Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review
Abstract
1. Introduction
2. Benefits and Application of Spirulina
3. Methods
- What is the prevalence of allergic reactions to spirulina consumption?
- What is the severity of allergic reaction to spirulina consumption?
- What are the management strategies for individuals experiencing allergic reactions to spirulina?
4. Results
Evaluation of the Severity of Analyzed Cases of Allergy Reaction to Spirulina
5. Discussion
5.1. Spirulina as an Allergy-Inducing Factor
5.2. Spirulina—As an Allergy-Alleviating Factor
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassoun, A.; Cropotova, J.; Trif, M.; Rusu, A.V.; Bobiş, O.; Nayik, G.A.; Jagdale, Y.D.; Saeed, F.; Afzaal, M.; Mostashari, P.; et al. Consumer acceptance of new food trends resulting from the fourth industrial revolution technologies: A narrative review of literature and future perspectives. Front. Nutr. 2022, 9, 972154. [Google Scholar] [CrossRef]
- Wojas, O.; Krzych-Fałta, E.; Samel-Kowalik, P.; Żalikowska-Gardocka, M.; Majsiak, E.; Mari, A.; Samoliński, B. A case of allergy to Silybum marianum (milk thistle) and Eragrostis tef (teff). Allergy Asthma Clin. Immunol. 2020, 16, 23. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. A new paradigm to search for allergenic proteins in novel foods by integrating proteomics analysis and in silico sequence homology prediction: Focus on spirulina and chlorella microalgae. Talanta 2022, 240, 123188. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Viera, I.; Roca, M. Study of the authentic composition of the novel green foods: Food colorants and coloring foods. Food Res. Int. 2023, 170, 112974. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Discovery of marker peptides of spirulina microalga proteins for allergen detection in processed foodstuffs. Food Chem. 2022, 393, 133319. [Google Scholar] [CrossRef]
- EUR-Lex—31997R0258-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31997R0258 (accessed on 31 October 2023).
- Food and Feed Information Portal Database | FIP. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 20 March 2024).
- Araújo, R.; Peteiro (César), C. Algae as Food and Food Supplements in Europe. Technical Report by the Joint Research Centre (JRC), Publications Office of the European Union. 2021, pp. 1–34. Available online: http:// www.seaweed.ie/pdf/AraujoPeteiro2021.pdf (accessed on 24 February 2024).
- Karkos, D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Hashemian, M.; Ahmadzadeh, H.; Hosseini, M.; Lyon, S.; Pourianfar, H.R. Production of microalgae-derived high-protein biomass to enhance food for animal feedstock and human consumption. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts: Technologies and Approaches for Scale-Up and Commercialization; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Kumar, A.; Mohanty, V.; Yashaswini, P. Development of high protein nutrition bar enriched with spirulina plantensis for undernourished children. Curr. Res. Nutr. Food Sci. 2018, 6, 835–844. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough ‘crostini’: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed]
- Global Seaweeds and Microalgae Production, 1950–2019 WAPI Factsheet to Facilitate Evidence-Based Policy-Making and Sector Management in Aquacult. 2021. Available online: http://unohrlls.org/about-sids/country-profiles/ (accessed on 23 November 2023).
- Spirulina Market Value | Size, Trends, Forecast | 2023–2028. Available online: https://www.marketdataforecast.com/market-reports/spirulina-market (accessed on 4 November 2023).
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae☆. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Dai, H.V.; Se, K.N.; Thanh-Sang, K. Chapter 19—Nutritional and Pharmaceutical Properties of Microalgal Spirulina. In Handbook of Marine Microalgae: Biotechnology Advances; Academic Press: Cambridge, MA, USA, 2015; pp. 299–308. [Google Scholar]
- Dillon, J.C.; Phuc, A.; Dubacq, J. Nutritional value of the alga Spirulina. In World Review of Nutrition and Dietetics; Karger Publishers: Berlin, Germany, 1995; Volume 77. [Google Scholar] [CrossRef]
- Kay, R.A.; Barton, L.L. Microalgae as food and supplement. Crit Rev Food Sci Nutr 1991, 30, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; Bhat, A.G.; Okeefe, J. Effects of spirulina on weight loss and blood lipids: A review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Lee, E.H.; Kim, H.M. Spirulina platensis inhibits anaphaylactic reaction. Life Sci. 1997, 61, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of Spirulina (Arthrospira platensis) Supplementation on Physical and Chemical Properties of Semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Kim, K.-J.; Song, J.-H.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Heo, H.J.; Lee, B.-Y. Spirulina maxima Extract Ameliorates Learning and Memory Impairments via Inhibiting GSK-3β Phosphorylation Induced by Intracerebroventricular Injection of Amyloid-β 1–42 in Mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Qu, R.; Fu, Y.; Zhou, C.; Yu, J. A network pharmacology and molecular docking approach to reveal the mechanism of Chaihu Anxin Capsule in depression. Front. Endocrinol. 2023, 14, 1256045. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, I.T.; Jeng, K.C.; Wang, M.F.; Hou, R.C.; Wu, S.M.; Chan, Y.C. Spirulina prevents memory dysfunction, reduces oxidative stress damage and augments antioxidant activity in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2011, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective Activities of Spirulina platensis in the 6-OHDA Model of Parkinson’s Disease Are Related to Its Anti-Inflammatory Effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef]
- Yousefi, R.; Mottaghi, A.; Saidpour, A. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: A randomized controlled trial. Complement. Ther. Med. 2018, 40, 106–112. [Google Scholar] [CrossRef]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement. Altern. Med. 2017, 17, 225. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Petrus, M.; Culerrier, R.; Campistron, M.; Barre, A.; Rougé, P. First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen. Allergy 2010, 65, 924–925. [Google Scholar] [CrossRef]
- Le, T.M.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef]
- Pimblett, P. Spirulina allergy; a case history of two patients. World Allergy Organ. J. 2020, 13, 100149. [Google Scholar] [CrossRef]
- Pescosolido, E.; Yerly, D.; Caubet, J.C.; Bergmann, M.M. Delayed IgE-mediated hypersensitivity to Arthrospira platensis (spirulina). Ann. Allergy Asthma Immunol. 2022, 129, 522–524. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World Allergy Organization Anaphylaxis Guida2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- McHugh, K.; Repanshek, Z. Anaphylaxis: Emergency Department Treatment. Emerg. Med. Clin. N. Am. 2022, 40, 19–32. [Google Scholar] [CrossRef]
- Ring, J.; Behrendt, H. Anaphylaxis and anaphylactoid reactions: Classification and pathophysiology. Clin. Rev. Allergy Immunol. 1999, 17, 387–399. [Google Scholar] [CrossRef]
- Mueller, H.L. Diagnosis and treatment of insect sensitivity. J. Asthma 1966, 3, 331–333. [Google Scholar] [CrossRef]
- Cox, L.S.; Sanchez-Borges, M.; Lockey, R.F. World Allergy Organization Systemic Allergic Reaction Grading System: Is a Modification Needed? J. Allergy Clin. Immunol. Pract. 2017, 5, 58–62. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Clark, A.; Eigenmann, P.A.; Halken, S.; Lack, G.; Moneret-Vautrin, A.; Niggemann, B.; Rancé, F.; EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: Position paper of the European academy of allergology and clinical immunology. Allergy 2007, 62, 857–871. [Google Scholar] [CrossRef]
- James, C.A.; Welham, S.; Rose, P. Edible algae allergenicity—A short report. J. Appl. Phycol. 2023, 35, 339–352. [Google Scholar] [CrossRef]
- Buczyłko, K.; Majsiak, E. Upper aero- and digestive tract allergy—Selected cross reactivity. Alergologia Polska. Pol. J. Allergol. 2017, 4, 139–145. [Google Scholar]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.N.; Nishad, D.K.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology and undefi 2001. Evaluation of Allergenicity of Genetically Modified Foods: Report of a Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from pesquisa.bvsalud.org. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/who-340572 (accessed on 12 December 2023).
- Weichel, M.; Glaser, A.G.; Ballmer-Weber, B.K.; Schmid-Grendelmeier; Crameri, R. Wheat and maize thioredoxins: A novel cross-reactive cereal allergen family related to baker’s asthma. J. Allergy Clin. Immunol. 2006, 117, 676–681. [Google Scholar] [CrossRef]
- trxh1—Thioredoxin h1 Protein—Zea mays (Maize) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q4W1F7/entry (accessed on 23 November 2023).
- Superoxide Dismutase—Arthrospira platensis AV | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/C3V6P3/entry (accessed on 23 November 2023).
- sod—Superoxide Dismutase—Hevea brasiliensis (Para Rubber Tree) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9STB5/entry (accessed on 23 November 2023).
- SODA—Superoxide Dismutase [Mn], Mitochondrial—Hevea brasiliensis (Para Rubber Tree) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P35017/entry (accessed on 23 November 2023).
- sod—Superoxide Dismutase—Hevea brasiliensis (Para Rubber Tree) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9FSJ2/entry (accessed on 23 November 2023).
- Superoxide Dismutase—Pistacia vera (Pistachio) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/B2BDZ8/entry (accessed on 23 November 2023).
- MnSOD—Superoxide Dismutase—Malassezia sympodialis (Atopic Eczema-Associated Yeast) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q873M4/entry (accessed on 23 November 2023).
- Mala s 11—Superoxide Dismutase—Malassezia sympodialis (Strain A42132) (Atopic Eczema-Associated Yeast) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/M5ECN9/entry (accessed on 23 November 2023).
- sodB—Superoxide Dismutase [Mn], Mitochondrial—Aspergillus fumigatus (Strain ATCC MYA-4609/101355/FGSC A1100/Af293) (Neosartorya fumigata) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q92450/entry (accessed on 23 November 2023).
- ga3pd—Glyceraldehyde-3-Phosphate Dehydrogenase—Triticum aestivum (Wheat) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/C7C4X1/entry (accessed on 23 November 2023).
- Glyceraldehyde-3-Phosphate Dehydrogenase—Pangasianodon hypophthalmus (Striped catfish) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0A5N5Q6M7/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Dermatophagoides farinae (American House Dust Mite) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0A088SAX2/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Dermatophagoides farinae (American House Dust Mite) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/L7UZA7/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Pangasianodon hypophthalmus (Striped catfish) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0A5N5Q6M9/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Scylla paramamosain (Mud crab) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0A1L5YRA2/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Crangon crangon (Brown shrimp) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/D7F1Q0/entry (accessed on 23 November 2023).
- Triosephosphate Isomerase—Procambarus clarkii (Red Swamp Crayfish) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/F5A6E9/entry (accessed on 23 November 2023).
- tpi1b—Triosephosphate Isomerase—Salmo salar (Atlantic salmon) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/B5DGL3/entry (accessed on 23 November 2023).
- WHO/IUIS Allergen Nomenclature Home Page. Available online: https://allergen.org/ (accessed on 20 March 2023).
- Kim, H.M.; Lee, E.H.; Cho, H.H.; Moon, Y.H. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by spirulina. Biochem. Pharmacol. 1998, 55, 1071–1076. [Google Scholar] [CrossRef]
- Hayashi, O.; Hirahashi, T.; Katoh, T.; Miyajima, H.; Hirano, T.; Okuwaki, Y. Class specific influence of dietary Spirulina platensis on antibody production in mice. J. Nutr. Sci. Vitaminol. 1998, 44, 841–851. [Google Scholar] [CrossRef][Green Version]
- Hirahashi, T.; Matsumoto, M.; Hazeki, K.; Saeki, Y.; Ui, M.; Seya, T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002, 2, 423–434. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, S.F.; Huang, D.N.; Tan, J.Q.; He, S.H. Experimental study of spirulina platensis in treating allergic rhinitis in rats. J. Cent. South Univ. (Med. Sci.) 2005, 30, 96–98. [Google Scholar]
- Mao, T.K.; Van De Water, J.; Gershwin, M.E. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J. Med. Food 2005, 8, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Cingi, C.; Conk-Dalay, M.; Cakli, H.; Bal, C. The effects of spirulina on allergic rhinitis. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Nourollahian, M.; Rasoulian, B.; Gafari, A.; Anoushiravani, M.; Jabari, F.; Bakhshaee, M. Clinical comparison of the efficacy of spirulina platensis and cetirizine for treatment of allergic rhinitis. Acta Otorhinolaryngol. Ital. 2020, 40, 224–229. [Google Scholar] [CrossRef] [PubMed]
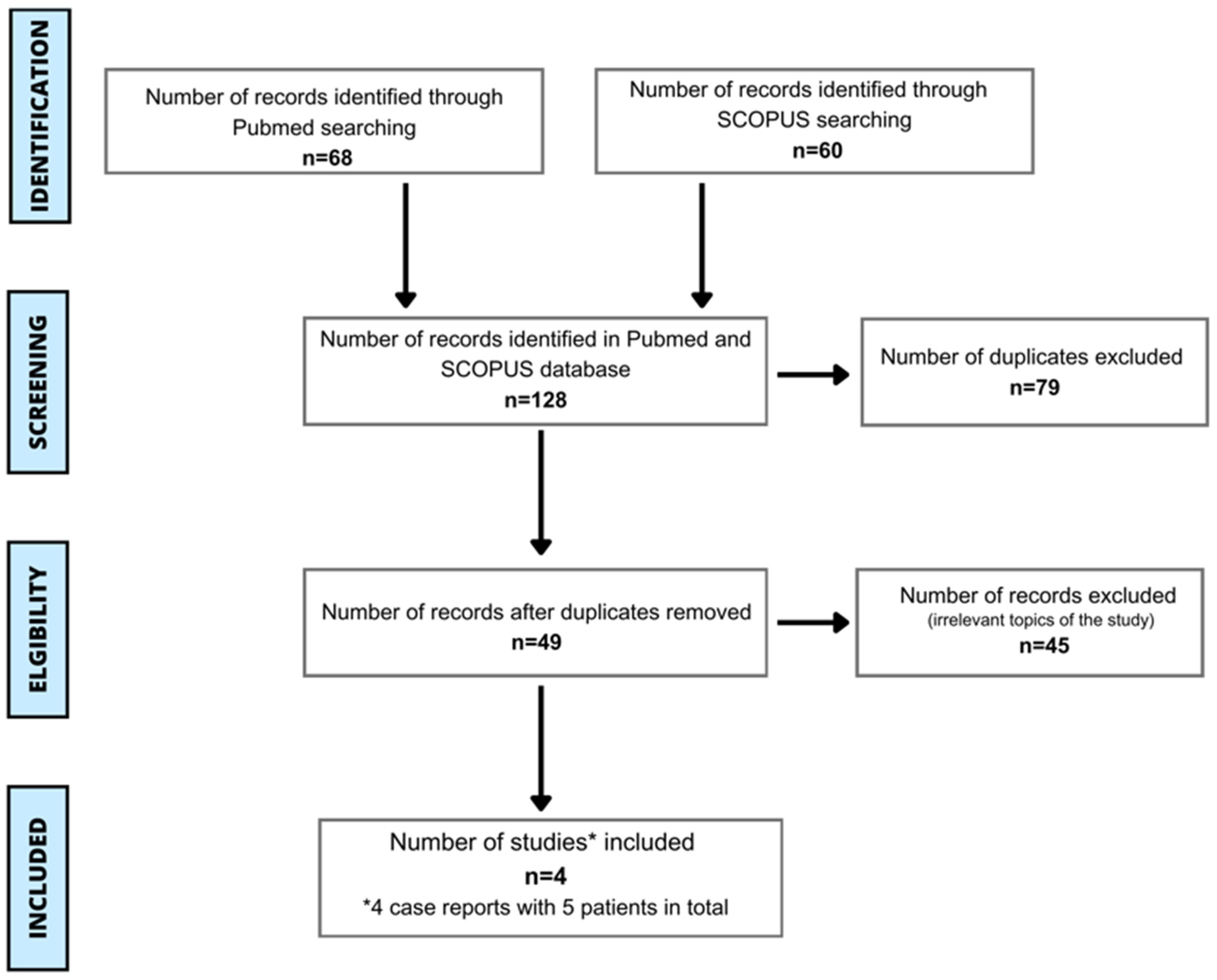

| Search | Search Term | PubMed | Scopus |
|---|---|---|---|
| 1 | (allergy) AND (spirulina) AND (human) | 19 | 30 |
| 2 | (allergy) AND (Arthrospira) AND (human) | 19 | 5 |
| 3 | (hypersensitivity) AND (spirulina) AND (human) | 13 | 15 |
| 4 | (hypersensitivity) AND (Arthrospira) AND (human) | 13 | 2 |
| 5 | (anaphylaxis) AND (spirulina) AND (human) | 2 | 5 |
| 6 | (anaphylaxis) AND (Arthrospira) AND (human) | 2 | 3 |
| Summary | Total = 128 | 68 | 60 |
| Patient | Author(s), Kind of Article, Year | Location | Age and Sex | Form of Ingested Spirulina | Diagnosis of FA/FH | Clinical Symptoms | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | Petrus et al. [32]; Case report, 2010 | France | 14 y/o male | tablets | Clinical symptoms of allergy, positive SPT and OC | urticaria, labial edema, asthma | 2nd-generation antihistamines, corticosteroids |
| 2 | Le et al. [33]; Short communication, 2014 | The Netherlands | 17 y/o male | tablets | Clinical symptoms of allergy, positive SPT | tingling of the lips, angioedema of the face, an itching exanthema, urticaria of arms and trunk, nausea, abdominal pain, wheezing, dyspnea, inspiratory stridor | im ephinephrine, iv antihistamines, iv glucocorticoids, nebulized beta-2 agonists |
| 3 and 4 | Pimblett [34]; Poster, 2020 | The United Kingdom | 2 patients (ND about age and sex) | powder | Clinical symptoms of allergy, positive SPT | generalized itching, burning in soles of feet, shortness of breath, urticaria | No data |
| 5 | Pescosolido et al. [35]; Letter, 2022 | Switzerland | 48 y/o female | tablets | Clinical symptoms of allergy, positive SPT, OC, BAT | mild left plantar aspect swelling, acute tongue swelling | iv antihistamines, iv glucocorticoids |
| Patient | Anaphylaxis | Grade | Symptoms | Assessment of Reaction Severity | |
|---|---|---|---|---|---|
| 1 [32] | No | 1 | Urticaria | 3 | |
| Labial edema | |||||
| 2 | - | ||||
| Yes | 3 | Asthma | |||
| 4 | - | ||||
| 5 | - | ||||
| 2 [33] | No | 1 | An itching exanthema | 4 | |
| Angioedema of the face | |||||
| Nausea | |||||
| Tingling of the lips | |||||
| Urticaria of arms and trunk | |||||
| 2 | Abdominal pain | ||||
| Yes | 3 | Dyspnea | |||
| Wheezing | |||||
| 4 | Inspiratory stridor | ||||
| 5 | - | ||||
| 3 and 4 [34] | No | 1 | Burning in soles of feet | 3 | |
| Generalized itching | |||||
| Urticaria | |||||
| 2 | - | ||||
| Yes | 3 | Shortness of breath | |||
| 4 | - | ||||
| 5 | - | ||||
| 5 [35] | No | 1 | Acute tongue swelling | 2 symptoms from different systems are characterized as grade 2 | 2 |
| Mild left plantar aspect swelling | |||||
| 2 | |||||
| Yes | 3 | - | |||
| 4 | - | ||||
| 5 | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods 2024, 13, 1052. https://doi.org/10.3390/foods13071052
Gromek W, Kołdej N, Kurowski M, Majsiak E. Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods. 2024; 13(7):1052. https://doi.org/10.3390/foods13071052
Chicago/Turabian StyleGromek, Weronika, Natalia Kołdej, Marcin Kurowski, and Emilia Majsiak. 2024. "Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review" Foods 13, no. 7: 1052. https://doi.org/10.3390/foods13071052
APA StyleGromek, W., Kołdej, N., Kurowski, M., & Majsiak, E. (2024). Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods, 13(7), 1052. https://doi.org/10.3390/foods13071052







