Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation
2.3. Liposomes Preparation
2.4. Encapsulation Efficiency Analysis
2.5. Size, PDI, and Zeta Potential Analysis
2.6. Stability Study
2.7. Differential Scanning Calorimetry
2.8. Fourier Transform Infrared Spectroscopy
2.9. Thiobarbituric Acid Reacting Substances Assay
2.10. In Vitro Release Study
2.11. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency
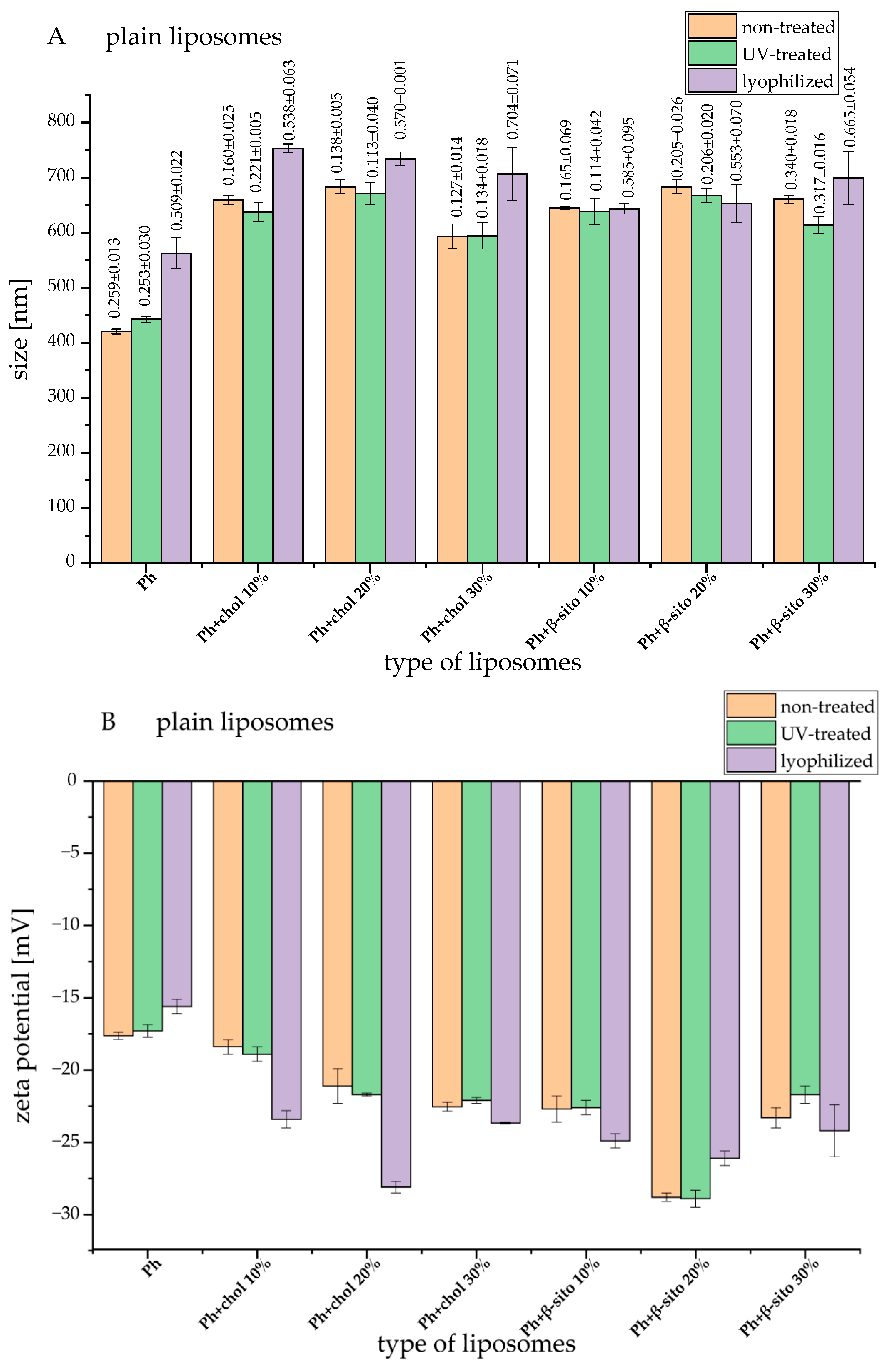
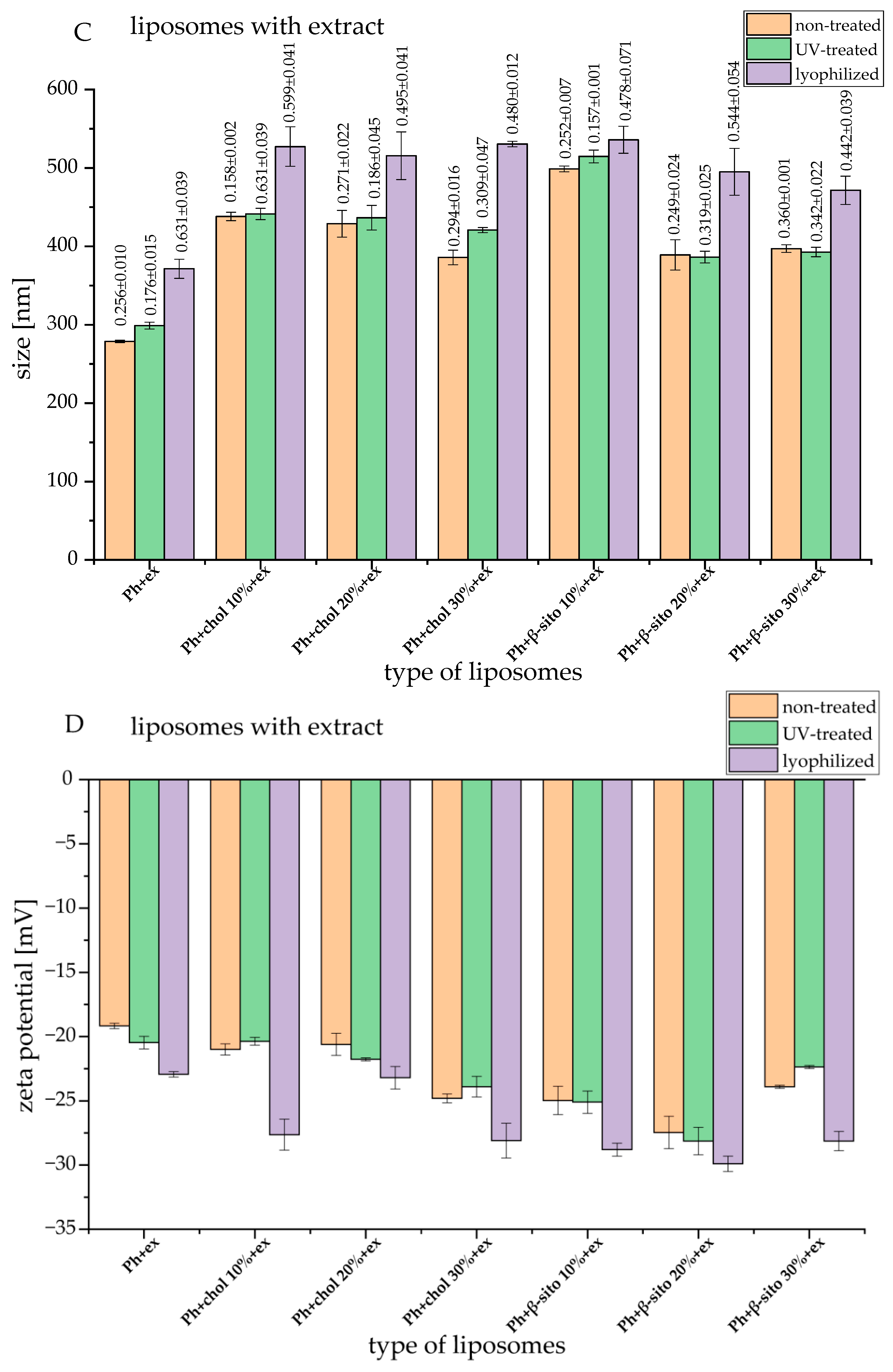
3.2. Liposomes Size, PDI, and Zeta Potential
3.3. The Stability of Liposomal Vesicles
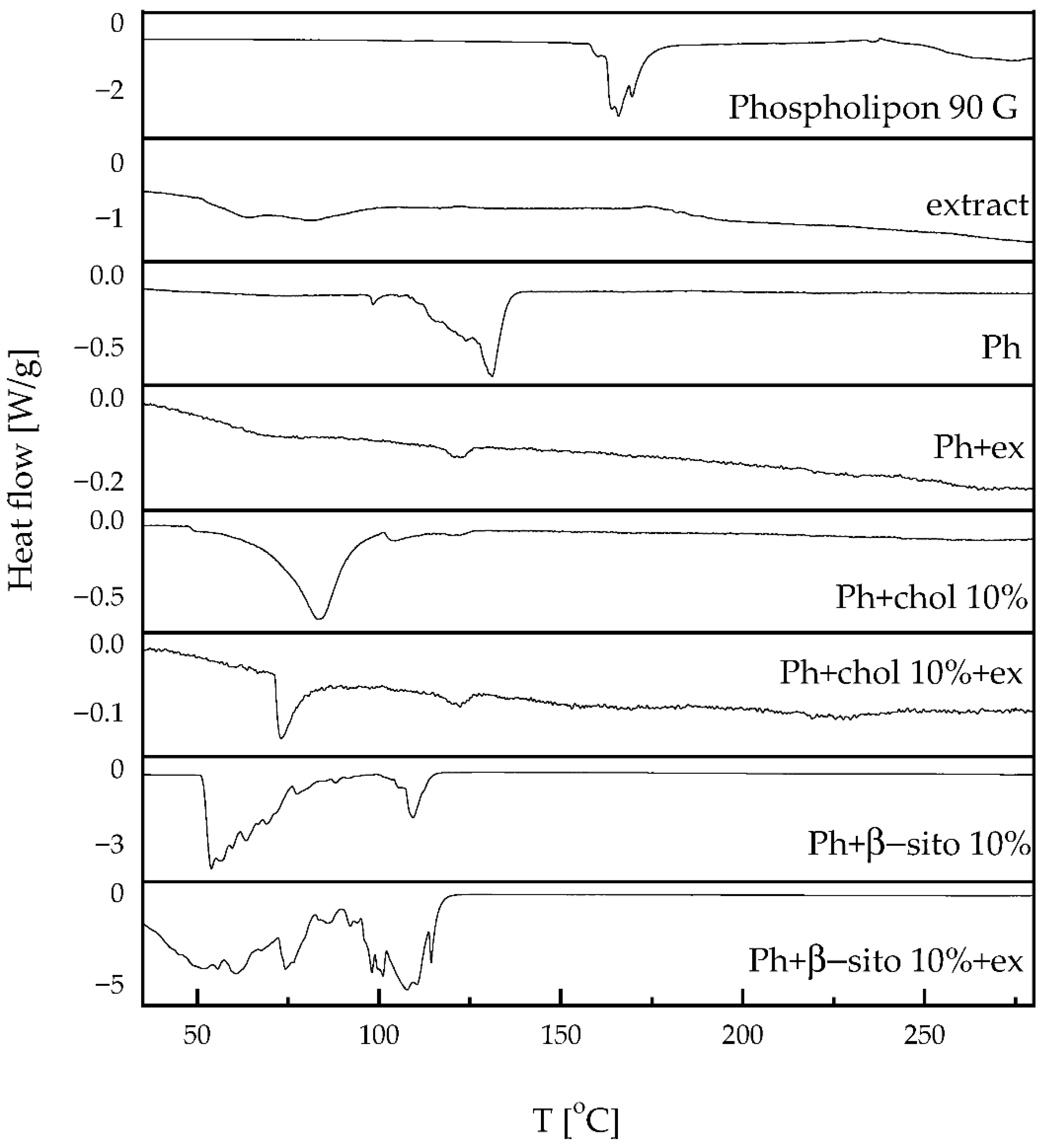
3.4. Thermotropic Properties of Liposomes
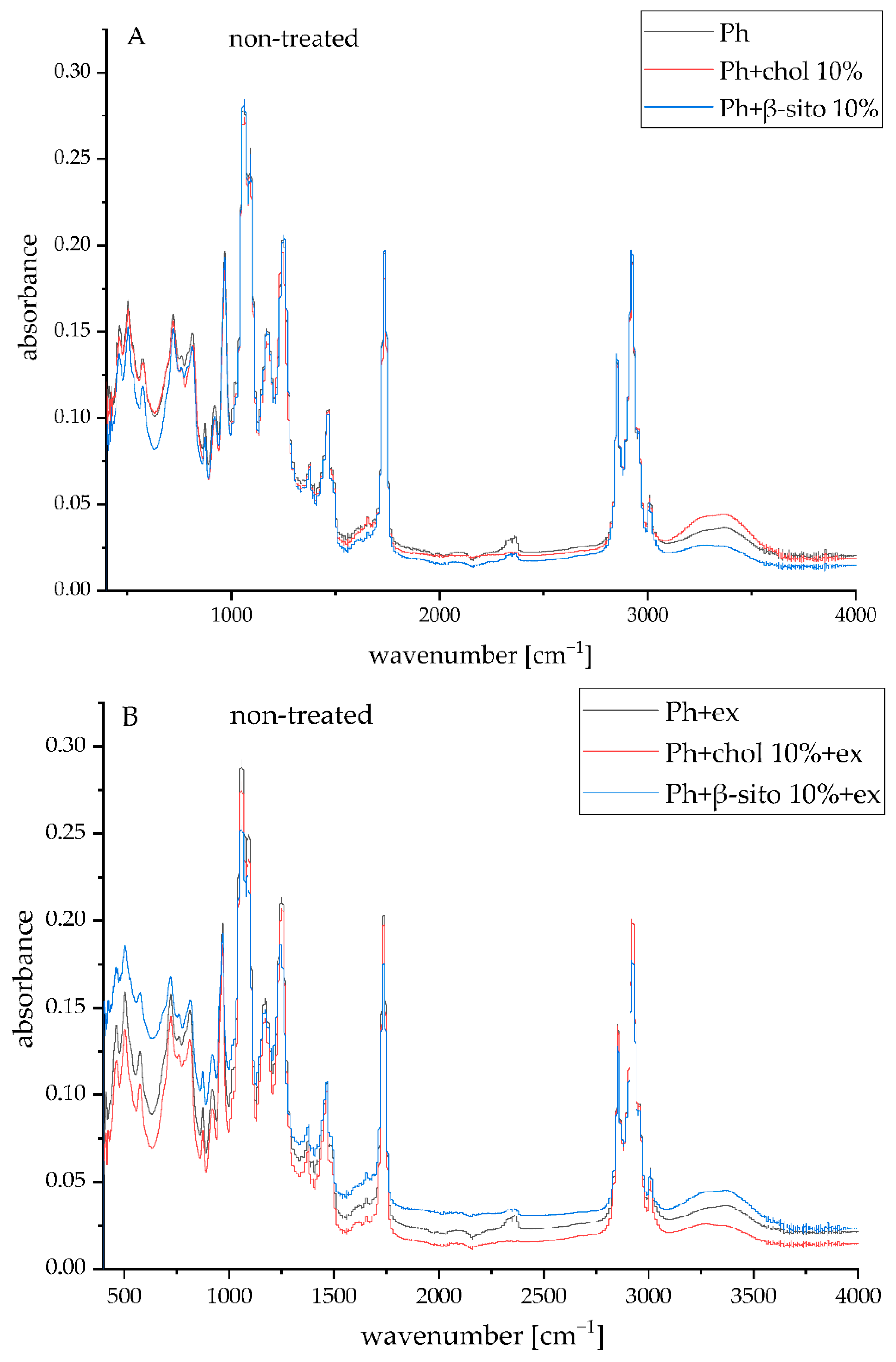
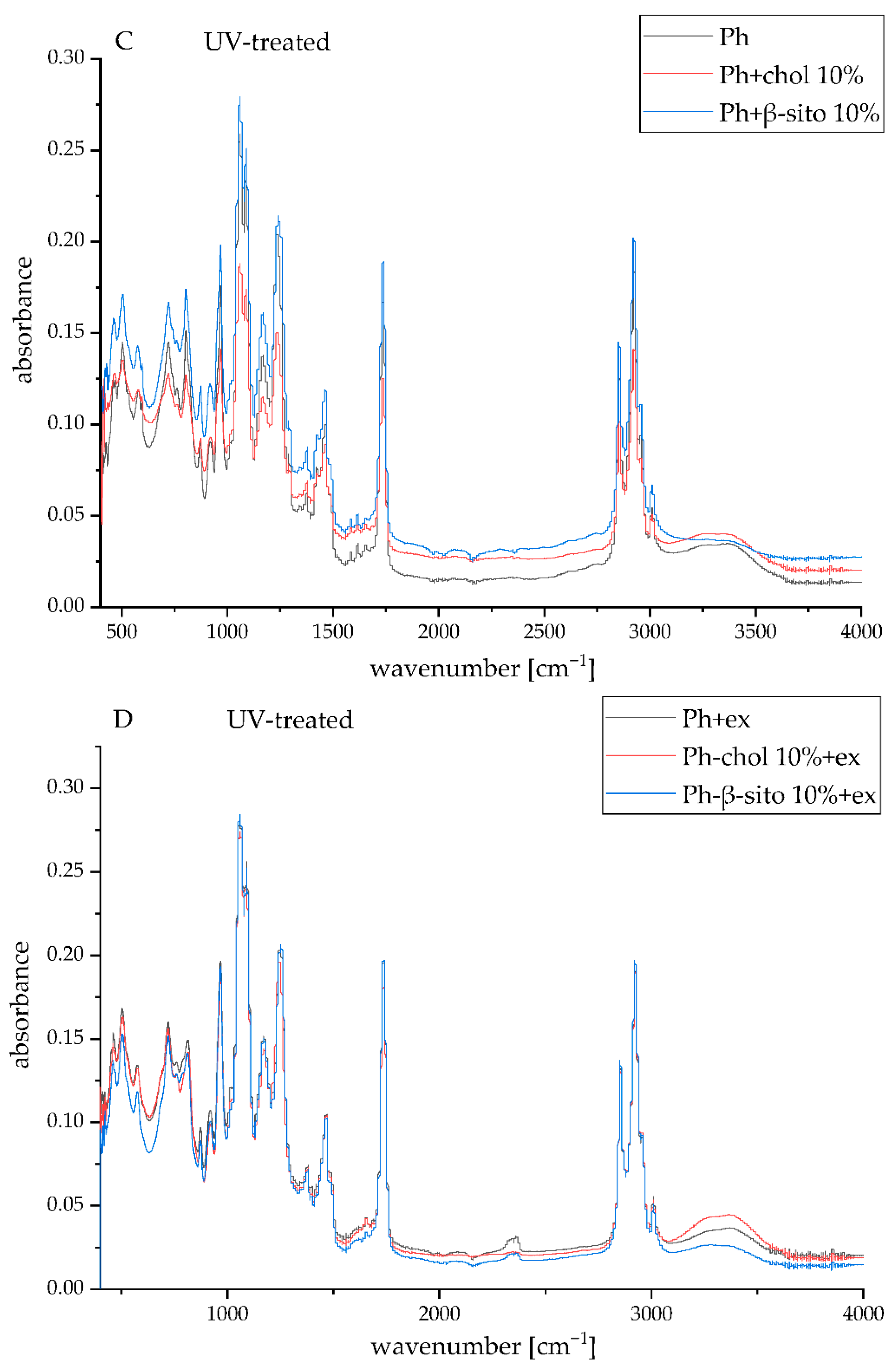
3.5. FT-IR Spectra of the Samples
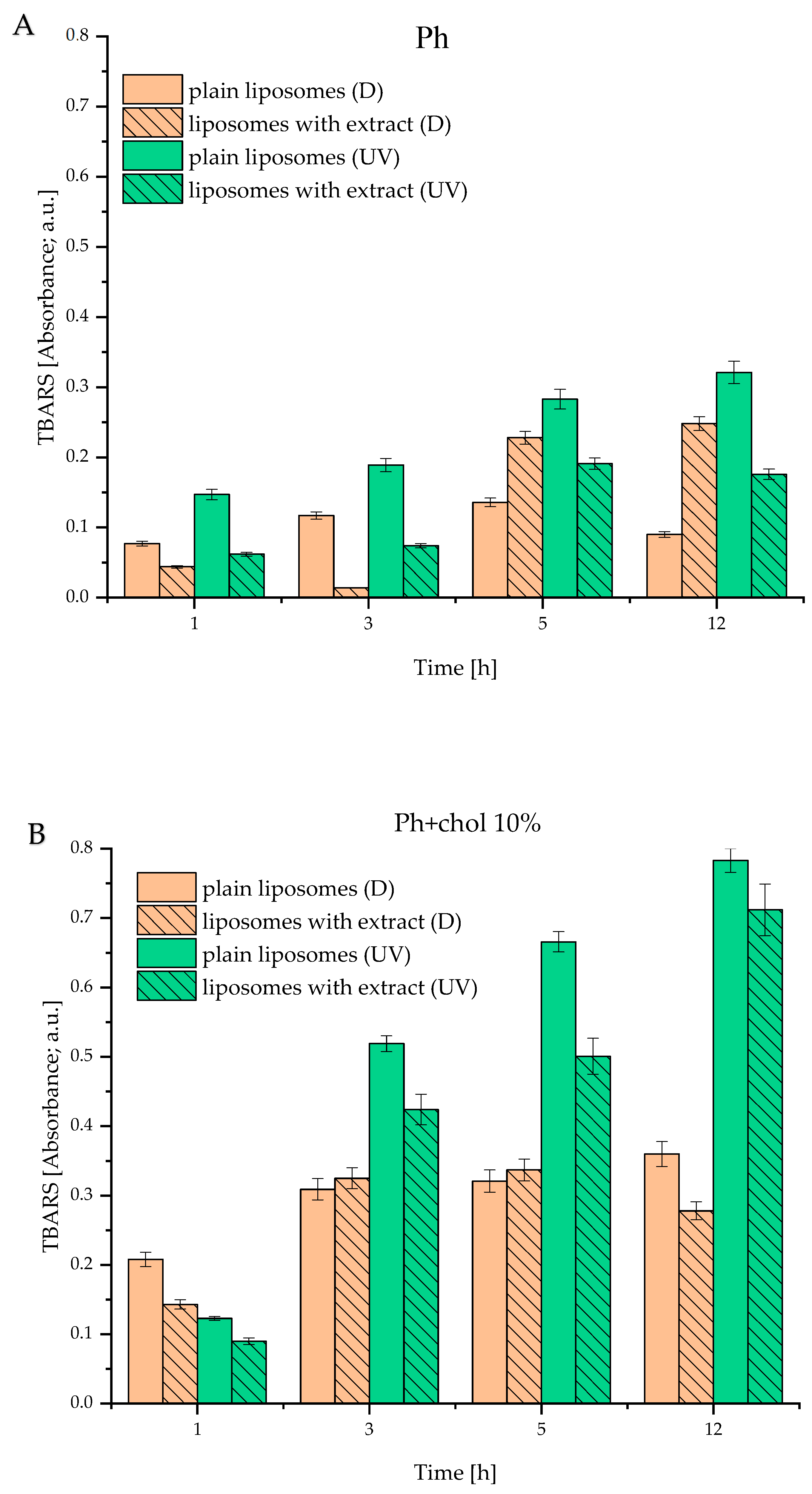
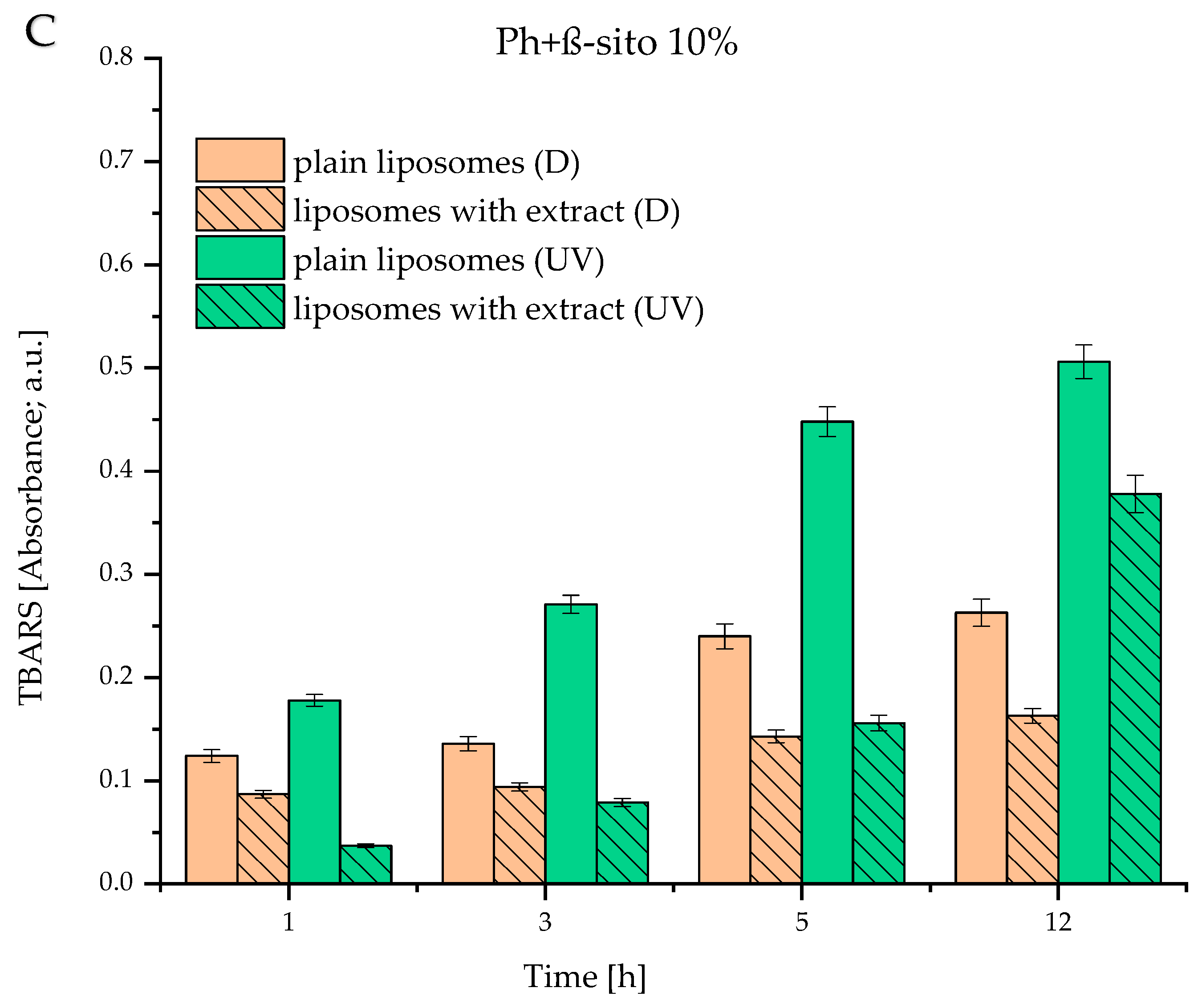
3.6. Lipid Peroxidation of Developed Liposomes
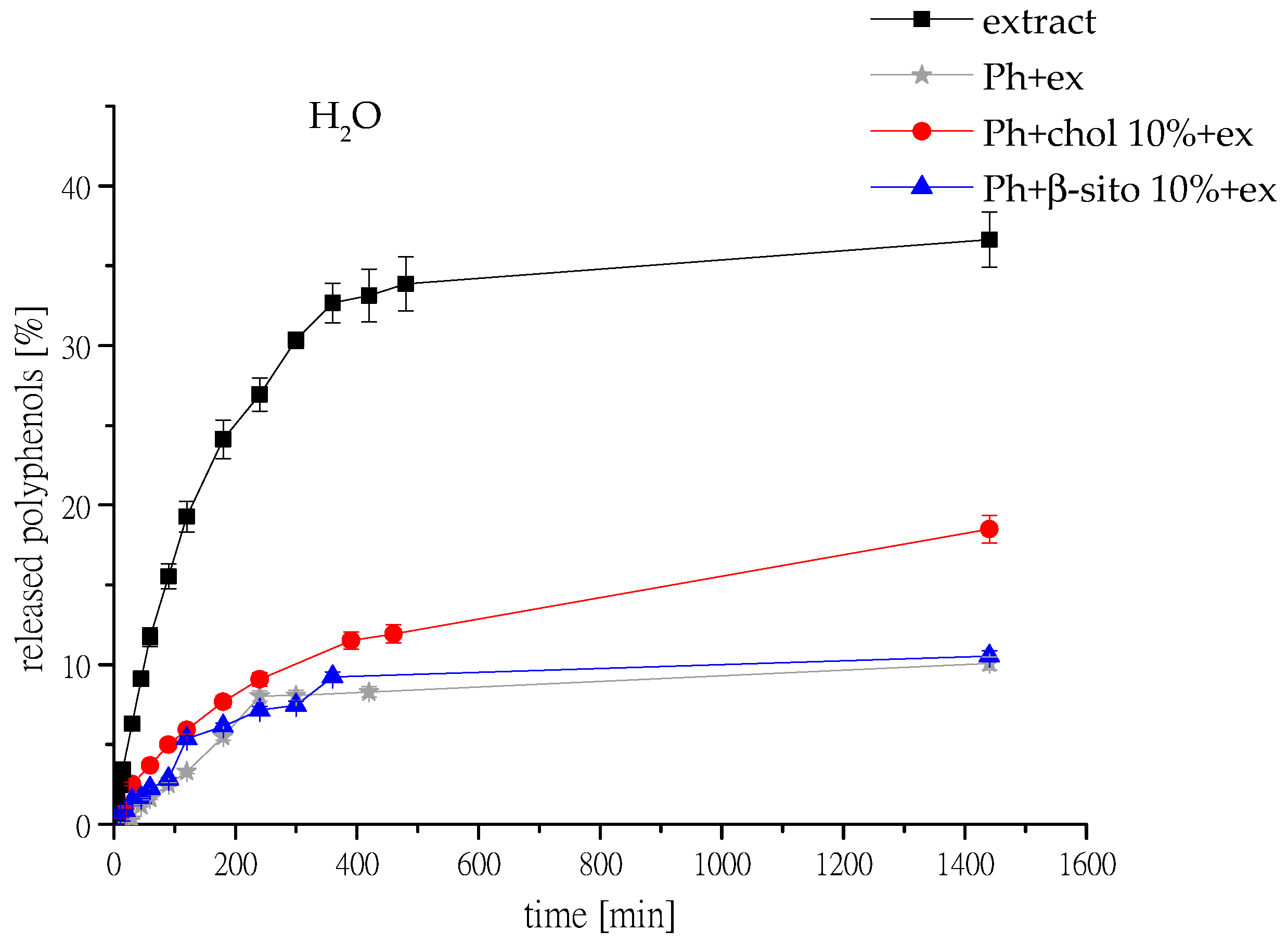
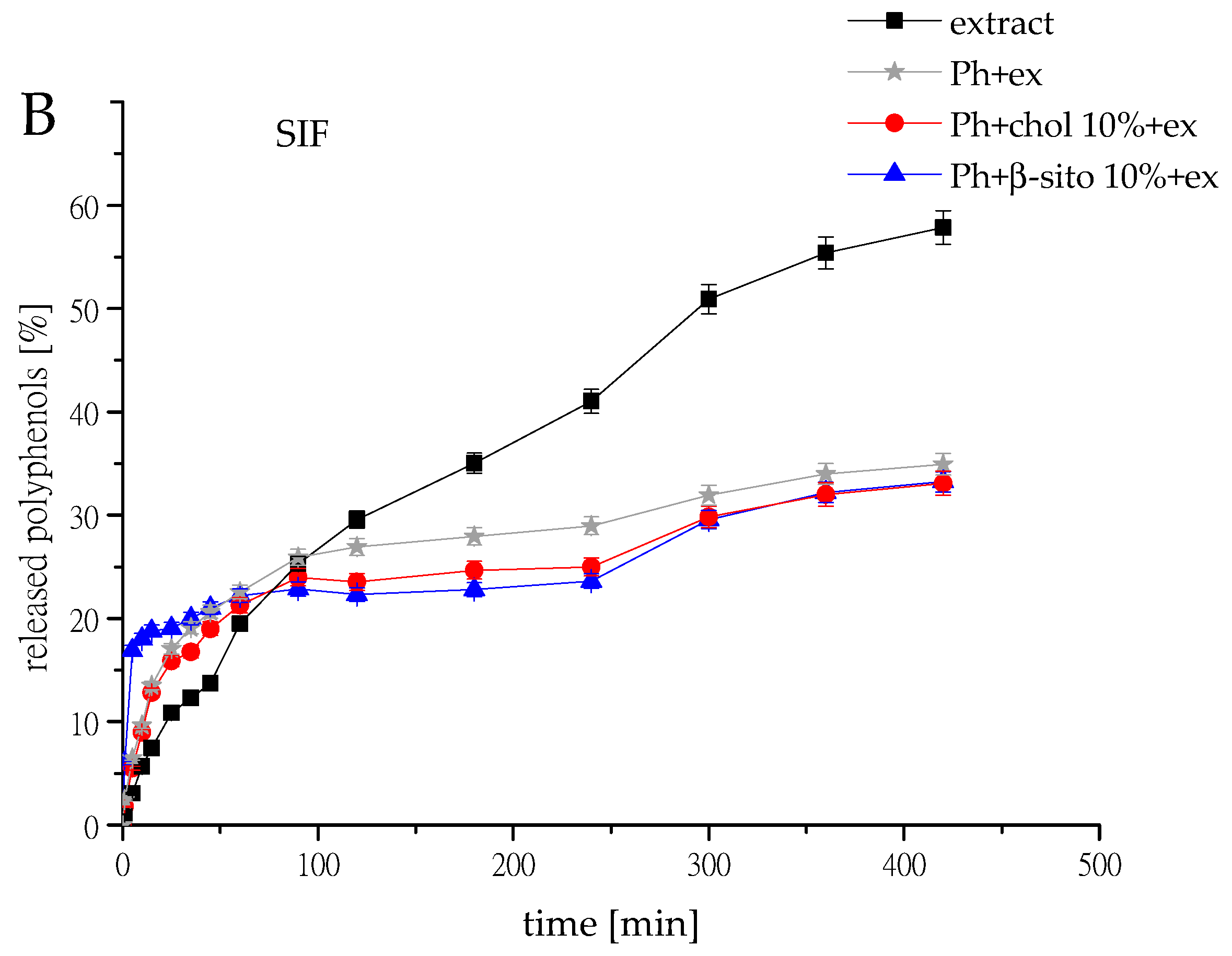
3.7. Polyphenol Release
| Conditions | Sample | D [m2/s] | R [s/m] |
|---|---|---|---|
| Water, 25 °C | extract | 1.41 · 10−9 | 2.17 · 106 |
| Ph+ex * | 2.68 · 10−10 | 1.14 · 107 | |
| Ph+chol 10%+ex | 4.68 · 10−10 | 6.52 · 106 | |
| Ph+β-sito 10%+ex | 2.01 · 10−10 | 1.52 · 107 | |
| Simulated gastric fluid, 37 °C | extract | 7.36 · 10−10 | 8.64 · 106 |
| Ph+ex | 2.74 · 10−9 | 7.42 · 105 | |
| Ph+chol 10%+ex | 6.76 · 10−9 | 3.01 · 105 | |
| Ph+β-sito 10%+ex | 9.57 · 10−9 | 2.13 · 105 | |
| Simulated intestinal Fluid, 37 °C | extract | 1.74 · 10−9 | 3.66 · 106 |
| Ph+ex | 5.96 · 10−8 | 3.42 · 104 | |
| Ph+chol 10%+ex | 6.22 · 10−9 | 3.27 · 105 | |
| Ph+β-sito 10%+ex | 2.50 · 10−8 | 8.18 · 104 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| EE | Encapsulation Efficiency |
| DSC | Differential Scanning Calorimetry |
| PCS | Photon Correlation Spectroscopy |
| PDI | Polydispersity Index |
| Ph | Phospholipids |
| SGF | Simulated Gastric Fluid |
| SIF | Simulated Intestinal Fluid |
| TPC | Total Polyphenol Content |
References
- Jarić, S.; Mitrović, M.; Karadžić, B. Plant resources used in Serbian medieval medicine. Genet. Resour. Crop Evol. 2014, 61, 1359–1379. [Google Scholar] [CrossRef]
- Jovanović, A.; Vajić, U.-J.; Mijin, D.; Zdunić, G.; Šavikin, K.; Branković, S.; Kitić, D.; Bugarski, B. Polyphenol extraction in microwave reactor using by-product of Thymus serpyllum L. and biological potential of the extract. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100417. [Google Scholar] [CrossRef]
- Brown da Rocha, C.; Noreña, C. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Ulrih, N.P. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Trifković, K.; Milašinović, N.; Đorđević, V.; Kalagasidis Kruščić, M.; Knežević-Jugović, Z.; Nedović, V.; Bugarski, B. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2024, 111, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Zuidam, N.J.; Shimoni, E. Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedović, V., Eds.; Springer: New York, NY, USA, 2010; pp. 3–29. [Google Scholar] [CrossRef]
- Ribeiro, H.; Schuchmann, H.; Engel, R.; Walz, E.; Briviba, K. Encapsulation of carotenoids. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedović, V., Eds.; Springer: New York, NY, USA, 2010; pp. 211–252. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics 2024, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, H.M.T.; Santos, C.M.M.; Silva, A.M.S. Cholesterol-based compounds: Recent advances in synthesis and applications. Molecules 2018, 24, 116. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Park, H.W.; Lee, S.C. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Rappolt, M.; He, X.; Wei, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 2019, 293, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Regulations on the Quality of Tea, Herbal Tea and Their Products of Republic of Serbia; Službeni glasnik RS, 4/12: Belgrade, Serbia, 2012. (In Serbian)
- Isailović, B.; Kostić, I.; Zvonar, A.; Đorđević, V.; Gašperlin, M.; Nedović, V.; Bugarski, B. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Galván d’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Balanč, B.; Ota, A.; Djordjević, V.; Šentjurc, M.; Nedović, V.; Bugarski, B.; Poklar Ulrih, N. Resveratrol-loaded liposomes: Interaction of resveratrol with phospholipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1615–1626. [Google Scholar] [CrossRef]
- Batinić, P.; Đorđević, V.; Stevanović, S.; Balanč, B.; Marković, S.; Luković, M.; Mijin, D.; Bugarski, B. Formulation and characterization of novel liposomes containing histidine for encapsulation of a poorly soluble vitamin. J. Drug Deliv. Sci. Technol. 2020, 59, 101920. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Temelli, F.; Curtis, J.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Jouyban, A.; Yung Chan, S. Impact of surface tension in pharmaceutical sciences. J. Pharm. Pharm. Sci. 2009, 12, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pinto, J.M.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Aliakbarian, B.; Perego, P.; Reverchon, E. Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J. Supercrit. Fluids 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Hunter, R.J.; Midmore, B.R.; Zhang, H. Zeta potential of highly charged thin double-layer systems. J. Colloid Interface Sci. 2001, 237, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Haldar, S. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochim. Biophys. Acta—Biomembr. 2000, 1467, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Olivia, R.; Del Vecchio, P.; Paolantoni, M.; Moressi, A.; Sassi, P. DMSO-induced perturbation of thermotropic properties of cholesterol-containing DPPC liposomes. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Djordjević, V.B.; Ota, A.; Skrt, M.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Ulrih, N.P. Effect of gentisic acid on the structural-functional properties of liposomes incorporating β-sitosterol. Colloids Surf. B Biointerfaces 2019, 183, 110422. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Murthy, M.S.; Shete, A.S.; Sakhar, S. Stability aspects of liposomes. Ind. J. Pharm. Edu. Res. 2011, 45, 402–413. [Google Scholar]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef] [PubMed]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Peter Tieleman, D.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Azam, M.; Nehdi, I.A.; Tan, C.P. Improving Vesicular integrity and antioxidant activity of novel mixed soy lecithin-based liposomes containing squalene and their stability against UV light. Molecules 2020, 25, 5873. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Ribovski, L.; Zhou, Q.; Chen, J.; Feringa, B.L.; van Rijn, P.; Zuhorn, I.S. Light-induced molecular rotation triggers on-demand release from liposomes. Chem. Commun. 2020, 56, 7115–7118. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Karkad, A.A.; Pirković, A.; Milošević, M.; Stojadinović, B.; Šavikin, K.; Marinković, A.; Jovanović, A.A. Silibinin-loaded liposomes: The influence of modifications on physicochemical characteristics, stability, and bioactivity associated with dermal application. Pharmaceutics 2024, 16, 1476. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Takechi, Y.; Tamai, N.; Matsuki, H.; Yomota, C.; Saito, H. Thermotropic phase behavior of hydrogenated soybean phosphatidylcholine–cholesterol binary liposome membrane. Chem. Pharm. Bull. 2014, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Saoji, S.D.; Raut, N.A.; Dhore, P.W.; Borkar, C.D.; Popielarczyk, M.; Dave, V.S. Preparation and evaluation of phospholipid-based complex of standardized centella extract (SCE) for the enhanced delivery of phytoconstituents. AAPS J. 2016, 18, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Yang, X.; Cheng, W.; Huang, Y.; Liang, P. Oxidative stability of marine phospholipids derived from large yellow croaker roe. Food Res. Int. 2022, 160, 111743. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Momoh, M.A.; Nnamani, P.O.; Attama, A.A. Solid lipid micro-dispersions (SLMs) based on PEGylated solidified reverse micellar solutions (SRMS): A novel carrier system for gentamicin. Pharm. Dev. Technol. 2015, 20, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Salve, P.S.; Hussain, U.M.H.; Tatode, A.; Qutub, M. Enhanced therapy for diabetic neuropathy utilizing venlafaxine hydrochloride-loaded transferosome-based transdermal gel. New Wet Nanotechnol. 2025, 9, 100085. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Bansal, A.K.; Beg, S.; Burrow, A.J.; Katare, O.P.; Singh, K.K.; Singh, B. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: Systematic development, characterization and evaluation. Int. J. Pharm. 2017, 518, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Nielsen, M.; Thewalt, J.; Ipsen, J.; Bloom, M.; Zuckermann, M.; Mouritsen, O. From lanosterol to cholesterol: Structural evolution and differential effects on lipid bilayers. Biophys. J. 2002, 82, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Rui-Guang, W.; Lin, C.; Zhi-Wu, Y. Structural properties of the liquid ordered phase of phosphatidylcholine/stigmasterol liposomes: A synchrotron X-ray diffraction study. Acta Phys. Chim. Sin. 2012, 28, 2008–2014. [Google Scholar] [CrossRef]
- Farkas, E.; Schubert, R.; Zelkó, R. Effect of β-sitosterol on the characteristics of vesicular gels containing chlorhexidine. Int. J. Pharm. 2004, 278, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR Spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, A.M.; Pinakoulaki, E. Linking the dynamic changes in the in vitro antioxidant activity of carob kibbles upon roasting to the chemical and structural changes revealed by FTIR spectroscopy. Antioxidants 2021, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Cesquini, M.; Torsoni, M.; Stoppa, G.; Ogo, S. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed. Pharmacother. 2003, 57, 124–129. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Cabrera, M.P.; Arcisio-Miranda, M.; Gorjão, R.; Leite, N.B.; de Souza, B.M.; Curi, R.; Procopio, J.; Ruggiero Neto, J.; Palma, M.S. Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry 2012, 51, 4898–4908. [Google Scholar] [CrossRef] [PubMed]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.B.; Almeda, R.A.; Vidallon, M.L.P.; Reyes, C.T. Enhanced bioactivity and efficient delivery of quercetin through nanoliposomal encapsulation using rice bran phospholipids. J. Sci. Food Agric. 2019, 99, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiong, H.; Peng, H.; Wang, Q.; Han, D.; Bai, C.; Liu, Y.; Shi, S.; Deng, B. PEG-coated lyophilized proliposomes: Preparation, characterizations and in vitro release evaluation of vitamin E. Eur. Food Res. Technol. 2011, 232, 647–654. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization, stability, and in vitro release evaluation of carboxymethyl chitosan coated liposomes containing fish oil. J. Food Sci. 2015, 80, C1460–C1467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, R.; Zhang, Z.; Zhong, C.; Wang, J.; Wang, M. Curcumin-loaded liposomes with the hepatic and lysosomal dual-targeted effects for therapy of hepatocellular carcinoma. Int. J. Pharm. 2021, 602, 120628. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion. Food Res. Int. 2012, 48, 499–506. [Google Scholar] [CrossRef]
- Chen, Y.; Capuano, E.; Stieger, M. Chew on it: Influence of oral processing behaviour on in vitro protein digestion of chicken and soya-based vegetarian chicken. Br. J. Nutr. 2021, 126, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, J.; Wang, X.; Li, X.; Deng, Y. N-trimethyl chitosan-coated multivesicular liposomes for oxymatrine oral delivery. Drug Dev. Ind. Pharm. 2009, 35, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.-I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.-A. Coating materials to increase the stability of liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]

| Samples | EE [%] | ||
|---|---|---|---|
| Non-Treated | UV | Lyophilized | |
| Ph+ex * | 89.4 ± 0.8 c,1,* | 87.8 ± 1.0 d,1 | 88.3 ± 0.1 d,1 |
| Ph+chol 10%+ex | 94.4 ± 0.4 a,1 | 94.8 ± 0.7 a,1 | 94.4 ± 1.1 a,1 |
| Ph+chol 20%+ex | 91.2 ± 0.8 bc,1 | 90.1 ± 0.4 c,1 | 91.7 ± 0.2 b,1 |
| Ph+chol 30%+ex | 82.2 ± 0.8 d,1 | 81.8 ± 0.6 e,1 | 80.7 ± 1.5 e,1 |
| Ph+β-sito 10%+ex | 92.8 ± 0.9 b,1 | 93.0 ± 0.6 b,1 | 92.2 ± 0.3 b,1 |
| Ph+β-sito 20%+ex | 88.9 ± 0.7 c,1 | 90.1 ± 0.9 c,1 | 90.0 ± 1.3 c,1 |
| Ph+β-sito 30%+ex | 79.1 ± 0.9 e,1 | 77.7 ± 1.0 f,1 | 78.4 ± 1.0 e,1 |
| Sample | Temperature (°C) | ΔH (J/g) | ||
|---|---|---|---|---|
| Onset | Peak | Offset | ||
| Phospholipon * | 162.4 | 165.8 | 170.9 | 110.1 |
| Extract | 50.9 | 63.6 | 96.7 | 65.1 |
| Ph | 125.1 | 131.1 | 135.1 | 42.7 |
| Ph+ex | 117.7 | 122.4 | 124.9 | 0.9 |
| Ph+chol 10% | 72.2 | 83 | 92.4 | 55.9 |
| Ph+chol 10%+ex | 71.4 | 73.1 | 78.99 | 3.28 |
| Ph+β-sito 10% | 51.7 | 53.9 | 63 | 347.7 |
| 106.9 | 109.3 | 113.4 | 58.6 | |
| Ph+β-sito 10%+ex | 71.2 | 60.7 | 82.1 | 813.7 |
| 103 | 107.8 | 113.4 | 459.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, A.A.; Balanč, B.; Petrović, P.M.; Čutović, N.; Marković, S.B.; Djordjević, V.B.; Bugarski, B.M. Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols. Foods 2025, 14, 2626. https://doi.org/10.3390/foods14152626
Jovanović AA, Balanč B, Petrović PM, Čutović N, Marković SB, Djordjević VB, Bugarski BM. Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols. Foods. 2025; 14(15):2626. https://doi.org/10.3390/foods14152626
Chicago/Turabian StyleJovanović, Aleksandra A., Bojana Balanč, Predrag M. Petrović, Natalija Čutović, Smilja B. Marković, Verica B. Djordjević, and Branko M. Bugarski. 2025. "Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols" Foods 14, no. 15: 2626. https://doi.org/10.3390/foods14152626
APA StyleJovanović, A. A., Balanč, B., Petrović, P. M., Čutović, N., Marković, S. B., Djordjević, V. B., & Bugarski, B. M. (2025). Liposome-Based Encapsulation of Extract from Wild Thyme (Thymus serpyllum L.) Tea Processing Residues for Delivery of Polyphenols. Foods, 14(15), 2626. https://doi.org/10.3390/foods14152626








