Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing
Abstract
1. Introduction
2. Type and Design Strategies of Conductive Hydrogels
2.1. Metal-Based Conductive Hydrogels for Wearable Sensors
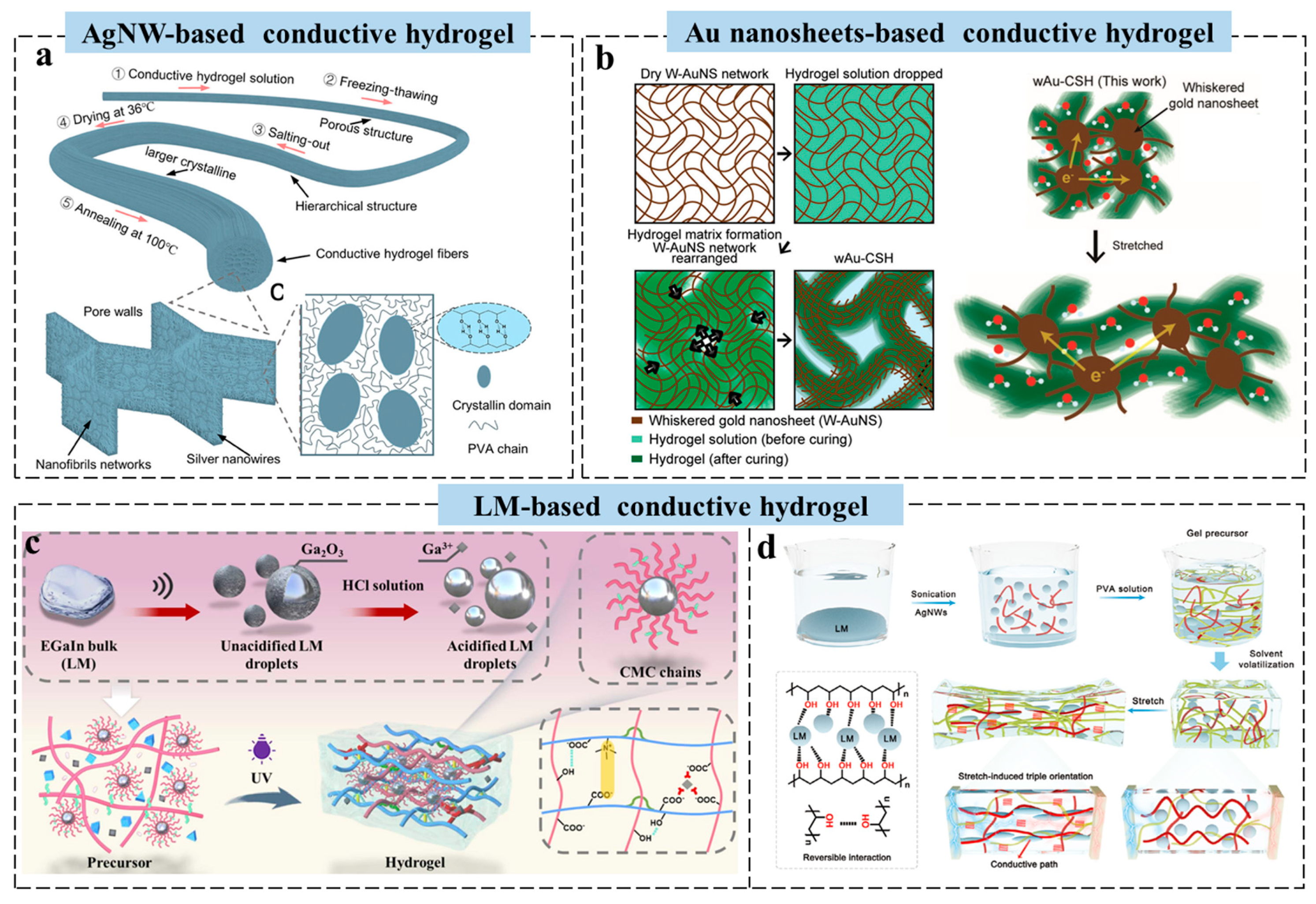
2.2. Carbon-Based Conductive Hydrogels for Wearable Sensors
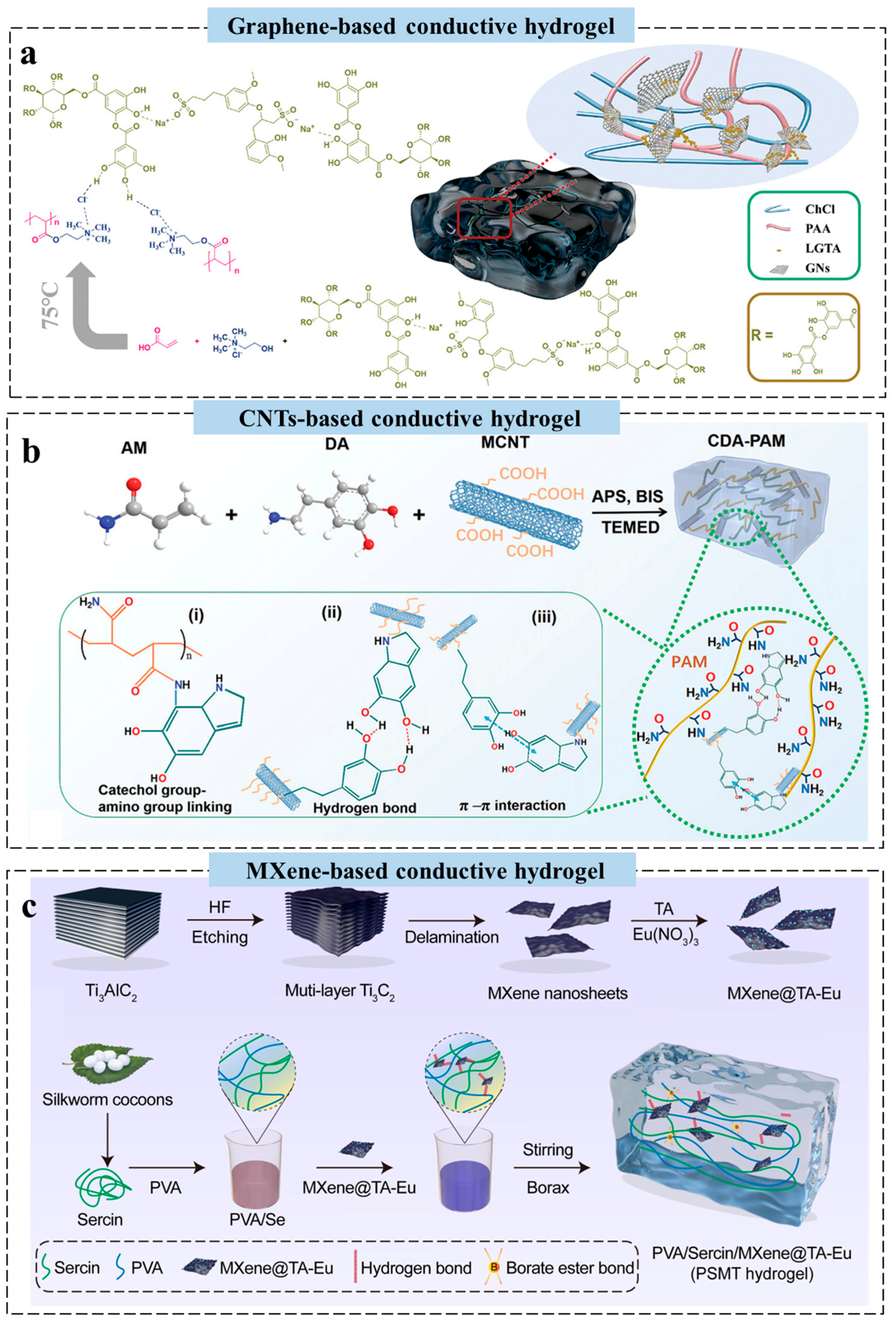
2.3. Conductive Polymer-Based Hydrogels for Wearable Sensors
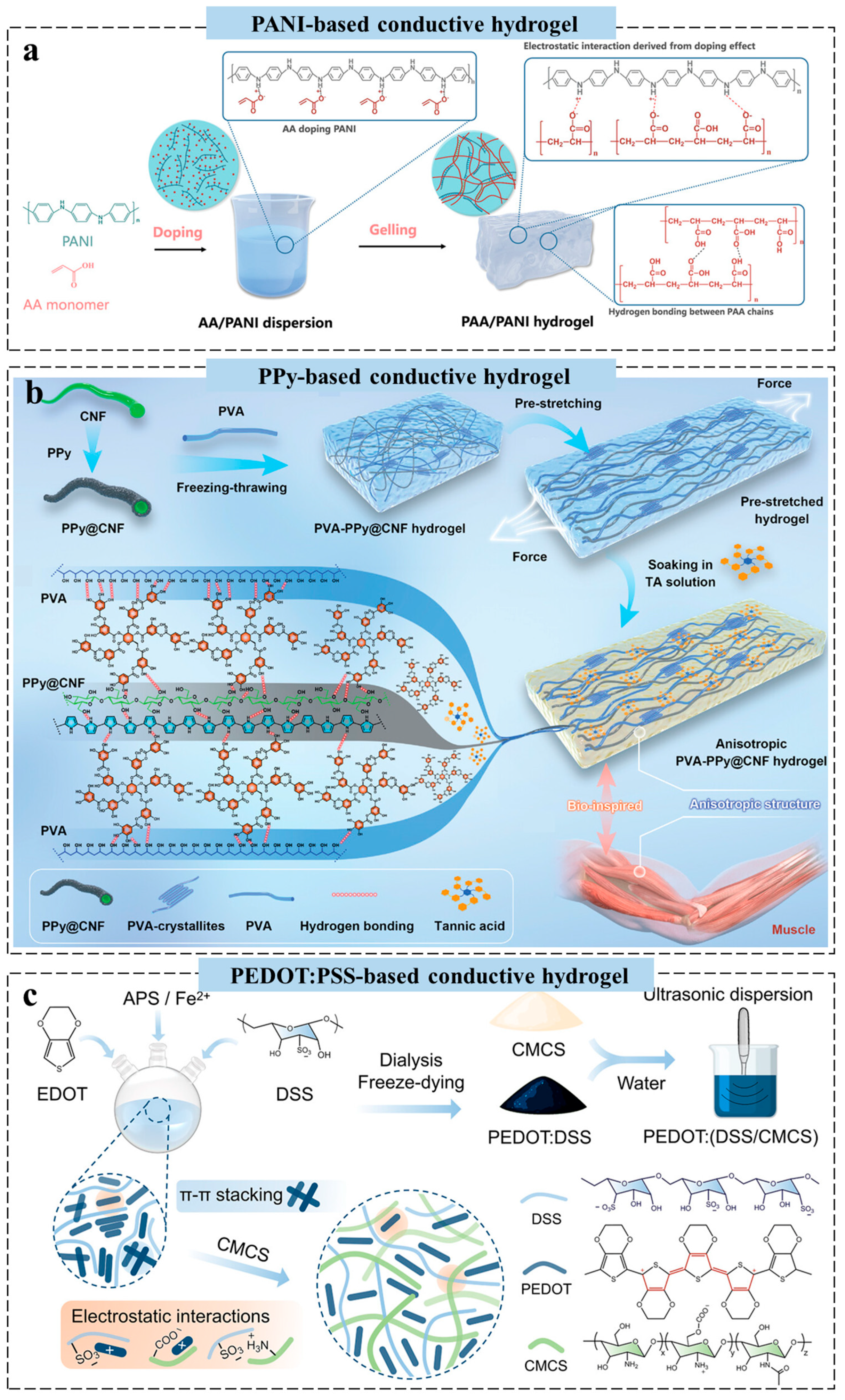
2.4. Ionic Conductive Hydrogels for Wearable Sensors

2.5. Hybrid Conductive Hydrogels for Wearable Sensors
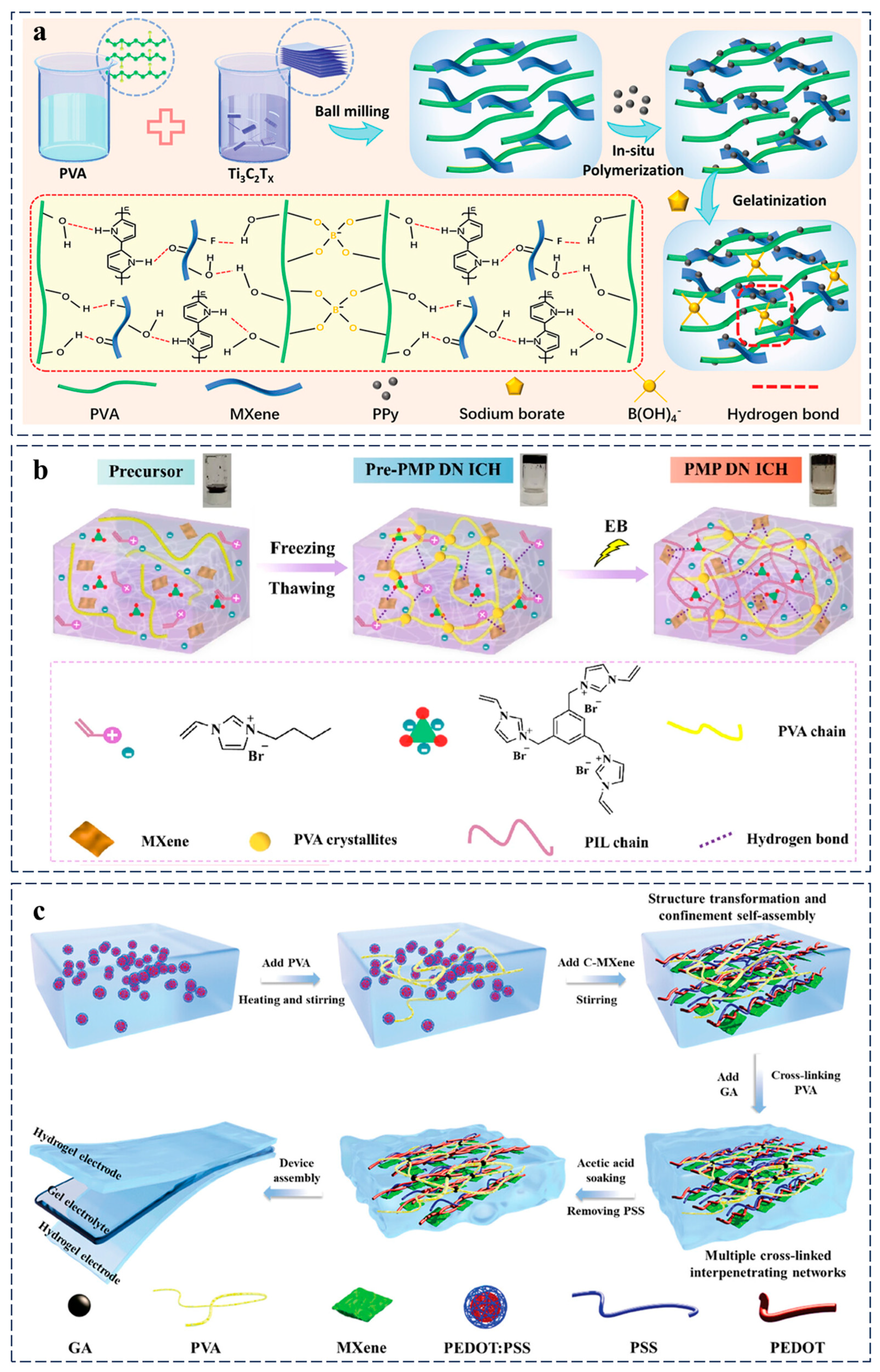
2.6. Analysis of Limitations and Challenges in Conductive Hydrogels
2.7. Environmental and Ethical Considerations of Conductive Hydrogels
3. Application of Conductive Hydrogels in Wearable Sensors
3.1. Physiological Monitoring
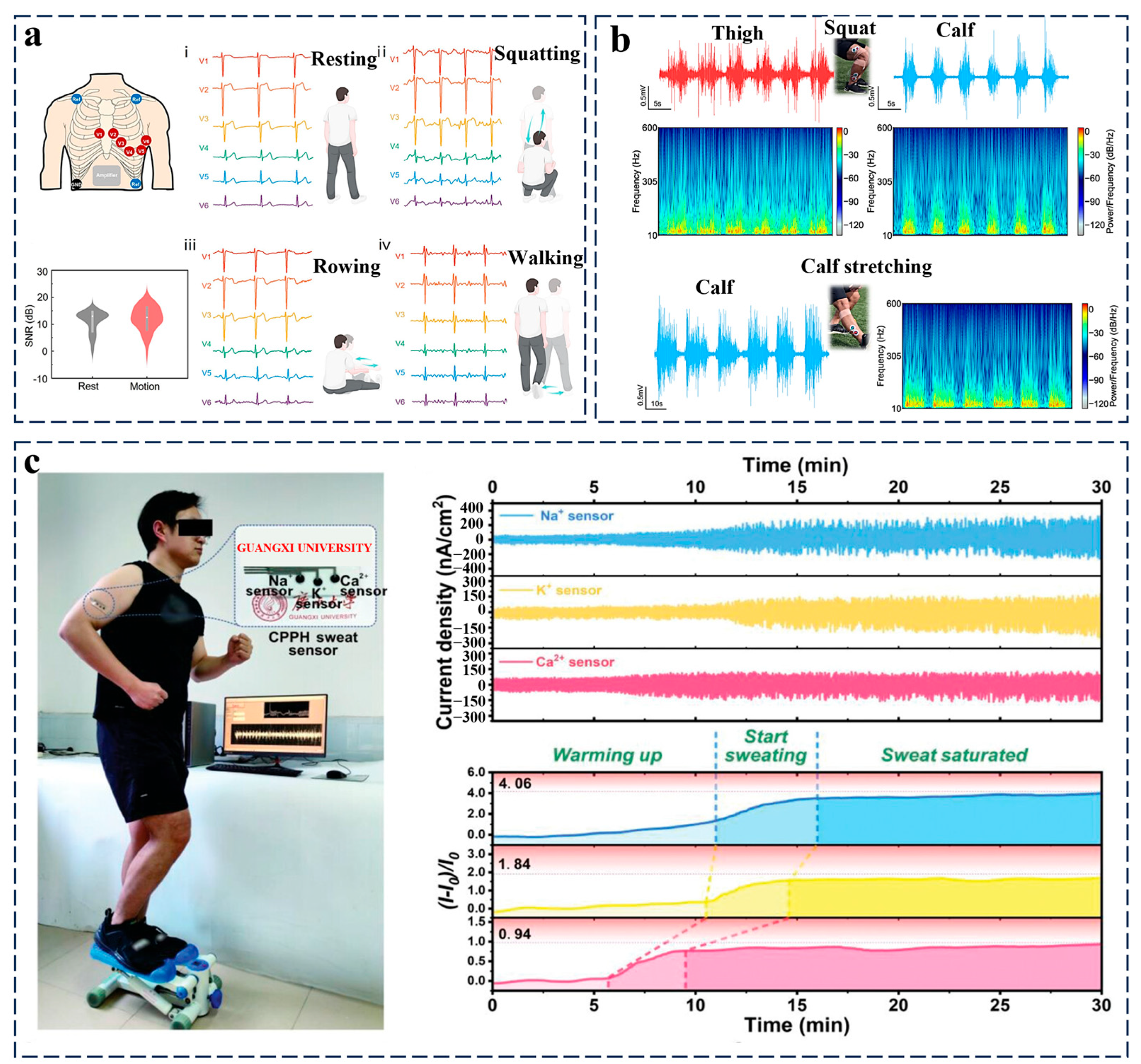
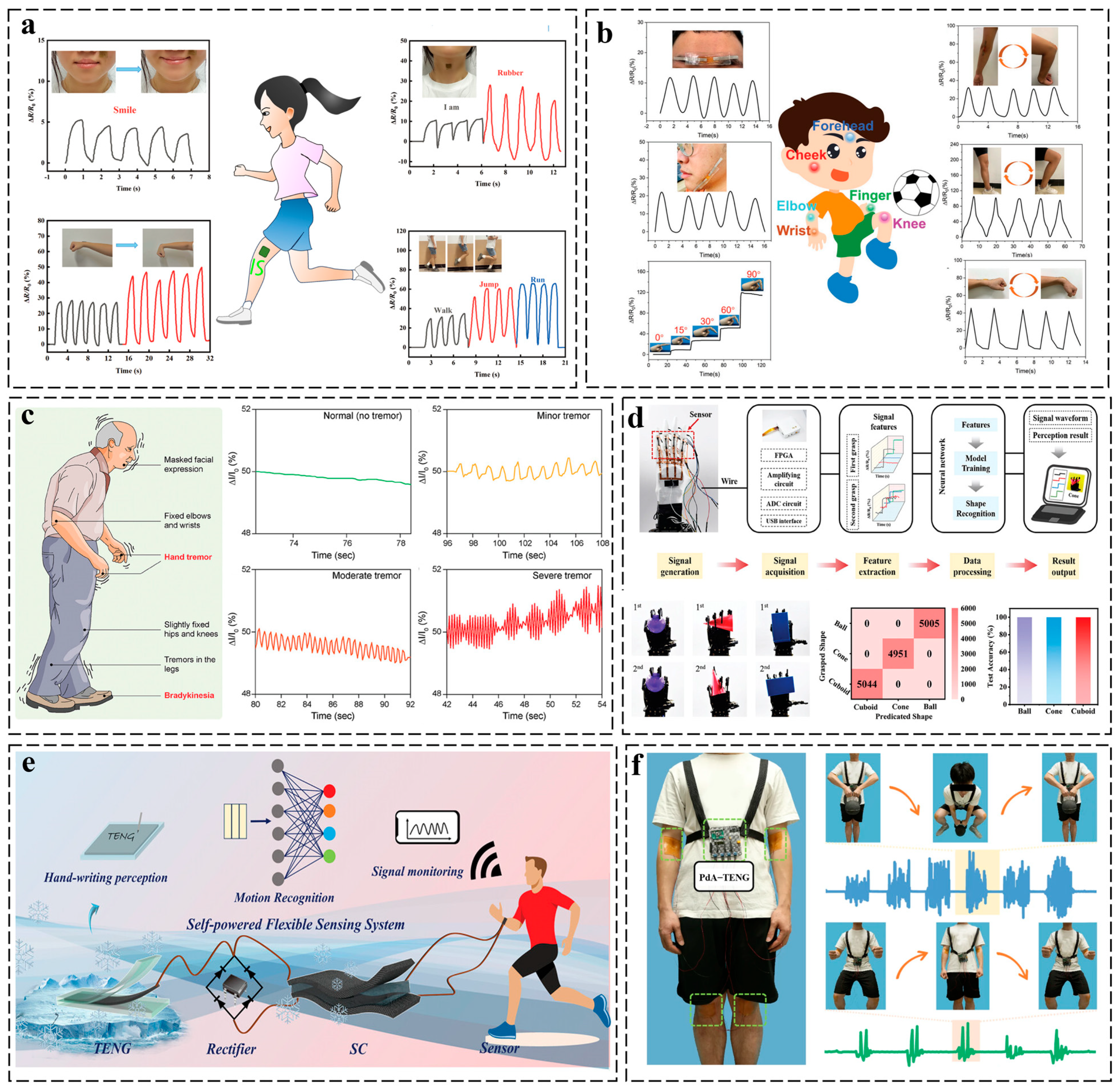
3.2. Mechano-Responsive Systems
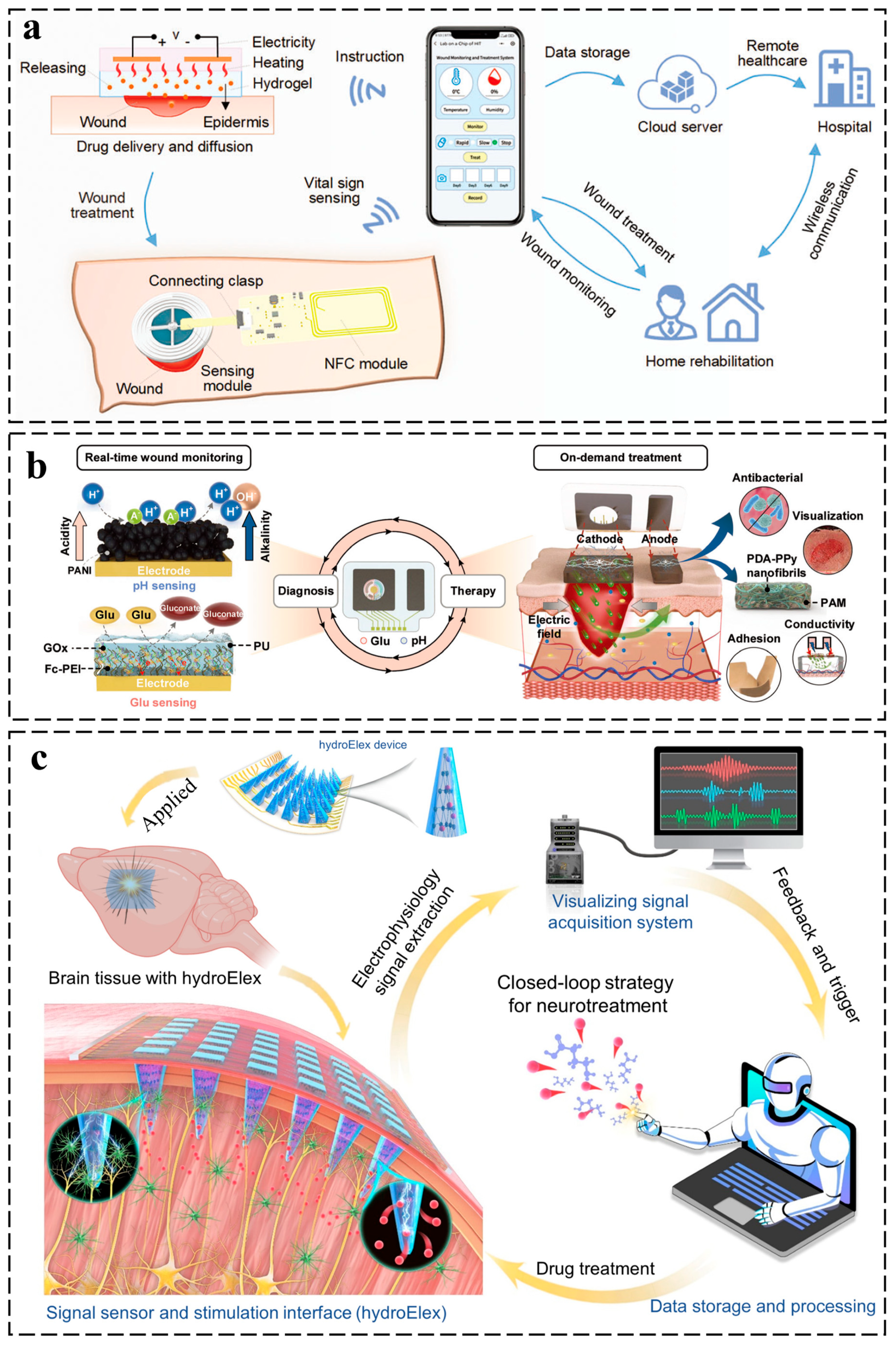
3.3. Closed-Loop Diagnostic–Therapeutic Systems
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, S.C.; Saha, T.; Yin, L.; Liu, R.X.; Khan, M.I.; Chang, A.Y.; Lee, H.; Zhao, H.; Liu, Y.Z.; Nazemi, A.S.; et al. A fingertip-wearable microgrid system for autonomous energy management and metabolic monitoring. Nat. Electron. 2024, 7, 788–799. [Google Scholar]
- Shi, X.L.; Fan, X.Q.; Zhu, Y.B.; Liu, Y.; Wu, P.Q.; Jiang, R.H.; Wu, B.; Wu, H.A.; Zheng, H.; Wang, J.B.; et al. Pushing detectability and sensitivity for subtle force to new limits with shrinkable nanochannel structured aerogel. Nat. Commun. 2022, 13, 1119. [Google Scholar] [PubMed]
- Fu, X.M.; Cheng, W.; Wan, G.X.; Yang, Z.J.; Tee, B.C.K. Toward an ai era: Advances in electronic skins. Chem. Rev. 2024, 124, 9899–9948. [Google Scholar] [PubMed]
- Luo, Y.F.; Abidian, M.R.; Ahn, J.H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.N.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology roadmap for flexible sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar]
- Liang, Y.N.; Wu, Z.X.; Wei, Y.N.; Ding, Q.L.; Zilberman, M.; Tao, K.; Xie, X.; Wu, J. Self-healing, self-adhesive and stable organohydrogel-based stretchable oxygen sensor with high performance at room temperature. Nano-Micro Lett. 2022, 14, 52. [Google Scholar]
- Araromi, O.A.; Graule, M.A.; Dorsey, K.L.; Castellanos, S.; Foster, J.R.; Hsu, W.H.; Passy, A.E.; Vlassak, J.J.; Weaver, J.C.; Walsh, C.J.; et al. Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature 2020, 587, 219. [Google Scholar]
- Qin, R.Z.; Nong, J.; Wang, K.Q.; Liu, Y.S.; Zhou, S.B.; Hu, M.J.; Zhao, H.B.; Shan, G.C. Recent advances in flexible pressure sensors based on MXene materials. Adv. Mater. 2024, 36, 2312761. [Google Scholar]
- Khang, D.-Y.; Jiang, H.; Huang, Y.; Rogers, J.A. A stretchable form of single-crystal silicon for high-performance electronics on rubber substrates. Science 2006, 311, 208–212. [Google Scholar]
- Mo, F.; Zhou, P.C.; Lin, S.H.; Zhong, J.W.; Wang, Y. A review of conductive hydrogel-based wearable temperature sensors. Adv. Healthc. Mater. 2024, 13, 2401503. [Google Scholar]
- Liang, Y.P.; Qiao, L.P.; Qiao, B.W.; Guo, B.L. Conductive hydrogels for tissue repair. Chem. Sci. 2023, 14, 3091–3116. [Google Scholar]
- Liu, C.C.; Wang, Y.Y.; Shi, S.T.; Zheng, Y.B.; Ye, Z.W.; Liao, J.Q.; Sun, Q.F.; Dang, B.K.; Shen, X.P. Myelin sheath-inspired hydrogel electrode for artificial skin and physiological monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [PubMed]
- Wang, H.; Shang, R.; Chen, J.; Jin, X.; Chen, K.; Huang, B.; Chen, H.; Lu, Q.-L. Flexible chitosan sensing hydrogel enabled by phytic acid coordination effect with high-conductivity and ultra-sensitivity for self-powered handwriting recognition and multimodal sensors. Nano Energy 2024, 128, 109843. [Google Scholar]
- Shan, M.; Chen, X.; Zhang, X.; Zhang, S.; Zhang, L.; Chen, J.; Wang, X.; Liu, X. Injectable conductive hydrogel with self-healing, motion monitoring, and bacteria theranostics for bioelectronic wound dressing. Adv. Healthc. Mater. 2024, 13, 2303876. [Google Scholar]
- Li, Y.C.; Zheng, C.R.; Liu, S.; Huang, L.; Fang, T.S.; Li, J.X.Z.; Xu, F.; Li, F. Smart glove integrated with tunable MWNTs/PDMS fibers made of a one-step extrusion method for finger dexterity, gesture, and temperature recognition. ACS Appl. Mater. Interfaces 2020, 12, 23764–23773. [Google Scholar]
- Wang, W.; Zhou, H.L.; Xu, Z.S.; Li, Z.H.; Zhang, L.Q.; Wan, P.B. Flexible conformally bioadhesive MXene hydrogel electronics for machine learning-facilitated human-interactive sensing. Adv. Mater. 2024, 36, 2401035. [Google Scholar]
- Liu, Y.; Tian, G.X.; Du, Y.J.; Shi, P.J.; Li, N.; Li, Y.F.; Qin, Z.H.; Jiao, T.F.; He, X.M. Highly stretchable, low-hysteresis, and adhesive ta@mxene-composited organohydrogels for durable wearable sensors. Adv. Funct. Mater. 2024, 34, 2315813. [Google Scholar]
- Li, Y.F.; Yang, X.; Ding, Y.R.; Zhang, H.W.; Cheng, Y.F.; Li, X.F.; Sun, J.C.; Liu, Y.N.; Li, Y.C.; Fan, D.D. A wireless health monitoring system accomplishing bimodal decoupling based on an “IS”-shaped multifunctional conductive hydrogel. Small 2025. [Google Scholar] [CrossRef]
- Wang, W.Y.; Guo, P.S.; Liu, X.; Chen, M.J.; Li, J.H.; Hu, Z.G.; Li, G.D.; Chang, Q.; Shi, K.M.; Wang, X.L.; et al. Fully polymeric conductive hydrogels with low hysteresis and high toughness as multi-responsive and self-powered wearable sensors. Adv. Funct. Mater. 2024, 34, 2316346. [Google Scholar]
- Hao, S.W.; Dai, R.A.; Fu, Q.J.; Wang, Y.C.; Zhang, X.R.; Li, H.; Liu, X.D.; Yang, J. A robust and adhesive hydrogel enables interfacial coupling for continuous temperature monitoring. Adv. Funct. Mater. 2023, 33, 2302840. [Google Scholar]
- Li, X.Y.; Liu, Y.N.; Ding, Y.R.; Zhang, M.; Lin, Z.H.; Hao, Y.; Li, Y.C.; Chang, J.J. Capacitive pressure sensor combining dual dielectric layers with integrated composite electrode for wearable healthcare monitoring. ACS Appl. Mater. Interfaces 2024, 16, 12974–12985. [Google Scholar]
- Li, J.; Li, J.L.; Tang, Y.T.; Liu, Z.H.; Zhang, Z.L.; Wu, H.; Shen, B.; Su, M.; Liu, M.J.; Li, F.Y. Touchable gustation via a hoffmeister gel iontronic sensor. ACS Nano 2023, 17, 5129–5139. [Google Scholar] [PubMed]
- Wang, W.Y.; Yao, D.J.; Wang, H.; Ding, Q.L.; Luo, Y.B.; Ding, H.J.; Yu, J.H.; Zhang, H.; Tao, K.; Zhang, S.; et al. A breathable, stretchable, and self-calibrated multimodal electronic skin based on hydrogel microstructures for wireless wearables. Adv. Funct. Mater. 2024, 34, 2316339. [Google Scholar]
- Zhang, J.; Yan, K.; Huang, J.R.; Sun, X.D.; Li, J.; Cheng, Y.; Sun, Y.Q.; Shi, Y.; Pan, L.J. Mechanically robust, flexible, fast responding temperature sensor and high-resolution array with ionically conductive double cross-linked hydrogel. Adv. Funct. Mater. 2024, 34, 2314433. [Google Scholar]
- Chen, K.; Liang, K.W.; Liu, H.; Liu, R.A.; Liu, Y.Y.; Zeng, S.J.; Tian, Y. Skin-inspired ultra-tough supramolecular multifunctional hydrogel electronic skin for human-machine interaction. Nano-Micro Lett. 2023, 15, 102. [Google Scholar]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate complexes and their hydrogels: A powerful toolkit for the development of next-generation sustainable functional materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar]
- Ding, J.J.; Zhang, H.; Wang, W.B.; Zhu, Y.F.; Wang, Q.; Wang, A.Q. Synergistic effect of palygorskite nanorods and ion crosslinking to enhance sodium alginate-based hydrogels. Eur. Polym. J. 2021, 147, 110306. [Google Scholar]
- Ni, Y.M.; Zang, X.R.; Yang, Y.; Gong, Z.H.; Li, H.Q.; Chen, J.J.; Wu, C.; Huang, J.Y.; Lai, Y.K. Environmental stability stretchable organic hydrogel humidity sensor for respiratory monitoring with ultrahigh sensitivity. Adv. Funct. Mater. 2024, 34, 2402853. [Google Scholar]
- Lei, T.; Wang, Y.; Feng, Y.; Duan, X.; Zhang, Q.; Wan, A.; Xia, Z.; Shou, W.; Fan, J. PNIPAAm-based temperature responsive ionic conductive hydrogels for flexible strain and temperature sensing. J. Colloid Interface Sci. 2025, 678, 726–741. [Google Scholar]
- Lu, Y.-N.; Mo, K.; Liang, X.-H.; Xie, J.-S.; Yang, Y.; Zheng, L.; Gu, M.; Liu, X.-R.; Lu, Y.; Ge, J. High ion-conductive hydrogel: Soft, elastic, with wide humidity tolerance and long-term stability. ACS Appl. Mater. Interfaces 2024, 16, 60992–61003. [Google Scholar]
- Li, T.; Qi, H.; Dong, X.; Li, G.; Zhai, W. Highly robust conductive organo-hydrogels with powerful sensing capabilities under large mechanical stress. Adv. Mater. 2024, 36, 2304145. [Google Scholar]
- Lv, R.; Cao, X.; Zhang, T.; Ji, W.; Muhammad, U.; Chen, J.; Wei, Y. A highly stretchable, self-healing, self-adhesive polyacrylic acid/chitosan multifunctional composite hydrogel for flexible strain sensors. Carbohyd. Polym. 2025, 351, 123111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.X.; Ni, Y.M.; Biesold, G.M.; Cheng, Y.; Ge, M.Z.; Li, H.Q.; Huang, J.Y.; Lin, Z.Q.; Lai, Y.K. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Xu, T.; Zhang, H.; Wang, X.; Qi, J.J.; Dong, Q.; Zhu, L.Y.; Yuan, Z.H.; Si, C.L. Functionalities and properties of conductive hydrogel with nanocellulose integration. Chem. Eng. J. 2025, 506, 159872. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Zhao, K.; Qian, R.; Yu, Z.M.; Ye, C.Q. Interfacial engineering of liquid metal nanoparticles for the fabrication of conductive hydrogels: A review. Chem. Eng. J. 2024, 486, 150197. [Google Scholar] [CrossRef]
- Chen, Z.W.; Chen, X.G.; Wang, H.D.; Yang, T.T.; Huang, J.X.; Guo, Z.G. Metal ion mediated conductive hydrogels with low hysteresis and high resilience. Mater. Today Phys. 2025, 51, 101656. [Google Scholar] [CrossRef]
- Liu, R.A.; Liu, Y.Y.; Cheng, Y.G.; Liu, H.; Fu, S.M.; Jin, K.M.; Li, D.L.; Fu, Z.W.; Han, Y.X.; Wang, Y.P.; et al. Aloe inspired special structure hydrogel pressure sensor for real-time human-computer interaction and muscle rehabilitation system. Adv. Funct. Mater. 2023, 33, 2308175. [Google Scholar] [CrossRef]
- Li, Y.M.; Chen, S.E.; Yan, H.; Jiang, H.W.; Luo, J.J.; Zhang, C.; Pang, Y.K.; Tan, Y.Q. Biodegradable, transparent, and antibacterial alginate-based triboelectric nanogenerator for energy harvesting and tactile sensing. Chem. Eng. J. 2023, 468, 143572. [Google Scholar] [CrossRef]
- Yuan, X.M.; Wu, P.C.; Gao, Q.; Xu, J.; Guo, B.; He, Y. Multifunctionally wearable monitoring with gelatin hydrogel electronics of liquid metals. Mater. Horiz. 2022, 9, 961–972. [Google Scholar] [CrossRef]
- Li, X.F.; Jiang, M.; Du, Y.M.; Ding, X.; Xiao, C.; Wang, Y.Y.; Yang, Y.Y.; Zhuo, Y.Z.; Zheng, K.; Liu, X.L.; et al. Self-healing liquid metal hydrogel for human-computer interaction and infrared camouflage. Mater. Horiz. 2023, 10, 2945–2957. [Google Scholar] [CrossRef]
- Yuan, X.M.; Guo, C.R.; Wang, Z.J.; Jiang, H.W.; He, Y.; Xu, J.; Guo, B. Liquid metal-hydrogel biosensor for behavior and sweat monitoring. ACS Appl. Electron. Mater. 2023, 5, 1420–1428. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.Y.; Zhang, K.; Tan, R.J.; Zhang, S.; Su, Y.P.; Hu, J.L. Continuous phase separation induced tough hydrogel fibers with ultrahigh conductivity for multidimensional soft electronics. Adv. Funct. Mater. 2025, 35, 2413478. [Google Scholar]
- Lim, C.; Lee, S.; Kang, H.; Cho, Y.S.; Yeom, D.; Sunwoo, S.H.; Park, C.; Nam, S.; Kim, J.H.; Lee, S.P.; et al. Highly conductive and stretchable hydrogel nanocomposite using whiskered gold nanosheets for soft bioelectronics. Adv. Mater. 2024, 36, 2407931. [Google Scholar]
- Hao, F.Y.; Sun, S.; Xu, Y.Z.; Maimaitiyiming, X.R.L. 3D printing of flexible sensors based on polyvinyl alcohol/carboxylated chitosan/sodium alginate/silver nanowire high-strength hydrogels. Polymer 2024, 290, 126594. [Google Scholar]
- Hou, M.J.; Yu, M.L.; Liu, W.L.; Zhang, H.Y.; Wang, Z.S.; Du, J.J.; Xu, L.J.; Li, N.; Xu, J.X. Mxene hybrid conductive hydrogels with mechanical flexibility, frost-resistance, photothermoelectric conversion characteristics and their multiple applications in sensing. Chem. Eng. J. 2024, 483, 149299. [Google Scholar]
- Yang, Q.N.; Yu, M.L.; Zhang, H.Y.; Li, N.; Du, J.J.; Xu, L.J.; Xu, J.X. Triboelectric nanogenerator based on well-dispersed and oxide-free liquid metal-doped conductive hydrogel as self-powered wearable sensor for respiratory and thyroid cartilage signal monitoring. Nano Energy 2025, 134, 110530. [Google Scholar]
- Zhao, Z.B.; Soni, S.; Lee, T.; Nijhuis, C.A.; Xiang, D. Smart eutectic gallium-indium: From properties to applications. Adv. Mater. 2023, 35, 2203391. [Google Scholar] [CrossRef]
- Ma, Z.J.; Huang, Q.Y.; Xu, Q.; Zhuang, Q.N.; Zhao, X.; Yang, Y.H.; Qiu, H.; Yang, Z.L.; Wang, C.; Chai, Y.; et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 2021, 20, 859. [Google Scholar] [CrossRef]
- Vallem, V.; Roosa, E.; Ledinh, T.; Jung, W.; Kim, T.I.; Rashid-Nadimi, S.; Kiani, A.; Dickey, M.D. A soft variable-area electrical-double-layer energy harvester. Adv. Mater. 2021, 33, 2103142. [Google Scholar]
- Bao, W.; Fan, W.Z.; Luo, J.; Huo, S.K.; Hu, Z.Y.; Jing, X.; Chen, W.J.; Long, X.Y.; Zhang, Y.F. Imidazolium-type poly(ionic liquid) endows the composite polymer electrolyte membrane with excellent interface compatibility for all- solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 2022, 14, 55664–55673. [Google Scholar]
- Wang, X.W.; Zheng, S.J.; Xiong, J.F.; Liu, Z.Y.; Li, Q.N.; Li, W.Z.; Yan, F. Stretch-induced conductivity enhancement in highly conductive and tough hydrogels. Adv. Mater. 2024, 36, 2313845. [Google Scholar]
- Ma, J.N.; Zhang, Y.L.; Liu, Y.Q.; Han, D.D.; Mao, J.W.; Zhang, J.R.; Zhao, W.C.; Sun, H.B. Heterogeneous self-healing assembly of MXene and graphene oxide enables producing free-standing and self-reparable soft electronics and robots. Sci. Bull. 2022, 67, 501–511. [Google Scholar]
- Wang, Y.; Li, Y.X.; Zhang, Y.J.; You, L.X.; Song, Y.T.; Li, T.; Fang, Z.; Gui, A.; Li, Y.F.; Liao, L.; et al. Graphene-doped hydrogels with enhanced conductivity and stretchability for all-weather wearable devices. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Qin, Z.H.; Sun, X.; Yu, Q.Y.; Zhang, H.T.; Wu, X.J.; Yao, M.M.; Liu, W.W.; Yao, F.L.; Li, J.J. Carbon nanotubes/hydrophobically associated hydrogels as ultrastretchable, highly sensitive, stable strain, and pressure sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [PubMed]
- Wang, H.; Li, Z.; Liu, Z.; Fu, J.; Shan, T.; Yang, X.; Lei, Q.; Yang, Y.; Li, D. Flexible capacitive pressure sensors for wearable electronics. J. Mater. Chem. C 2022, 10, 1594–1605. [Google Scholar]
- Zhang, C.K.; Zhou, P.R.; Deng, Y.Y.; Ma, X.M.; Hu, Y.J.; Chen, Y.C.; Wang, X.; Tao, G.; He, Y.; Cai, R.; et al. Sericin-based conductive hydrogel loaded with MXene@TA-Eu nanosheets promotes the healing of infected wounds through photothermal antibacterial and electrical stimulation. Chem. Eng. J. 2025, 505, 159738. [Google Scholar]
- Chen, J.F.; Hou, R.; Li, S.; Sun, C.X.; Peng, K.; Dai, Y.C.; Chen, X.X. PAM/CNTs-Au microcrack sensor with high sensitivity and wide detection range for multi-scale human motion detection. Sens. Actuat. A-Phys. 2024, 370, 115203. [Google Scholar]
- Peng, L.; Su, Y.T.; Yang, X.P.; Sui, G. A liquid metal/carbon nanotubes complex enabling ultra-fast polymerization of super-robust, stretchable adhesive hydrogels for highly sensitive sensor. J. Colloid Interface Sci. 2023, 638, 313–323. [Google Scholar]
- Li, J.Y.; Luo, Y.B.; Tao, K.; Wu, J. Graphene-modified hydrogels for bioelectronic interface. Matter 2024, 7, 4139–4142. [Google Scholar]
- Yue, J.; Li, C.; Ji, X.; Tao, Y.; Lu, J.; Cheng, Y.; Du, J.; Wang, H. Highly tough and conductive hydrogel based on defect-patched reduction graphene oxide for high-performance self-powered flexible sensing micro-system. Chem. Eng. J. 2023, 466, 143358. [Google Scholar]
- Xu, Z.Y.; Qiao, X.J.; Tao, R.Z.; Li, Y.X.; Zhao, S.J.; Cai, Y.C.; Luo, X.L. A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar]
- Wang, Z.; Hu, Q.H.; Yao, S.C.; Wang, S.B.; Liu, X.; Zhang, C.P.; Wang, Z.L.; Li, L.L. Flexible triboelectric nanogenerator patch for accelerated wound healing through the synergy of electrostimulation and photothermal effect. Small 2025, 21, 2409756. [Google Scholar]
- Zhang, S.; Zhao, B.; Zhang, D.; Yang, M.; Huang, X.; Han, L.; Chen, K.; Li, X.; Pang, R.; Shang, Y. Conductive hydrogels incorporating carbon nanoparticles: A review of synthesis, performance and applications. Particuology 2023, 83, 212–231. [Google Scholar]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (Mxenes). Science 2021, 372, 1165. [Google Scholar]
- Geng, L.; Liu, W.; Fan, B.; Wu, J.; Shi, S.; Huang, A.; Hu, J.; Peng, X. Anisotropic double-network hydrogels integrated superior performance of strength, toughness and conductivity for flexible multi-functional sensors. Chem. Eng. J. 2023, 462, 142226. [Google Scholar]
- Ogawa, R.; Arakaki, R.; Oya, T. Development and geometrical considerations of unique conductive and reversible carbon-nanotube hydrogel without need for gelators. Gels 2024, 10, 457. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Y.; Gui, Y.; Wang, C.; Wu, Y.; Yao, J.; Wang, J. Ultrastretchable E-skin based on conductive hydrogel microfibers for wearable sensors. Small 2024, 20, 2305951. [Google Scholar]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.C.; Lim, H.R. Conductive polymer-based hydrogels for wearable electrochemical biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef]
- Wan, L.Y.; Li, P.J.; Yan, M.L.; Wang, J.H.; Li, X.B. Strong, self-healing, shape memory PAA-PANI/PVA/PDA/AOP conductive hydrogels with interpenetrating network and hydrogen bond interaction. Eur. Polym. J. 2023, 191, 112034. [Google Scholar]
- Hu, M.Y.; Qiu, L.H.; Huang, Y.L.; Wang, D.H.; Li, J.L.; Liang, C.Y.; Wu, G.; Peng, F. An adhesive, low swelling and conductive tri-network hydrogel for wearable electronic devices. J. Mater. Chem. C 2024, 12, 8534–8544. [Google Scholar]
- Yang, M.; Ren, X.N.; Yang, T.T.; Xu, C.; Ye, Y.Q.; Sun, Z.W.; Kong, L.H.; Wang, B.; Luo, Z.Q. Polypyrrole/sulfonated multi-walled carbon nanotubes conductive hydrogel for electrochemical sensing of living cells. Chem. Eng. J. 2021, 418, 129483. [Google Scholar]
- Kim, Y.W.; Park, J.M.; Park, C.S.; Na, H.; Kang, Y.W.; Lee, W.; Sun, J.Y. Anisotropically conductive hydrogels with directionally aligned PEDOT:PSS in a PVA matrix. ACS Appl. Mater. Interfaces 2024, 16, 4013–4023. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, H.H.; Zhao, Y.Y.; Huyan, C.X.; Wang, Z.B.; Torun, H.; Guo, Z.H.; Dai, S.; Xu, B.B.; Chen, F. A strand entangled supramolecular PANI/PAA hydrogel enabled ultra-stretchable strain sensor. Small 2022, 18, 2203258. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.C.; Yang, W.S.; Lu, B.L.; Xu, Y.L.; Chen, J.P.; Zheng, X.X.; Liu, S.Y.; Lin, C.S.; Zeng, H.B.; Huang, B. Muscle-inspired robust anisotropic cellulose conductive hydrogel for multidirectional strain sensors and implantable bioelectronics. Adv. Funct. Mater. 2024, 35, 2416419. [Google Scholar] [CrossRef]
- Yang, T.T.; Xu, C.; Liu, C.L.; Ye, Y.Q.; Sun, Z.W.; Wang, B.; Luo, Z.Q. Conductive polymer hydrogels crosslinked by electrostatic interaction with PEDOT:PSS dopant for bioelectronics application. Chem. Eng. J. 2022, 429, 132430. [Google Scholar] [CrossRef]
- Li, H.; Cao, J.; Wan, R.; Feig, V.R.; Tringides, C.M.; Xu, J.; Yuk, H.; Lu, B. PEDOTs-based conductive hydrogels: Design, fabrications, and applications. Adv. Mater. 2025, 37, 2415151. [Google Scholar] [CrossRef]
- Yu, C.J.; Yue, Z.W.; Zhang, H.; Shi, M.Y.; Yao, M.M.; Yu, Q.Y.; Liu, M.; Guo, B.Y.; Zhang, H.T.; Tian, L.Q.; et al. Ultra-histocompatible and electrophysiological-adapted PEDOT-based hydrogels designed for cardiac repair. Adv. Funct. Mater. 2023, 33, 2211023. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.P.; Zhang, S.Q.; Zhang, X.H.; Liu, Y.; Wu, X.G.; Xue, Y.R.; Qin, Y.X.; Wang, Y.Q.; Chen, W.Y. Composite biomaterial for mimetic electric skin generated by conductive polymer/anion synergistic effect. J. Mater. Chem. C 2023, 11, 13300–13310. [Google Scholar] [CrossRef]
- Liu, X.L.; Shi, H.Y.; Song, F.F.; Yang, W.H.; Yang, B.W.; Ding, D.Y.; Liu, Z.; Hui, L.F.; Zhang, F.S. A highly sensitive and anti-freezing conductive strain sensor based on polypyrrole/cellulose nanofiber crosslinked polyvinyl alcohol hydrogel for human motion detection. Int. J. Biol. Macromol. 2024, 257, 128800. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, Y.; Wang, S.L.; Tian, G.; Yang, T.; Huang, L.C.; Ao, Y.; Lan, B.L.; Zhang, J.L.; Xu, T.P.; et al. Body temperature-triggered adhesive ionic conductive hydrogels for bioelectrical signal monitoring. Chem. Eng. J. 2024, 498, 155195. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Deen, G.R.; Bates, J.S.; Maiti, C.; Lam, C.Y.K.; Pachauri, A.; AlAnsari, R.; Belsky, P.; Yoon, J.; Dodda, J.M. Smart skin-adhesive patches: From design to biomedical applications. Adv. Funct. Mater. 2023, 33, 2213560. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wei, H.; Huang, Y.J.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Chen, S.M.; Wang, F.; Liu, M.; Li, J.H.; Liu, H.; Yang, Y.S.; Zhang, H.Q.; Liu, D.; He, R.Y.; et al. High-conductivity, self-healing, and adhesive ionic hydrogels for health monitoring and human-machine interactions under extreme cold conditions. Adv. Sci. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.H.; Zhang, Y.F.; Chen, Y.; Han, X.S.; Jiang, F. Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Duan, X.Y.; Mi, Y.Z.; Lei, T.Y.; Ma, X.Y.D.; Chen, Z.; Kong, J.H.; Lu, X.H. Highly elastic spongelike hydrogels for impedance-based multimodal sensing. ACS Nano 2025, 19, 2909–2921. [Google Scholar]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive ionic conductive alginate/gelatin organohydrogels with tunable functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar]
- Liu, R.J.; Wang, T.J.; Li, G.F.; Fan, Z.Y.; Zhou, Q.; Wang, K.; Li, P.; Huang, W. Self-reinforced hydrogel-based skin-contactable flexible electronics for multimodal electrophysiological signal monitoring and emergency alarming system. Adv. Funct. Mater. 2023, 33, 2214917. [Google Scholar]
- Cheng, T.; Liu, Z.T.; Qu, J.; Meng, C.F.; He, L.J.; Li, L.; Yang, X.L.; Cao, Y.J.; Han, K.; Zhang, Y.Z.; et al. High-performance organic-inorganic hybrid conductive hydrogels for stretchable elastic all-hydrogel supercapacitors and flexible self-powered integrated systems. Adv. Sci. 2024, 11, 2403358. [Google Scholar]
- Wang, L.Y.; Daoud, W.A. Hybrid conductive hydrogels for washable human motion energy harvester and self-powered temperature-stress dual sensor. Nano Energy 2019, 66, 104080. [Google Scholar]
- Qin, Z.P.; Zhao, G.; Zhang, Y.Y.; Gu, Z.H.; Tang, Y.H.; Aladejana, J.T.; Ren, J.N.; Jiang, Y.H.; Guo, Z.H.; Peng, X.F.; et al. A simple and effective physical ball-milling strategy to prepare super-tough and stretchable PVA@Mxene@PPy hydrogel for flexible capacitive electronics. Small 2023, 19, 2303038. [Google Scholar]
- Zhao, W.C.; Zhou, H.F.; Li, W.K.; Chen, M.L.; Zhou, M.; Zhao, L. An environment-tolerant ion-conducting double-network composite hydrogel for high-performance flexible electronic devices. Nano-Micro Lett. 2024, 16, 99. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, M.; Lv, W.; Li, J.; Huang, R.; Wang, Y. Engineering carbon nanotube-based photoactive COF to synergistically arm a multifunctional antibacterial hydrogel. Adv. Funct. Mater. 2024, 34, 2310845. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Gao, C.; Zhang, Z.; Wang, Q.; Pei, Y.; Wang, H.; Tang, Y.; Li, K.; Yu, Y. Biodegradable piezoelectric-conductive integrated hydrogel scaffold for repair of osteochondral defects. Adv. Mater. 2024, 36, 2409400. [Google Scholar] [CrossRef]
- Liu, N.; Ma, H.; Li, M.; Qin, R.; Li, P. Electroconductive hydrogels for bioelectronics: Challenges and opportunities. FlexMat 2024, 1, 269–301. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Cui, W.; Leng, L.; Zhao, X.; Ji, G.; Zhao, J.; Zhu, Q.; Zheng, J. An ultrahighly stretchable and recyclable starch-based gel with multiple functions. Adv. Mater. 2023, 35, 2303632. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ren, P.; Yu, S.; Cui, P.; Nielsen, C.B.; Abrahams, I.; Briscoe, J.; Lu, Y. Versatile and recyclable double-network PVA/cellulose hydrogels for strain sensors and triboelectric nanogenerators under harsh conditions. Nano Energy 2024, 125, 109599. [Google Scholar] [CrossRef]
- Fu, H.; Wang, B.; Li, J.; Xu, J.; Li, J.; Zeng, J.; Gao, W.; Chen, K. A self-healing, recyclable and conductive gelatin/nanofibrillated cellulose/Fe 3+ hydrogel based on multi-dynamic interactions for a multifunctional strain sensor. Mater. Horiz. 2022, 9, 1412–1421. [Google Scholar] [CrossRef]
- Li, N.; Yu, Q.; Duan, S.; Du, Y.; Shi, X.; Li, X.; Jiao, T.; Qin, Z.; He, X. Anti-swelling, high-strength, anisotropic conductive hydrogel with excellent biocompatibility for implantable electronic tendon. Adv. Funct. Mater. 2024, 34, 2309500. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Yang, J.W.; Wang, H.Y.; Wang, C.Y.; Gu, Y.H.; Xu, Y.M.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, 2375–2548. [Google Scholar] [CrossRef]
- Lao, J.; Jiao, Y.; Zhang, Y.; Xu, H.; Wang, Y.; Ma, Y.; Feng, X.; Yu, J. Intrinsically adhesive and conductive hydrogel bridging the bioelectronic-tissue interface for biopotentials recording. ACS Nano 2025, 19, 7755–7766. [Google Scholar] [CrossRef]
- Li, T.; Qi, H.B.; Zhao, C.C.; Li, Z.M.; Zhou, W.; Li, G.J.; Zhuo, H.; Zhai, W. Robust skin-integrated conductive biogel for high-fidelity detection under mechanical stress. Nat. Commun. 2025, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Aggas, J.R.; Walther, B.K.; Abasi, S.; Kotanen, C.N.; Karunwi, O.; Wilson, A.M.; Guiseppi-Elie, A. On the intersection of molecular bioelectronics and biosensors: 20 years of C3B. Biosens. Bioelectron. 2021, 176, 112889. [Google Scholar] [CrossRef]
- Arwani, R.T.; Tan, S.C.L.; Sundarapandi, A.; Goh, W.P.; Liu, Y.; Leong, F.Y.; Yang, W.F.; Zheng, X.T.; Yu, Y.; Jiang, C.Y.; et al. Stretchable ionic-electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 2024, 23, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Mo, J.L.; Liu, Y.H.; Zhang, S.; Wang, J.L.; Fu, Q.; Wang, S.F.; Nie, S.X. Stretchable triboelectric self-powered sweat sensor fabricated from self-healing nanocellulose hydrogels. Adv. Funct. Mater. 2022, 32, 2201846. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, C.; Huang, W.Y.; Liu, C.L.; Zhang, Y. Wearable sensor based on a tough conductive gel for real-time and remote human motion monitoring. ACS Appl. Mater. Interfaces 2024, 16, 11957–11972. [Google Scholar] [CrossRef]
- Dang, C.; Shao, Y.Z.; Ding, S.W.; Qi, H.B.; Zhai, W. Polyfunctional and multisensory bio-ionoelastomers enabled by covalent adaptive networks with hierarchically dynamic bonding. Adv. Mater. 2024, 36, 2406967. [Google Scholar] [CrossRef]
- Pi, M.; Wu, D.; Wang, J.; Chen, K.; He, J.; Yang, J.; Zhang, D.; Chen, S.; Tang, X. Real-time and ultrasensitive humidity sensor based on lead-free Cs2SnCl6 perovskites. Sens. Actuat. B-Chem. 2022, 354, 131084. [Google Scholar] [CrossRef]
- Wu, K.L.; Li, J.W.; Li, Y.; Wang, H.L.; Zhang, Y.C.; Guo, B.B.; Yu, J.; Wang, Y.F. 3D printed silk fibroin-based hydrogels with tunable adhesion and stretchability for wearable sensing. Adv. Funct. Mater. 2024, 34, 2404451. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, H.Q.; Shen, S.T.; Gong, Y.; Zhou, P.P.; Zou, Z.G. Multi-modal sensing ionogels with tunable mechanical properties and environmental stability for aquatic and atmospheric environments. Adv. Mater. 2024, 36, 2410572. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.F.; Guan, Y.; Zhang, Y.J. Peptide-enhanced tough, resilient and adhesive eutectogels for highly reliable strain/pressure sensing under extreme conditions. Nat. Commun. 2022, 13, 6671. [Google Scholar] [CrossRef]
- Huo, H.X.; Shi, H.R.; Yang, H.X.; Zhang, X.; Wan, J.Y.; Shen, J.J.; Du, G.B.; Yang, L. A conductive hydrogel with excellent self-adhesion, sensitivity, and stability for wearable strain sensors to monitor human motion. J. Mater. Chem. A 2024, 12, 27506–27517. [Google Scholar]
- Zou, J.; Jing, X.; Chen, Z.; Wang, S.J.; Hu, X.S.; Feng, P.Y.; Liu, Y.J. Multifunctional organohydrogel with ultralow-hysteresis, ultrafast-response, and whole-strain-range linearity for self-powered sensors. Adv. Funct. Mater. 2023, 33, 2213895. [Google Scholar]
- Rovini, E.; Maremmani, C.; Cavallo, F. How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front. Neurosci. 2017, 11, 555. [Google Scholar]
- Roy, A.; Zenker, S.; Jain, S.; Afshari, R.; Oz, Y.; Zheng, Y.T.; Annabi, N. A highly stretchable, conductive, and transparent bioadhesive hydrogel as a flexible sensor for enhanced real-time human health monitoring. Adv. Mater. 2024, 36, 2412792. [Google Scholar] [CrossRef]
- Wu, M.; Pan, M.F.; Qiao, C.Y.; Ma, Y.H.; Yan, B.; Yang, W.S.; Peng, Q.Y.; Han, L.B.; Zeng, H.B. Ultra stretchable, tough, elastic and transparent hydrogel skins integrated with intelligent sensing functions enabled by machine learning algorithms. Chem. Eng. J. 2022, 450, 138212. [Google Scholar]
- Chen, G.Q.; Zhang, Y.T.; Li, S.N.; Zheng, J.X.; Yang, H.L.; Ren, J.Y.; Zhu, C.J.; Zhou, Y.C.; Chen, Y.M.; Fu, J. Flexible artificial tactility with excellent robustness and temperature tolerance based on organohydrogel sensor array for robot motion detection and object shape recognition. Adv. Mater. 2024, 36, 2408193. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wang, S.; Wang, S.; Wang, D.; Yu, L.; Xu, X.; Liu, H.; Chen, C. Cellulose nanofiber-mediated manifold dynamic synergy enabling adhesive and photo-detachable hydrogel for self-powered E-skin. Nat. Commun. 2024, 15, 3859. [Google Scholar]
- Wang, Y.; Chen, P.; Ding, Y.; Zhu, P.; Liu, Y.; Wang, C.; Gao, C. Multifunctional nano-conductive hydrogels with high mechanical strength, toughness and fatigue resistance as self-powered wearable sensors and deep learning-assisted recognition system. Adv. Funct. Mater. 2024, 34, 2409081. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, R.; Li, M.Z.; Song, H.Z.; Zhao, X.Y.; Zhang, L.; Zhou, Y.Z.; Chen, J.; Li, J.L.; Chen, M. High antimicrobial electrotherapy and wound monitoring hydrogel with bimetal phenolic networks for smart healthcare. Adv. Funct. Mater. 2025, 35, 2413080. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhang, J.M.; Song, J.Y.; Yang, J.; Du, Z.; Zhao, W.Q.; Guo, H.S.; Wen, C.Y.; Li, Q.S.; Sui, X.J.; et al. A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of ph and glucose in diabetic wound treatment. Adv. Funct. Mater. 2020, 30, 1905493. [Google Scholar]
- Xu, Z.; Fan, J.L.; Tian, W.G.; Ji, X.; Cui, Y.Q.; Nan, Q.Y.; Sun, F.F.; Zhang, J. Cellulose-based ph-responsive janus dressing with unidirectional moisture drainage for exudate management and diabetic wounds healing. Adv. Funct. Mater. 2024, 34, 2307449. [Google Scholar]
- Ma, H.; Liu, Z.Y.; Lu, X.Q.; Zhang, S.T.; Tang, C.L.; Cheng, Y.F.; Zhang, H.; Liu, G.L.; Sui, C.; Ding, C.B.; et al. 3D printed multi-coupled bioinspired skin-electronic interfaces with enhanced adhesion for monitoring and treatment. Acta. Biomater. 2024, 187, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [PubMed]
- Ge, Z.Y.; Guo, W.S.; Tao, Y.; Sun, H.X.; Meng, X.Y.; Cao, L.Y.; Zhang, S.G.; Liu, W.Y.; Akhtar, M.L.; Li, Y.; et al. Wireless and closed-loop smart dressing for exudate management and on-demand treatment of chronic wounds. Adv. Mater. 2023, 35, 2304005. [Google Scholar]
- Gong, X.; Yang, J.; Zheng, Y.; Chen, S.J.; Duan, H.; Gao, J.; Haick, H.; Yi, C.Q.; Jiang, L.L. Polymer hydrogel-based multifunctional theranostics for managing diabetic wounds. Adv. Funct. Mater. 2024, 34, 2315564. [Google Scholar]
- Ohm, Y.; Pan, C.F.; Ford, M.J.; Huang, X.N.; Liao, J.H.; Majidi, C. An electrically conductive silver-polyacrylamide-alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185. [Google Scholar]
- Qu, J.; Xie, K.; Chen, S.; He, X.D.; Wang, Y.; Chamberlin, M.; Zhao, X.; Zhu, G.Y.; Xu, C.J.; Shi, P. Multifunctional hydrogel electronics for closed-loop antiepileptic treatment. Sci. Adv. 2024, 10, 2375–2548. [Google Scholar]

| Conductive Hydrogels | Mechanical Properties | Conductivity | Biocompatibility | Cost |
|---|---|---|---|---|
| Metal | High strength and flexibility due to metal reinforcement (e.g., nanoparticles or liquid metal). | Excellent (high electrical conductivity from metal nanoparticles or liquid metal). | Good (if using biocompatible metals like gold or silver; liquid metals may require coating). | High (due to the cost of metal nanoparticles or liquid metals). |
| Carbon | Moderate strength; brittle if not combined with polymers. | High (due to graphene, carbon nanotubes, or conductive polymers). | Moderate (carbon materials can cause inflammation if not properly functionalized). | Moderate to high (graphene and carbon nanotubes are expensive). |
| Conductive polymer | Moderate strength and flexibility; tunable based on polymer type and doping. | High (intrinsically conductive polymers like PEDOT:PSS or polypyrrole). | Good (can be tailored for biocompatibility; some polymers may require modification). | Moderate (conductive polymers are cheaper than metals or carbon materials). |
| Ionic | Soft and stretchable, but mechanical strength is often low. | Moderate (ionically conductive, but lower than electronic conductors). | Excellent (biocompatible and often used in biomedical applications). | Low (ionic hydrogels are typically made from inexpensive materials). |
| Hybrid | Combines strengths of components (e.g., high strength and flexibility). | High (combines ionic and electronic conductivity). | Good to excellent (depends on the combination of materials used). | Moderate to high (depends on the complexity and materials used). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 258. https://doi.org/10.3390/gels11040258
Li Y, Tan S, Zhang X, Li Z, Cai J, Liu Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels. 2025; 11(4):258. https://doi.org/10.3390/gels11040258
Chicago/Turabian StyleLi, Yingchun, Shaozhe Tan, Xuesi Zhang, Zhenyu Li, Jun Cai, and Yannan Liu. 2025. "Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing" Gels 11, no. 4: 258. https://doi.org/10.3390/gels11040258
APA StyleLi, Y., Tan, S., Zhang, X., Li, Z., Cai, J., & Liu, Y. (2025). Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels, 11(4), 258. https://doi.org/10.3390/gels11040258







