Tripeptides Featuring Dehydrophenylalanine and Homophenylalanine: Homo- Versus Hetero-Chirality and Sequence Effects on Self-Assembly and Gelation
Abstract
:1. Introduction
2. Results and Discussion
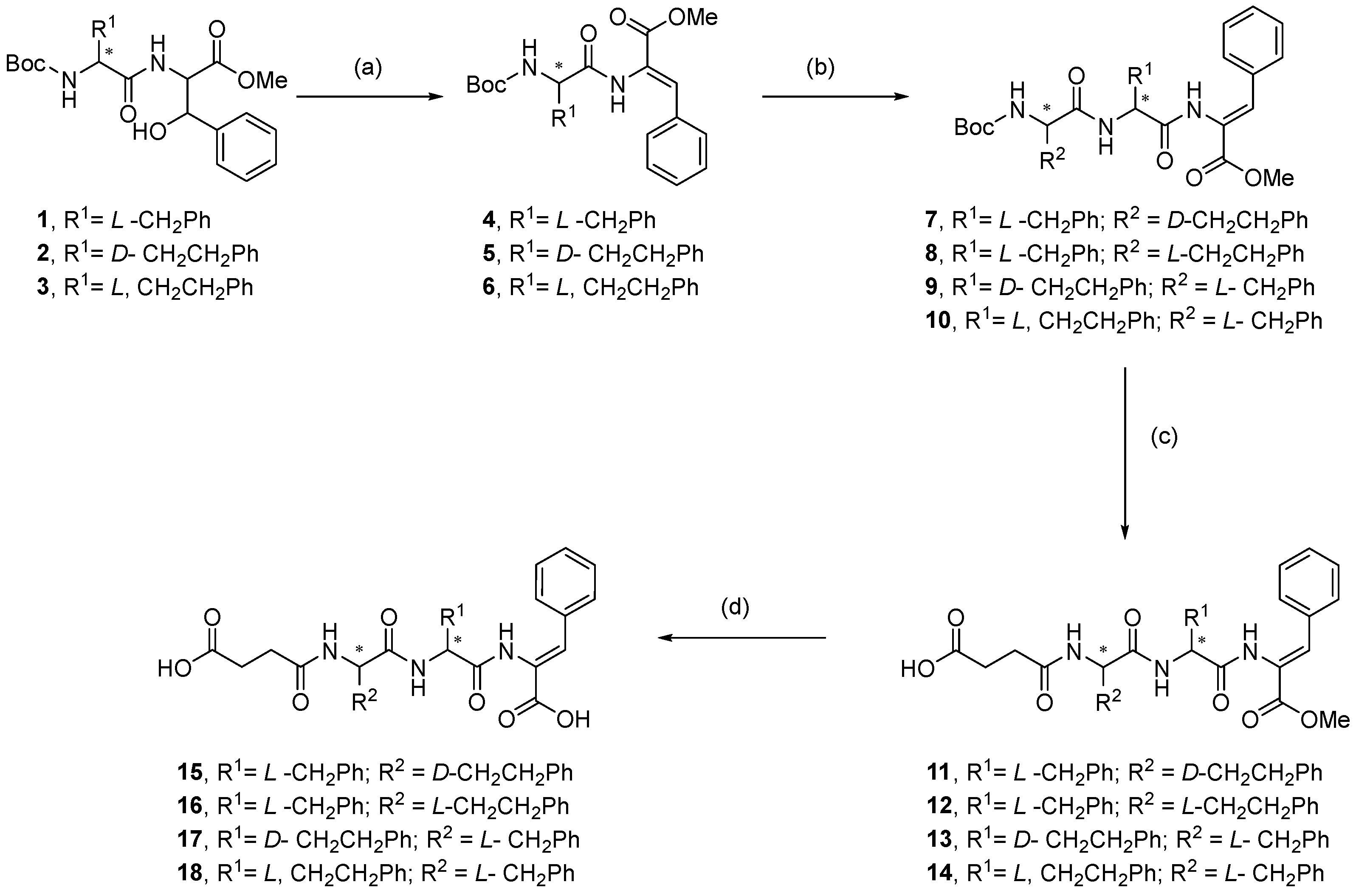
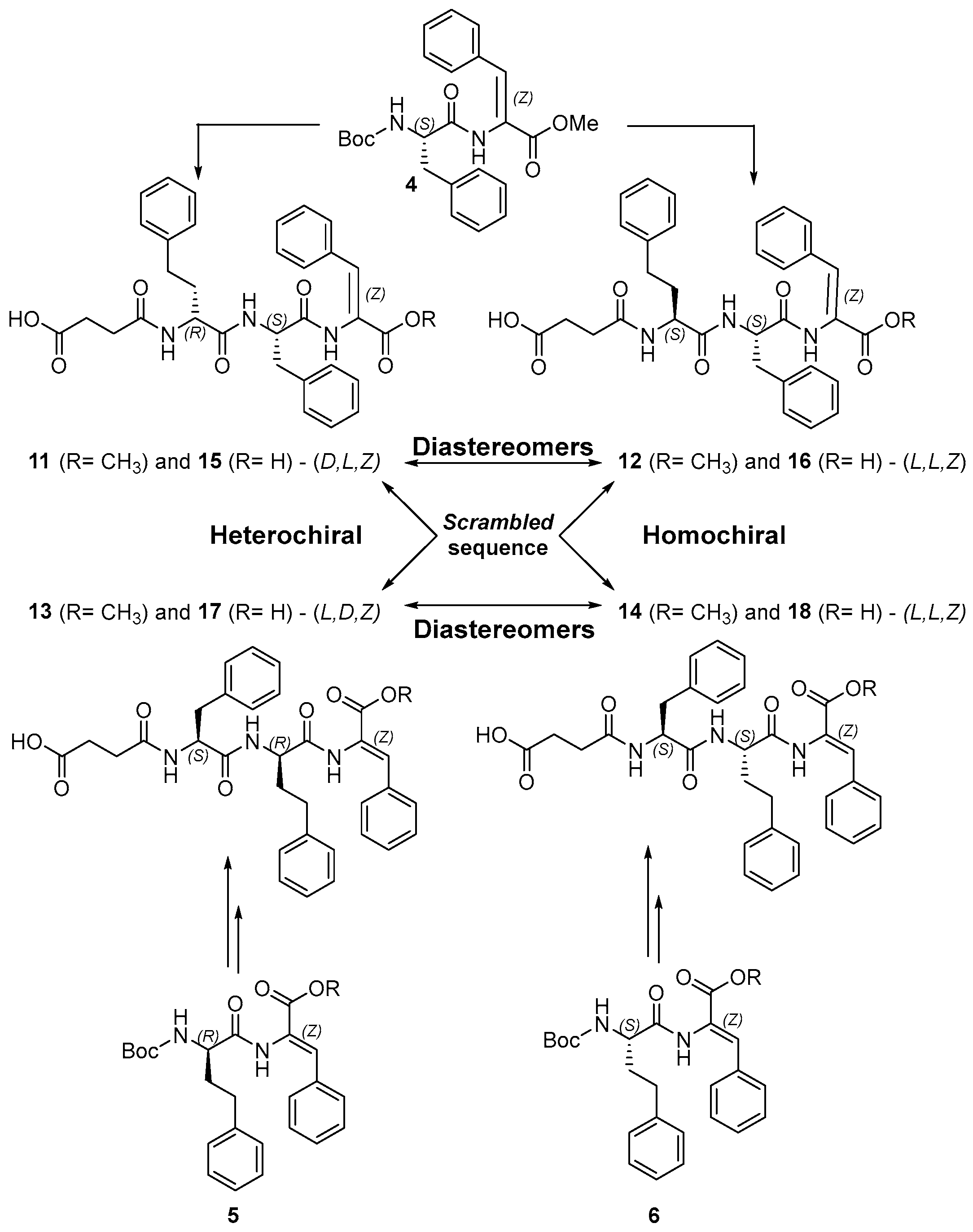
2.1. Synthesis
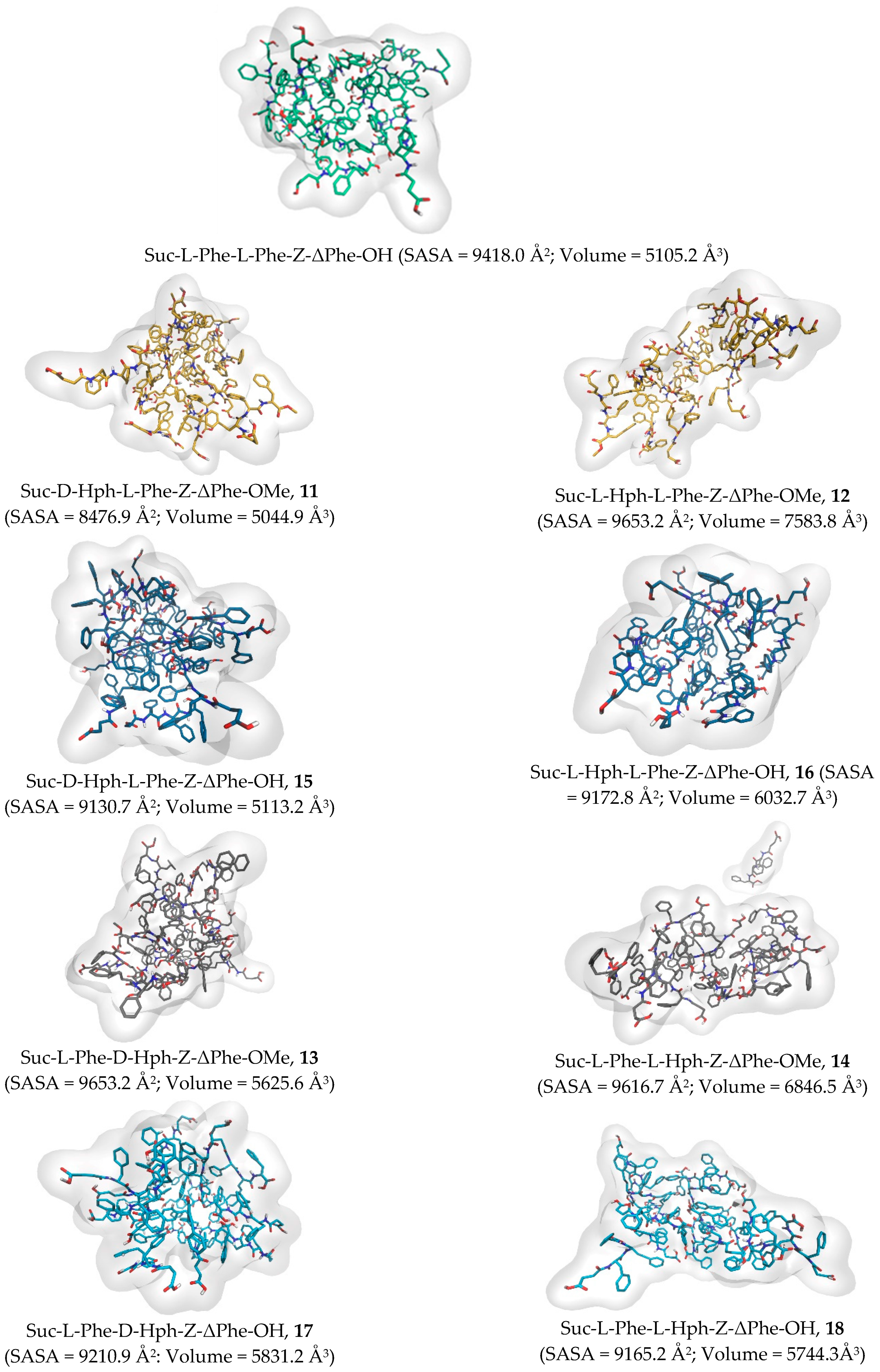
2.2. Molecular Dynamics Simulations
2.3. Hydrogelation
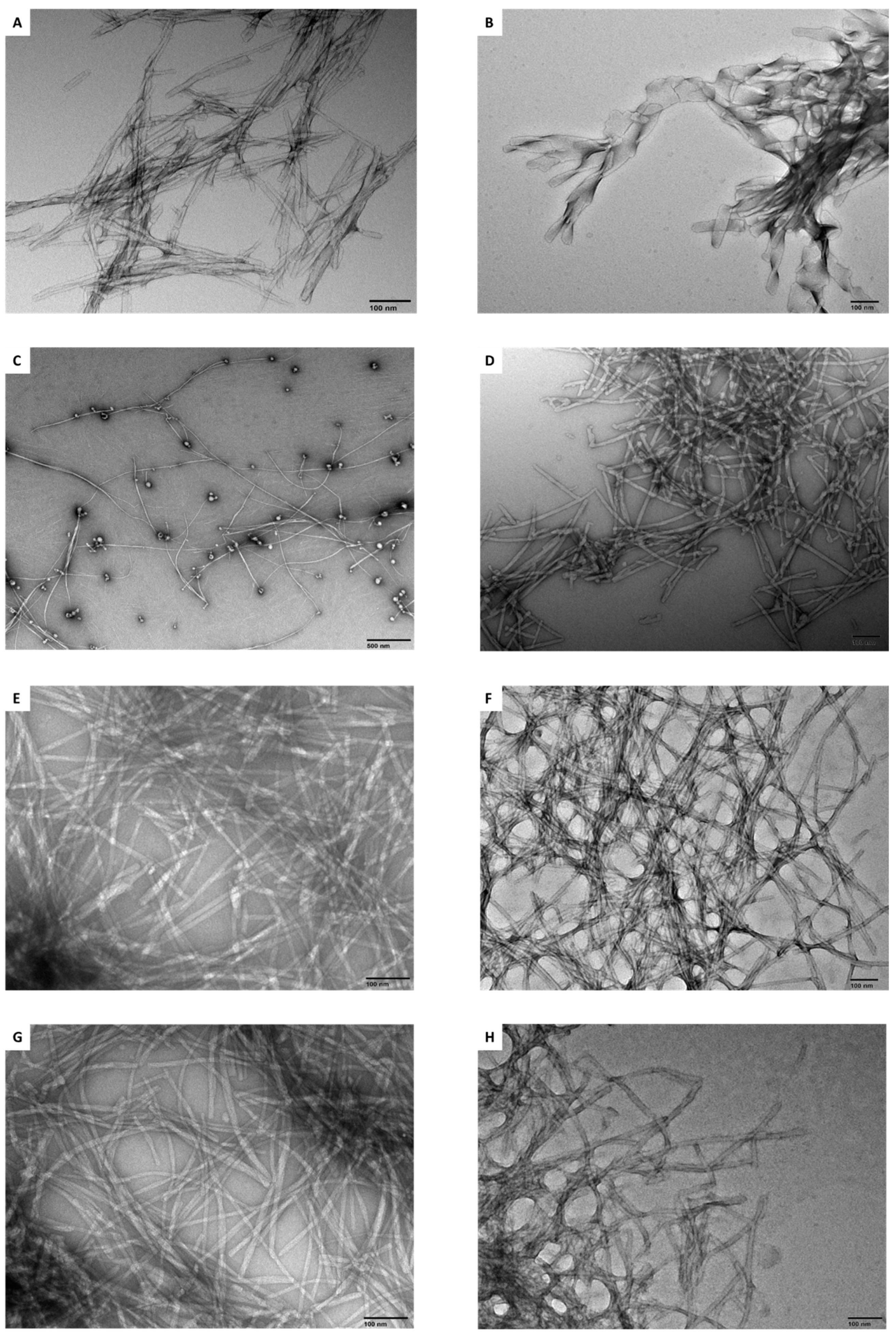
2.4. Transmission Electron Microscopy
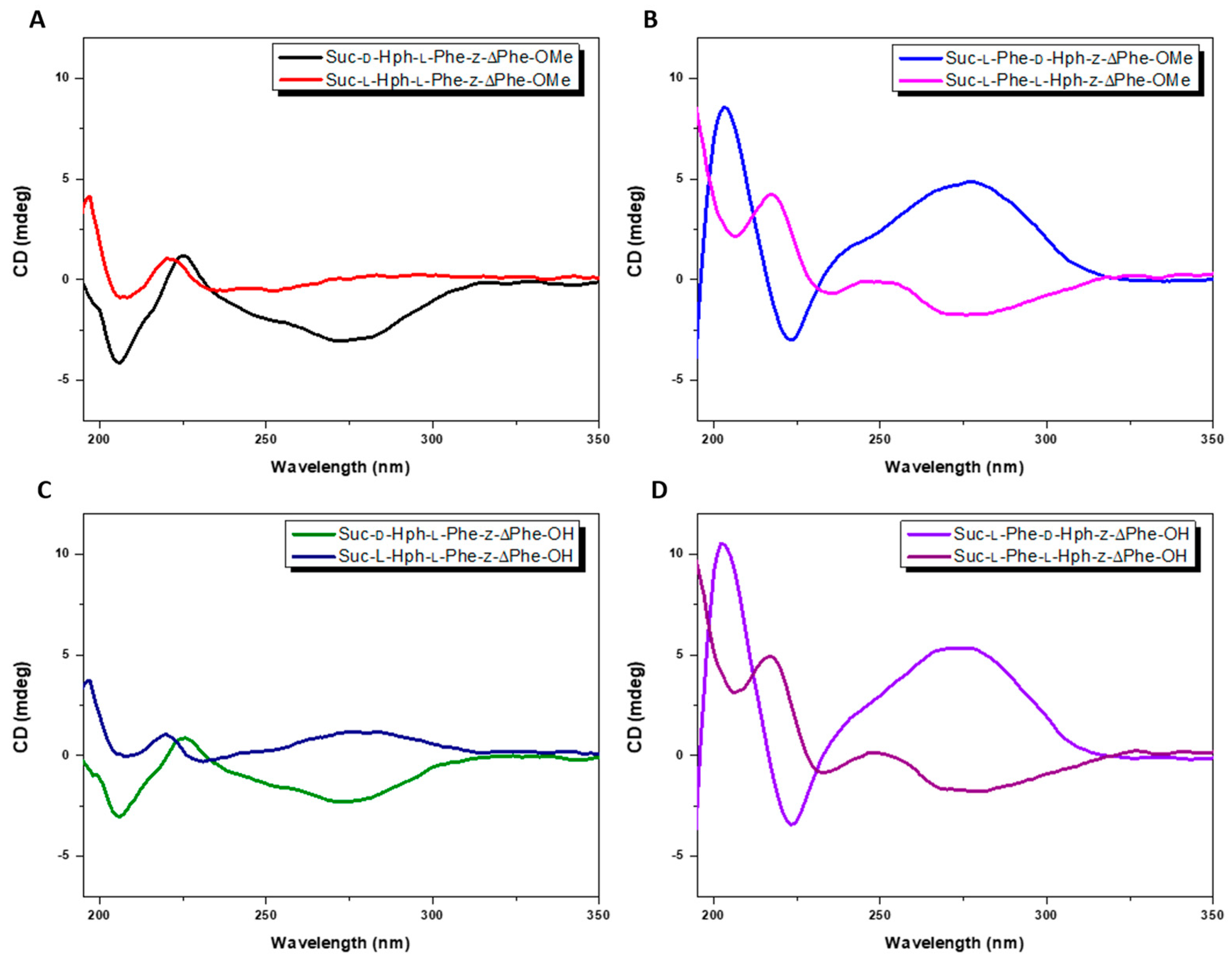
2.5. Circular Dichroism
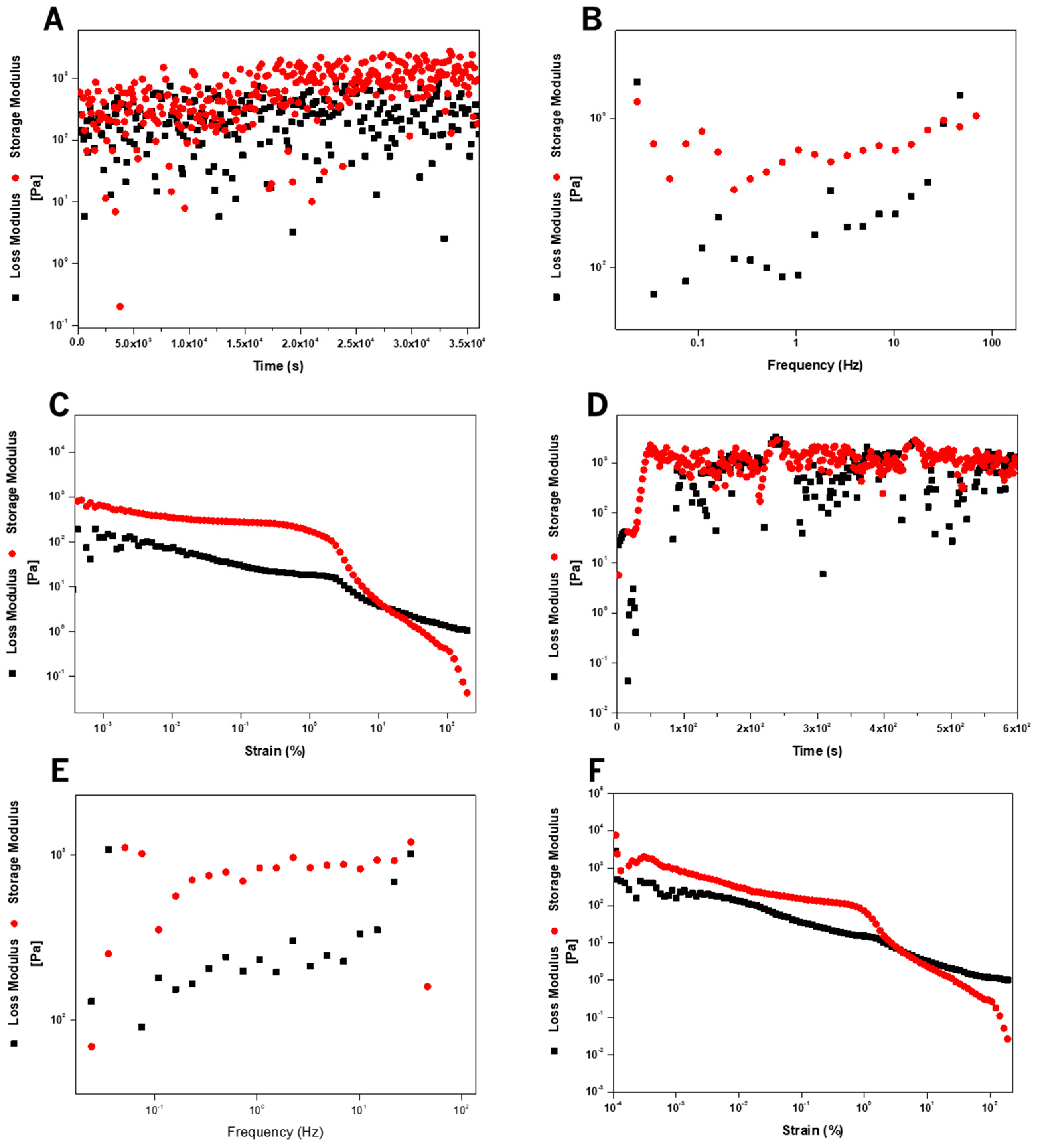
2.6. Rheology
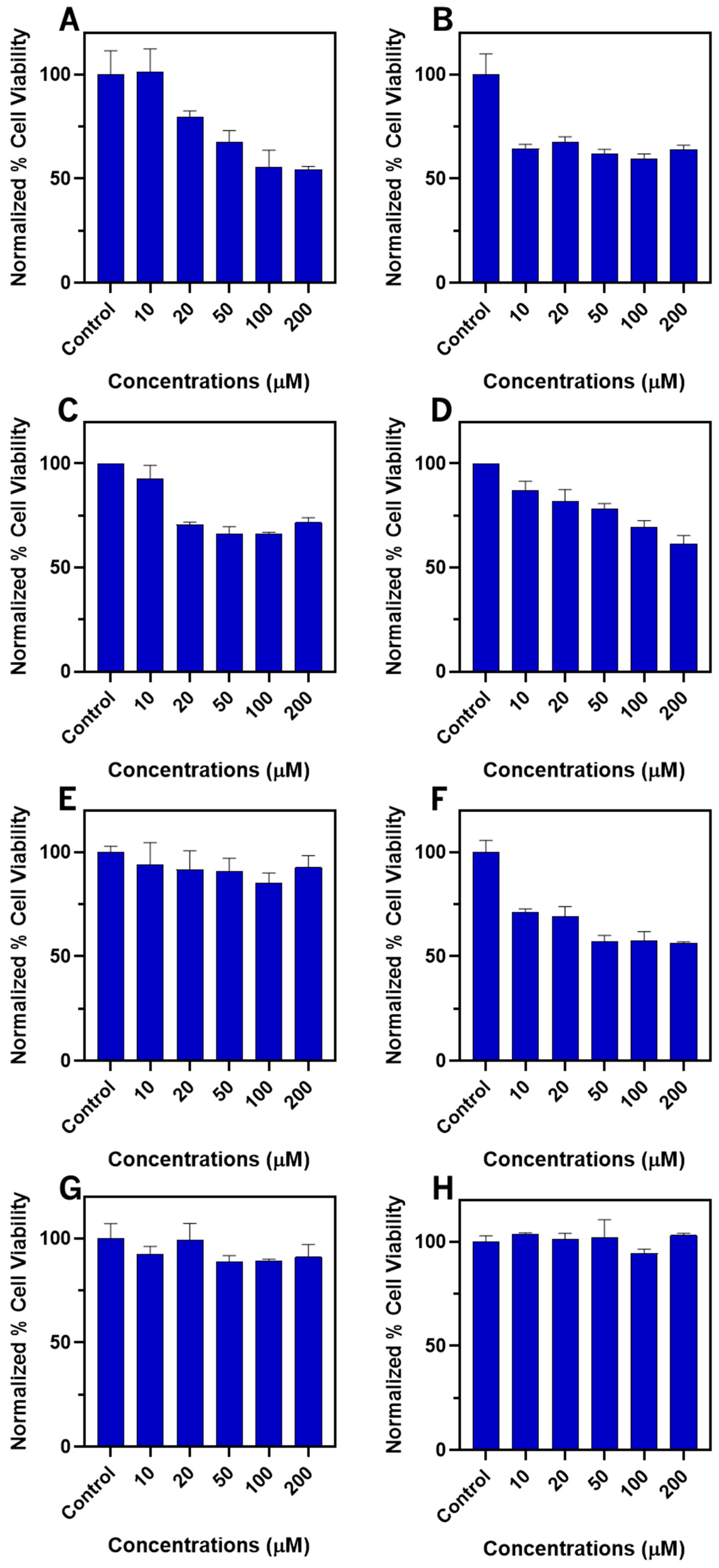
2.7. Biological Assays
3. Conclusions
4. Materials and Methods
4.1. General Procedures
4.2. Hydrogel Preparation
4.3. Transmission Electron Microscopy (TEM)
4.4. Circular Dichroism (CD) Spectroscopy
4.5. Rheological Studies
4.6. Cell Culture and MTT Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-Based Nanomaterials: Self-Assembly, Properties and Applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-Assembled Peptide and Protein Nanostructures for Anti-Cancer Therapy: Targeted Delivery, Stimuli-Responsive Devices and Immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-Assembled Peptide-Based Hydrogels as Scaffolds for Anchorage-Dependent Cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide Self-Assembly: Thermodynamics and Kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Morales-Reina, S.; Giri, C.; Leclercq, M.; Vela-Gallego, S.; de la Torre, I.; Castón, J.R.; Surin, M.; de la Escosura, A. Programmed Recognition between Complementary Dinucleolipids To Control the Self-Assembly of Lipidic Amphiphiles. Chem. A Eur. J. 2020, 26, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Grinberg, V.Y.; Burova, T.V.; Concheiro, A. Stimuli-Sensitive Cross-Linked Hydrogels as Drug Delivery Systems: Impact of the Drug on the Responsiveness. Int. J. Pharm. 2020, 579, 119157. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, D.E.; Petrovici, A.-R.; Rusu, D.; Avadanei, M.; Mihaila, A.C.; Butoi, E.; Ciolacu, F. 3D Matrices for Enhanced Encapsulation and Controlled Release of Anti-Inflammatory Bioactive Compounds in Wound Healing. Int. J. Mol. Sci. 2023, 24, 4213. [Google Scholar] [CrossRef]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-Assembling Peptide Hydrogel for Intervertebral Disc Tissue Engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Tasdemiroglu, Y.; Gourdie, R.G.; He, J.-Q. In Vivo Degradation Forms, Anti-Degradation Strategies, and Clinical Applications of Therapeutic Peptides in Non-Infectious Chronic Diseases. Eur. J. Pharmacol. 2022, 932, 175192. [Google Scholar] [CrossRef]
- He, S.; Yang, Z.; Li, X.; Wu, H.; Zhang, L.; Shan, A.; Wang, J. Boosting Stability and Therapeutic Potential of Proteolysis-Resistant Antimicrobial Peptides by End-Tagging β-Naphthylalanine. Acta Biomater. 2023, 164, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.; Banerjee, A. β-Amino Acid Containing Proteolitically Stable Dipeptide Based Hydrogels: Encapsulation and Sustained Release of Some Important Biomolecules at Physiological PH and Temperature. Soft Matter 2012, 8, 3380–3386. [Google Scholar] [CrossRef]
- Ito, T.; Matsunaga, N.; Kurashima, M.; Demizu, Y.; Misawa, T. Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids. Antibiotics 2023, 12, 1326. [Google Scholar] [CrossRef]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli Responsive Self-Assembled Hydrogel of a Low Molecular Weight Free Dipeptide with Potential for Tunable Drug Delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, H.; Pereira, G.; Castro, T.G.; Hermenegildo, B.F.; Shi, J.; Faria, T.Q.; Micaêlo, N.; Brito, R.M.M.M.; Xu, B.; Castanheira, E.M.S.S.; et al. New Self-Assembled Supramolecular Hydrogels Based on Dehydropeptides. J. Mater. Chem. B 2015, 3, 6355–6367. [Google Scholar] [CrossRef]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef]
- Amorim, C.; Veloso, S.R.S.; Castanheira, E.M.S.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.A.; Jervis, P.J.; Ferreira, P.M.T. Bolaamphiphilic Bis-Dehydropeptide Hydrogels as Potential Drug Release Systems. Gels 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.P.; Pereira, R.B.; Pereira, D.M.; Hilliou, L.; Castro, T.G.; Martins, J.A.; Jervis, P.J.; Ferreira, P.M.T. Aryl-Capped Lysine-Dehydroamino Acid Dipeptide Supergelators as Potential Drug Release Systems. Int. J. Mol. Sci. 2022, 23, 1811. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, H.; Carvalho, A.; Castro, T.; Castanheira, E.M.S.S.; Hilliou, L.; Hamley, I.; Melle-Franco, M.; Ferreira, P.M.T.T.; Martins, J.A. Unveiling the Role of Capping Groups in Naphthalene N-Capped Dehydrodipeptide Hydrogels. Gels 2023, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Veloso, S.R.S.; Carvalho, A.; Hilliou, L.; Coutinho, P.J.G.; Moura, C.; Martins, J.A.; Castanheira, E.M.S.; Ferreira, P.M.T. Multifunctional Magneto-plasmonic Lipogel Based on Peptide Hydrogel for Application in Combined Cancer Therapy. J. Pept. Sci. 2024, 31, e3650. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.P.; Carvalho, A.; Pereira, R.B.; Pereira, D.M.; Hilliou, L.; Jervis, P.J.; Martins, J.A.; Ferreira, P.M.T. New Supramolecular Hydrogels Based on Diastereomeric Dehydrotripeptide Mixtures for Potential Drug Delivery Applications. Gels 2024, 10, 629. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Giordano, S.; Rosa, E.; Carrese, B.; Piccialli, G.; Borbone, N.; Morelli, G.; Oliviero, G.; Accardo, A. Ultrashort Cationic Peptide Fmoc-FFK as Hydrogel Building Block for Potential Biomedical Applications. Gels 2023, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Choe, R.; Il Yun, S. Fmoc-Diphenylalanine-Based Hydrogels as a Potential Carrier for Drug Delivery. e-Polymers 2020, 20, 458–468. [Google Scholar] [CrossRef]
- Basavalingappa, V.; Guterman, T.; Tang, Y.; Nir, S.; Lei, J.; Chakraborty, P.; Schnaider, L.; Reches, M.; Wei, G.; Gazit, E. Expanding the Functional Scope of the Fmoc-Diphenylalanine Hydrogelator by Introducing a Rigidifying and Chemically Active Urea Backbone Modification. Adv. Sci. 2019, 6, 1900218. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.T.; Monteiro, L.S.; Pereira, G. Synthesis of Substituted Oxazoles from N-Acyl-β-Hydroxyamino Acid Derivatives. Eur. J. Org. Chem. 2008, 2008, 4676–4683. [Google Scholar] [CrossRef]
- Adorinni, S.; Kurbasic, M.; Garcia, A.M.; Kralj, S.; Bellotto, O.; Scarel, E.; Pengo, P.; De Zorzi, R.; Melchionna, M.; Vargiu, A.V.; et al. A Water Playground for Peptide Re-Assembly from Fibrils to Plates. J. Mater. Chem. B 2024, 12, 12589–12596. [Google Scholar] [CrossRef] [PubMed]
- Birdi, K.S.; Singh, H.N.; Dalsager, S.U. Interaction of Ionic Micelles with the Hydrophobic Fluorescent Probe 1-Anilino-8-Naphthalenesulfonate. J. Phys. Chem. 1979, 83, 2733–2737. [Google Scholar] [CrossRef]
- McAulay, K.; Dietrich, B.; Su, H.; Scott, M.T.; Rogers, S.; Al-Hilaly, Y.K.; Cui, H.; Serpell, L.C.; Seddon, A.M.; Draper, E.R.; et al. Using Chirality to Influence Supramolecular Gelation. Chem. Sci. 2019, 10, 7801–7806. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, O.; Kralj, S.; De Zorzi, R.; Geremia, S.; Marchesan, S. Supramolecular Hydrogels from Unprotected Dipeptides: A Comparative Study on Stereoisomers and Structural Isomers. Soft Matter 2020, 16, 10151–10157. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.M.; Casanovas, J.; Mayans, E.; Jiménez, A.I.; Puiggalí, J.; Alemán, C. Heterochirality Restricts the Self-Assembly of Phenylalanine Dipeptides Capped with Highly Aromatic Groups. J. Phys. Chem. B 2020, 124, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, H.; Tao, Y.; Zhang, H.; Wang, H. Controlling Supramolecular Filament Chirality of Hydrogel by Co-Assembly of Enantiomeric Aromatic Peptides. J. Nanobiotechnology 2022, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Styan, K.E.; Easton, C.D.; Waddington, L.; Vargiu, A.V. Higher and Lower Supramolecular Orders for the Design of Self-Assembled Heterochiral Tripeptide Hydrogel Biomaterials. J. Mater. Chem. B 2015, 3, 8123–8132. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 2827. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Cheeseman, J.R.; Scalmani, G.; Caricato, M.; Hratchian, H.P.; Li, X.; Barone, V.; Bloino, J.; Zheng, G.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Ashvar, C.S.; Devlin, F.J.; Bak, K.L.; Taylor, P.R.; Stephens, P.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra: 6,8-Dioxabicyclo[3.2.1]Octane. J. Phys. Chem. 1996, 100, 9262–9270. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges: The RESP Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A.V. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. WIREs Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Tanner, S.W.; Thompson, N.; Cheatham, T.E. Clustering Molecular Dynamics Trajectories: 1. Characterizing the Performance of Different Clustering Algorithms. J. Chem. Theory Comput. 2007, 3, 2312–2334. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, version 3.0.3. PyMOL. The PyMOL Molecular Graphics System, LLC: New York, NY, USA, 2024.
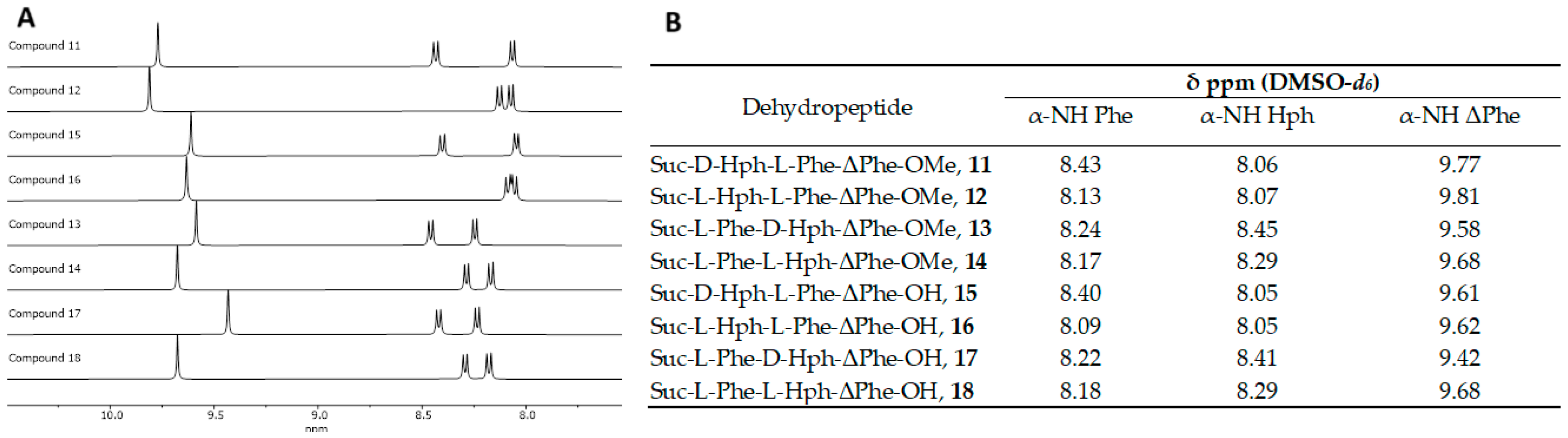
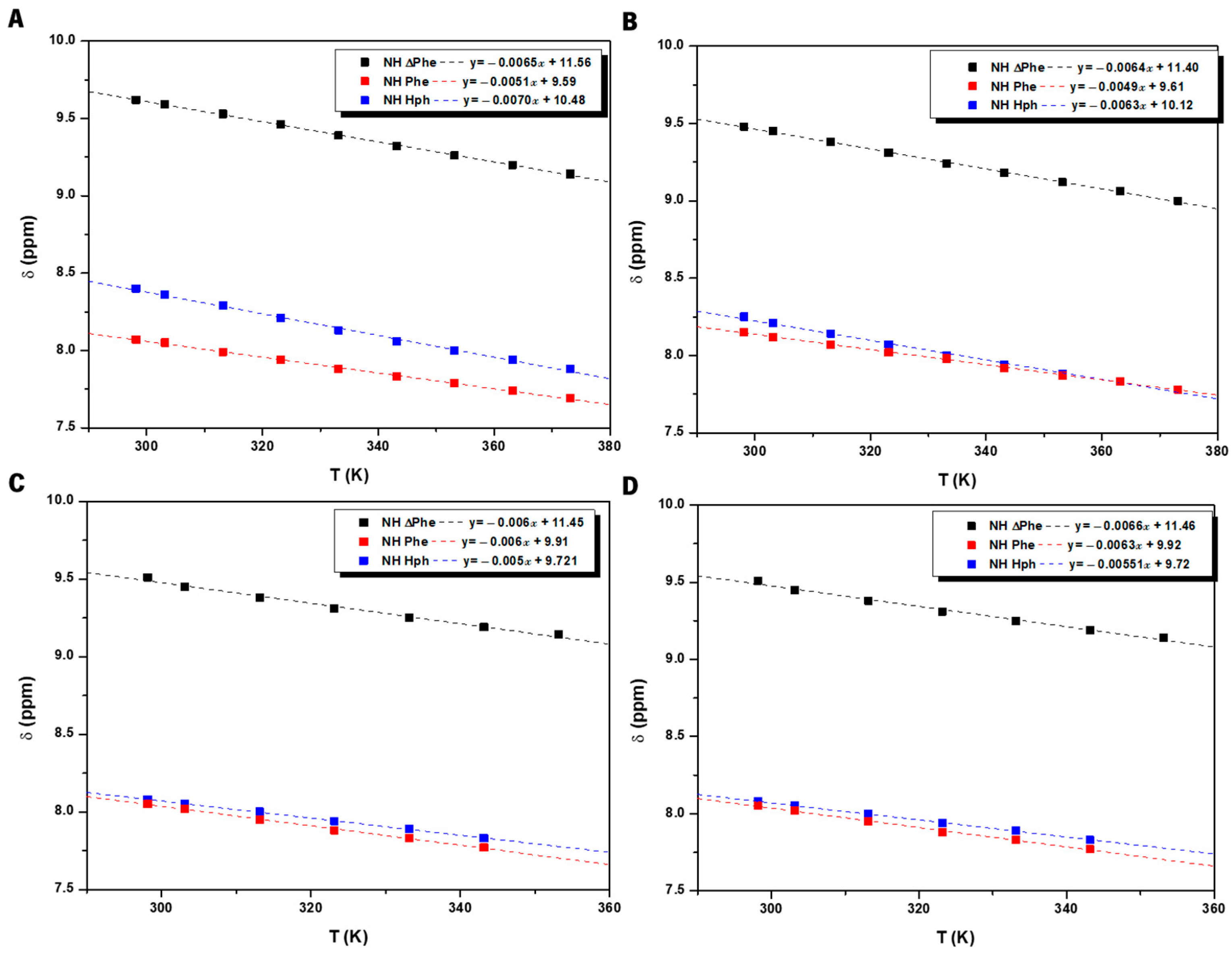




| Hydrogelator | Critical Aggregation Concentration (µM) |
|---|---|
| Suc-D-Hph-L-Phe-Z-ΔPhe-OMe, 11 | 34.36 |
| Suc-L-Hph-L-Phe-Z-ΔPhe-OMe, 12 | 40.74 |
| Suc-L-Phe-D-Hph-Z-ΔPhe-OMe, 13 | 38.19 |
| Suc-L-Phe-L-Hph-Z-ΔPhe-OMe, 14 | 35.80 |
| Suc-D-Hph-L-Phe-Z-ΔPhe-OH, 15 | 34.83 |
| Suc-L-Hph-L-Phe-Z-ΔPhe-OH, 16 | 38.64 |
| Suc-L-Phe-D-Hph-Z-ΔPhe-OH, 17 | 33.27 |
| Suc-L-Phe-L-Hph-Z-ΔPhe-OH, 18 | 37.76 |
| Hydrogel | Gelation Concentration wt% | Before Breaking G′ (Pa) | After Breaking G′ (Pa) | Recovery (%) |
|---|---|---|---|---|
| Suc-D-Hph-L-Phe-ΔPhe-OMe, 11 | 0.2 | 6.15 × 102 | 2.15 × 102 | 35% |
| 0.6 | 3.19 × 105 | 2.88 × 104 | 9% | |
| Suc-L-Hph-L-Phe-ΔPhe-OMe, 12 | 0.2 | 4.66 × 104 | 2.28 × 104 | 49% |
| 0.6 | 5.79 × 103 | 6.92 × 103 | 120% | |
| Suc-L-Phe-D-Hph-ΔPhe-OMe, 13 | 0.2 | 3.62 × 104 | 1.98 × 103 | 5% |
| 0.6 | 3.15 × 105 | 3.80 × 103 | 1% | |
| Suc-L-Phe-L-Hph-ΔPhe-OMe, 14 | 0.2 | 3.31 × 104 | 3.82 × 105 | 1154% |
| 0.6 | 2.73 × 105 | 1.25 × 105 | 46% | |
| Suc-D-Hph-L-Phe-ΔPhe-OH, 15 | 0.2 | 3.35 × 103 | 1.05 × 103 | 31% |
| 0.6 | 3.02 × 104 | 2.66 × 103 | 9% | |
| Suc-L-Hph-L-Phe-ΔPhe-OH, 16 | 0.2 | 1.75 × 104 | 1.39 × 103 | 8% |
| 0.6 | 1.27 × 105 | 2.02 × 104 | 16% | |
| Suc-L-Phe-D-Hph-ΔPhe-OH, 17 | 0.2 | 1.47 × 103 | 2.09 × 103 | 142% |
| 0.6 | 3.20 × 104 | 2.00 × 103 | 6% | |
| Suc-L-Phe-L-Hph-ΔPhe-OH, 18 | 0.2 | 2.09 × 106 | 5.44 × 106 | 260% |
| 0.6 | 8.73 × 104 | 5.28 × 103 | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.F.; Pereira, T.; Oliveira, C.; Figueiredo, P.; Carvalho, A.; Pereira, D.M.; Hilliou, L.; Bañobre-López, M.; Xu, B.; Ferreira, P.M.T.; et al. Tripeptides Featuring Dehydrophenylalanine and Homophenylalanine: Homo- Versus Hetero-Chirality and Sequence Effects on Self-Assembly and Gelation. Gels 2025, 11, 164. https://doi.org/10.3390/gels11030164
Carvalho AF, Pereira T, Oliveira C, Figueiredo P, Carvalho A, Pereira DM, Hilliou L, Bañobre-López M, Xu B, Ferreira PMT, et al. Tripeptides Featuring Dehydrophenylalanine and Homophenylalanine: Homo- Versus Hetero-Chirality and Sequence Effects on Self-Assembly and Gelation. Gels. 2025; 11(3):164. https://doi.org/10.3390/gels11030164
Chicago/Turabian StyleCarvalho, André F., Teresa Pereira, Carlos Oliveira, Pedro Figueiredo, Alexandra Carvalho, David M. Pereira, Loic Hilliou, Manuel Bañobre-López, Bing Xu, Paula M. T. Ferreira, and et al. 2025. "Tripeptides Featuring Dehydrophenylalanine and Homophenylalanine: Homo- Versus Hetero-Chirality and Sequence Effects on Self-Assembly and Gelation" Gels 11, no. 3: 164. https://doi.org/10.3390/gels11030164
APA StyleCarvalho, A. F., Pereira, T., Oliveira, C., Figueiredo, P., Carvalho, A., Pereira, D. M., Hilliou, L., Bañobre-López, M., Xu, B., Ferreira, P. M. T., & Martins, J. A. (2025). Tripeptides Featuring Dehydrophenylalanine and Homophenylalanine: Homo- Versus Hetero-Chirality and Sequence Effects on Self-Assembly and Gelation. Gels, 11(3), 164. https://doi.org/10.3390/gels11030164










