, M. A Comparison of the Enamel Remineralisation Potential of Self-Assembling Peptides. Int. Dent. J. 2024, 74, 187–194. [Google Scholar] [CrossRef] [PubMed]
- El Moshy, S.; Radwan, I.A.; Matoug-Elwerfell, M.; Abdou, A.; Abbass, M.M.S. A Novel Nano-Hydroxyapatite Agarose-Based Hydrogel for Biomimetic Remineralization of Demineralized Human Enamel: An In-Vitro Study. Clin. Cosmet. Investig. Dent. 2024, 16, 453–465. [Google Scholar] [CrossRef]
- Cao, Y.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.H. Enamel prism-like tissue regeneration using enamel matrix derivative. J. Dent. 2014, 42, 1535–1542. [Google Scholar] [CrossRef]
- El Moshy, S.; Abbass, M.M.; El-Motayam, A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000Research 2018, 7, 1476. [Google Scholar] [CrossRef]
- Moldovan, A.; Cuc, S.; Gasparik, C.; Sarosi, C.; Moldovan, M.; Ilie, N.; Petean, I.; Rusu, L.M.; Ionescu, A.; Pastrav, M. Effect of Experimental Bleaching Gels with Enzymes on Composite and Enamel. Int. Dent. J. 2024; in press. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of dental enamel remineralization: A systematic review of technique comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarosi, C.; Muntean, A.; Cuc, S.; Petean, I.; Balint, S.; Moldovan, M.; Mohan, A.G. In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization. Gels 2025, 11, 11. https://doi.org/10.3390/gels11010011
Sarosi C, Muntean A, Cuc S, Petean I, Balint S, Moldovan M, Mohan AG. In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization. Gels. 2025; 11(1):11. https://doi.org/10.3390/gels11010011
Chicago/Turabian StyleSarosi, Codruta, Alexandrina Muntean, Stanca Cuc, Ioan Petean, Sonia Balint, Marioara Moldovan, and Aurel George Mohan. 2025. "In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization" Gels 11, no. 1: 11. https://doi.org/10.3390/gels11010011
APA StyleSarosi, C., Muntean, A., Cuc, S., Petean, I., Balint, S., Moldovan, M., & Mohan, A. G. (2025). In Vitro Investigation of Novel Peptide Hydrogels for Enamel Remineralization. Gels, 11(1), 11. https://doi.org/10.3390/gels11010011








