Exploring Non-Thermal Plasma and UV Radiation as Biofilm Control Strategies against Foodborne Filamentous Fungal Contaminants
Abstract
1. Introduction
2. Materials and Methods
2.1. Microscopic Filamentous Fungi
2.2. Spore Suspension Preparation
2.3. UV Lamp and NTP Generator Specifications
2.4. Metabolic Activity Measurement
2.5. Monitoring the Effect of NTP and UV Radiation on Fungal Biofilms
2.6. Preparation of NTP/UV Treated Biofilms for SEM
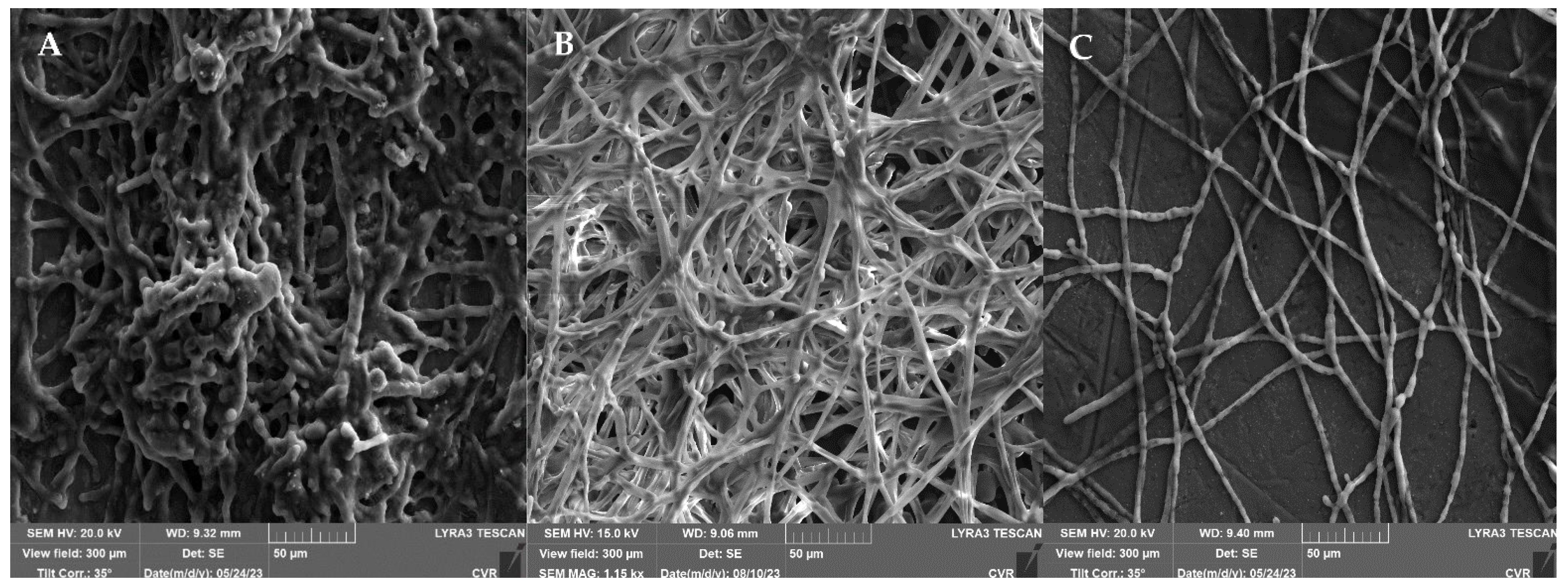
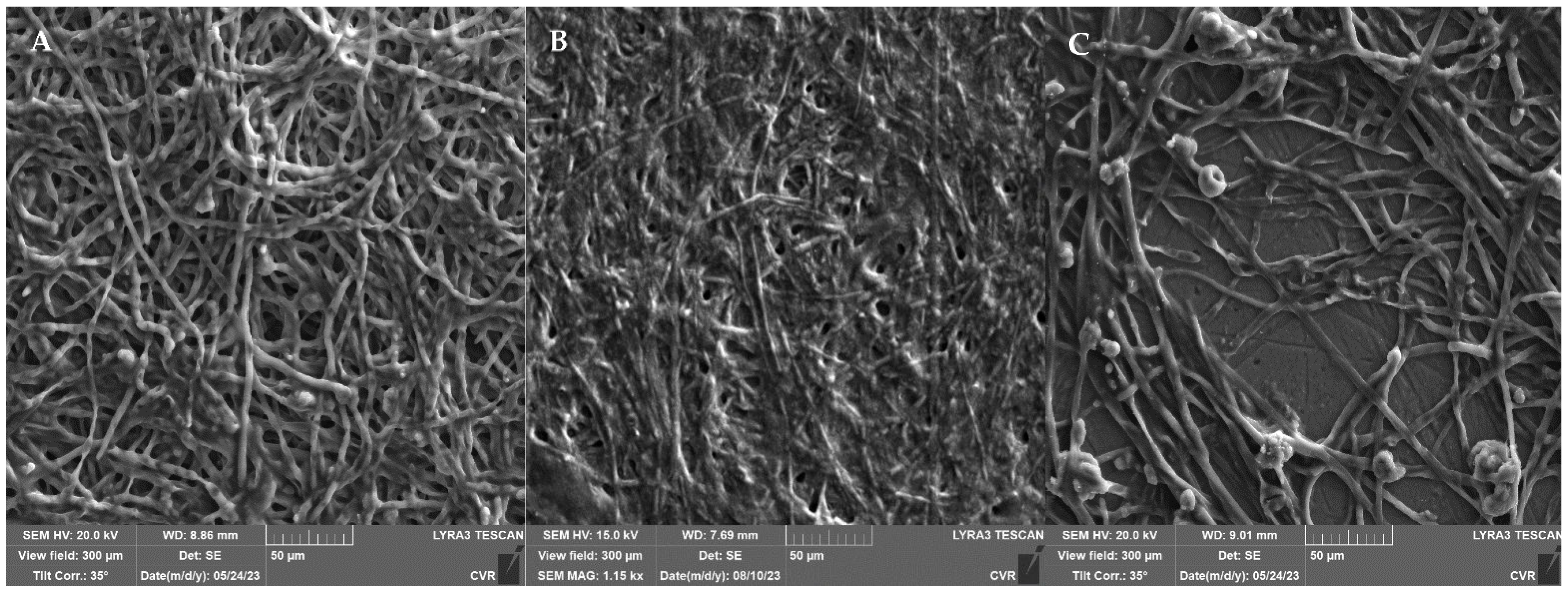
2.7. SEM Microscopy
2.8. Statistical Analysis
3. Results
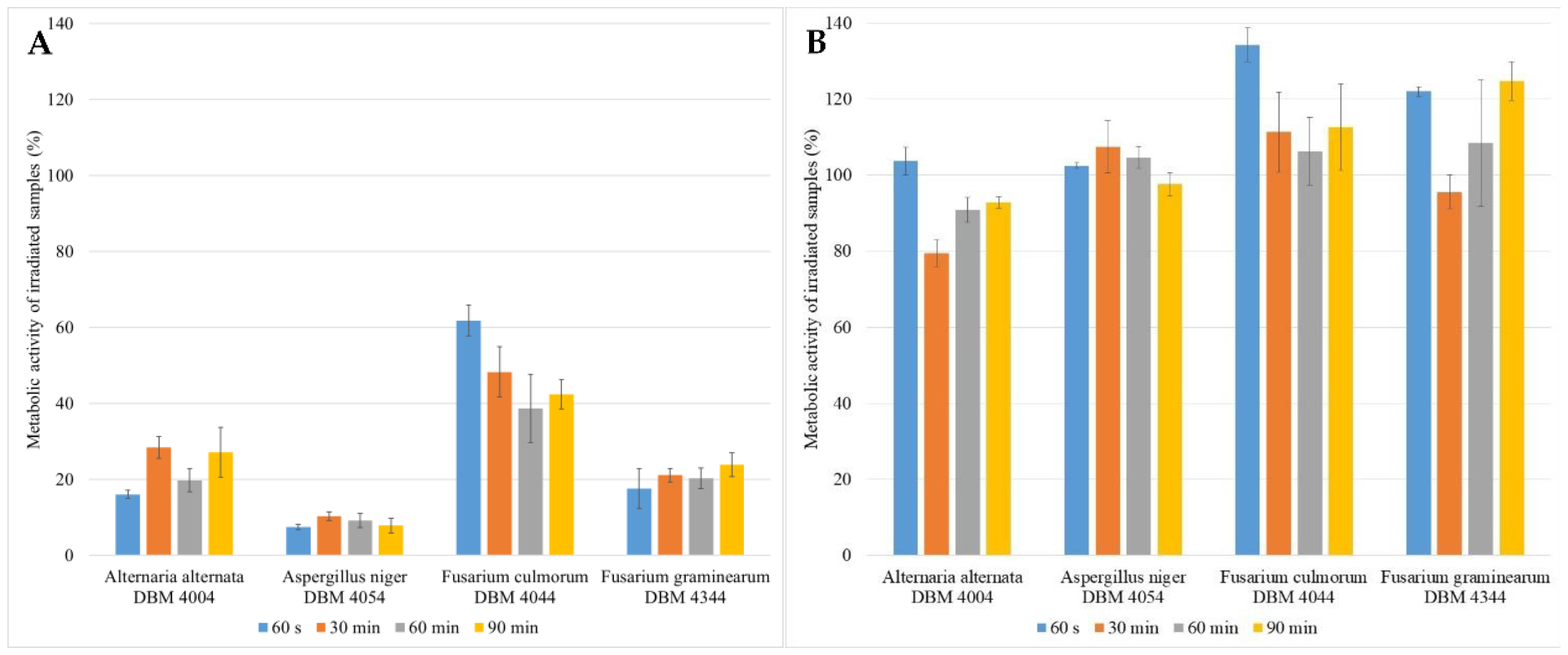
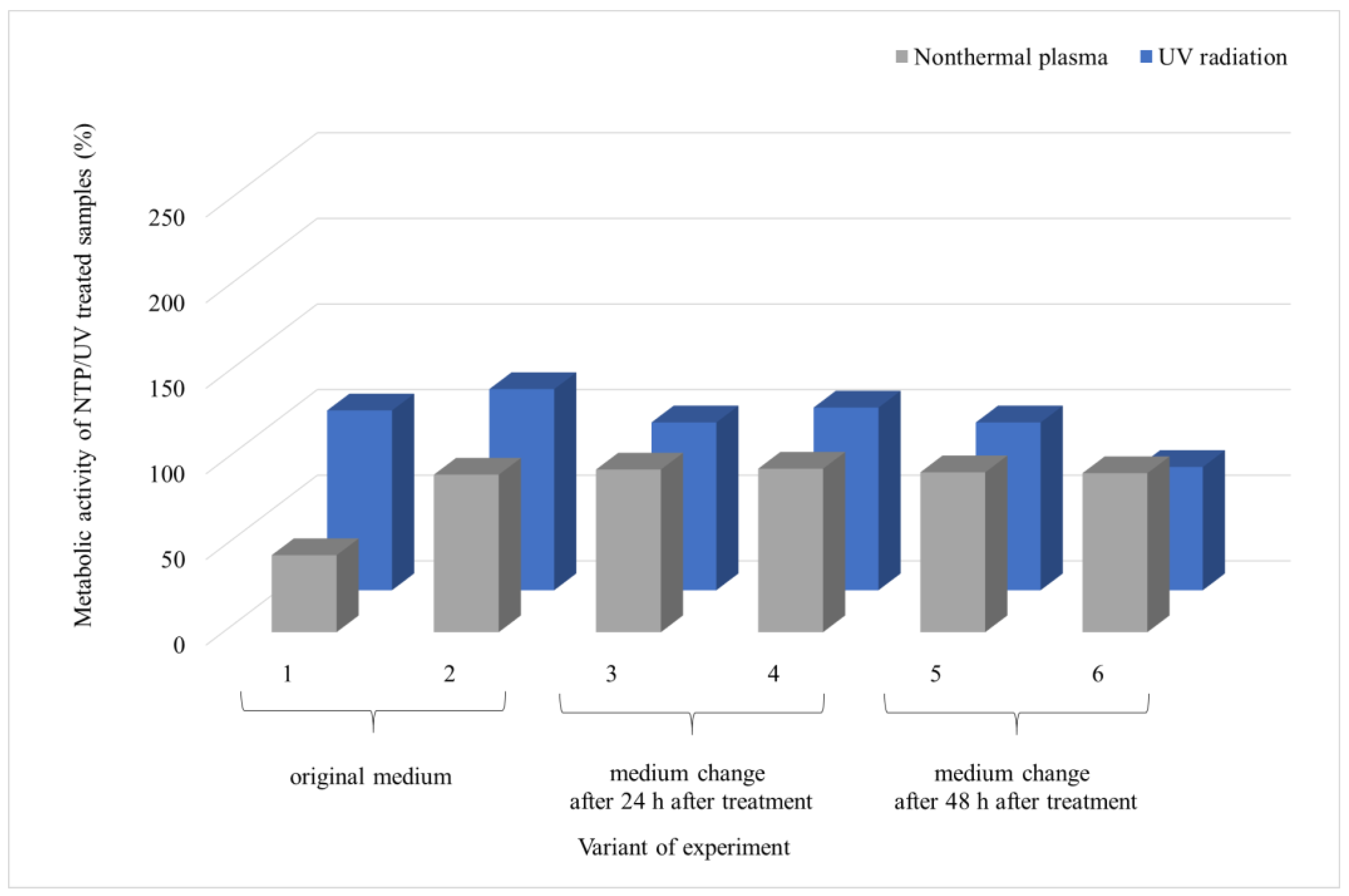
3.1. The Impact of UV Radiation on the Metabolic Activity of Microscopic Filamentous Fungal Biofilms
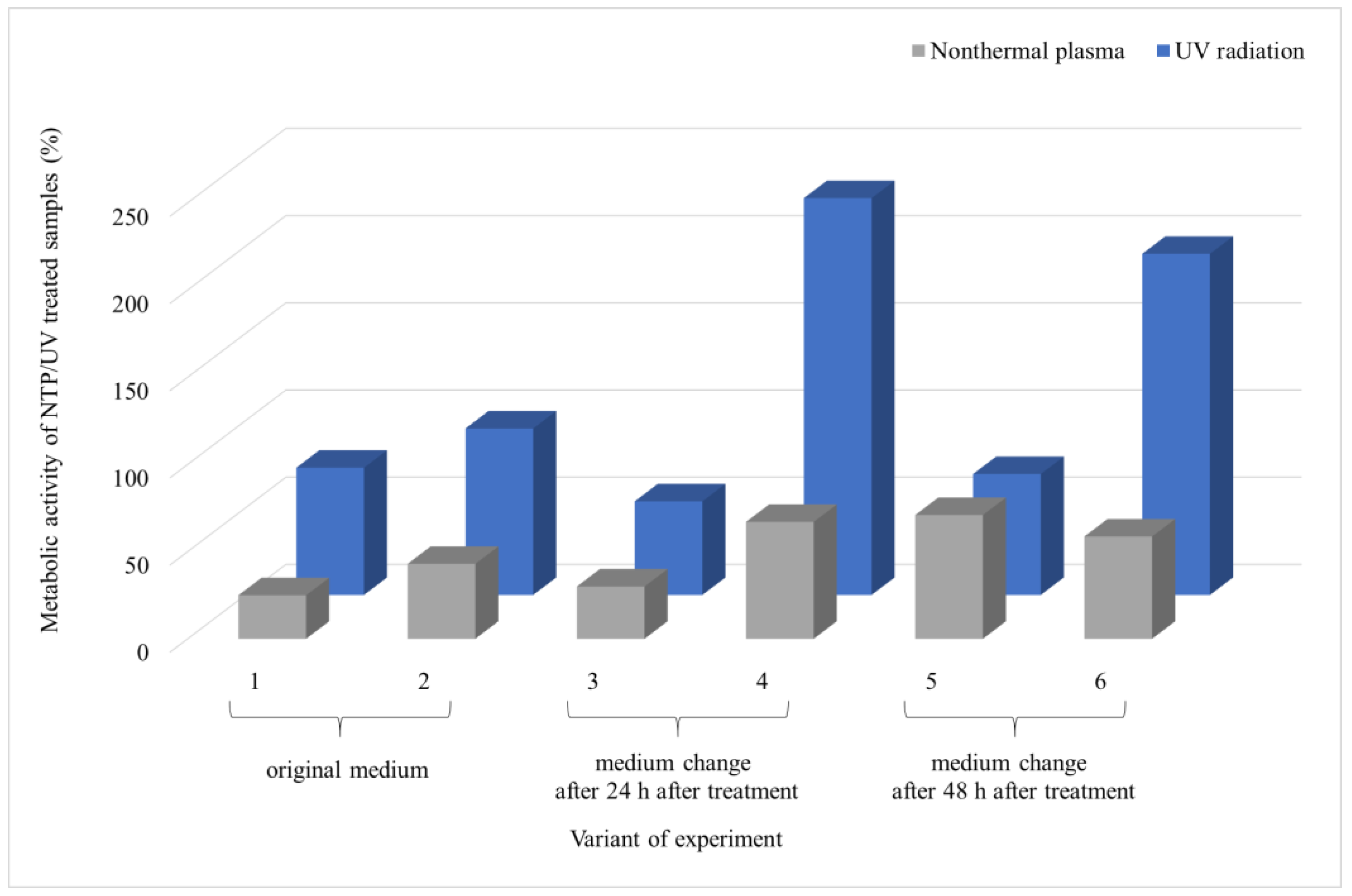
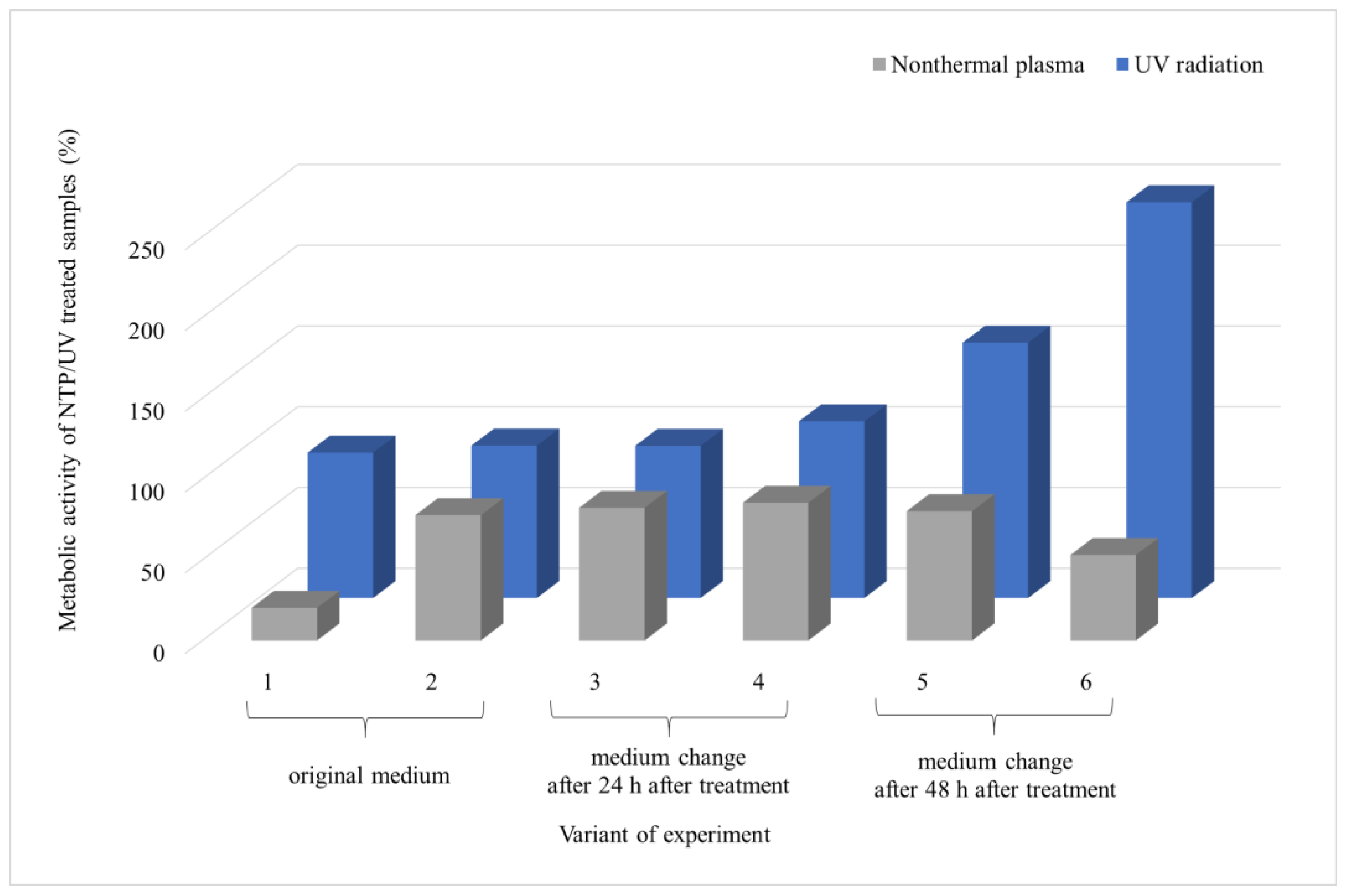
3.2. Comparison of the Effect of UV Radiation and NTP on Microscopic Filamentous Fungal Biofilms
4. Discussion
4.1. Impact of UV Radiation on the Metabolic Activity of Microscopic Filamentous Fungal Biofilms
4.2. Comparison of the Effect of UV Radiation and NTP on Microscopic Filamentous Fungal Biofilms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Köhler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019273. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.-M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Shay, R.; Wiegand, A.A.; Trail, F. Biofilm formation and structure in the filamentous fungus Fusarium graminearum, a plant pathogen. Microbiol. Spectr. 2022, 10, e00171-22. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, R.; Gerrits, R.; Dementyeva, P.; Knabe, N.; Schumacher, J.; Feldmann, I.; Radnik, J.; Ryo, M.; Gorbushina, A.A. The role of extracellular polymeric substances of fungal biofilms in mineral attachment and weathering. NPJ Mater. Degrad. 2022, 6, 42. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef]
- Watchaputi, K.; Jayasekara, L.C.B.; Ratanakhanokchai, K.; Soontorngun, N. Inhibition of cell cycle-dependent hyphal and biofilm formation by a novel cytochalasin 19, 20-epoxycytochalasin Q in Candida albicans. Sci. Rep. 2023, 13, 9724. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single-and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-albicans Candida species: Immune response, evasion mechanisms, and new plant-derived alternative therapies. J. Fungi 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Tekaia, F.; Latgé, J.-P. Aspergillus fumigatus: Saprophyte or pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Granillo, A.; Bautista-Hernández, L.A.; Bautista-De Lucío, V.M.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Córdova-Alcántara, I.M.; Pérez, N.O.; Martinez-Rivera, M.d.l.A.; Rodríguez-Tovar, A.V. Microbial warfare on three fronts: Mixed biofilm of Aspergillus fumigatus and Staphylococcus aureus on primary cultures of human limbo-corneal fibroblasts. Front. Cell. Infect. Microbiol. 2021, 11, 646054. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Prevention of fungal spoilage in food products using natural compounds: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2002–2016. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2009; Volume 519. [Google Scholar]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.V.; Bernardi, A.O.; Copetti, M.V. The fungal problem in bread production: Insights of causes, consequences, and control methods. Curr. Opin. Food Sci. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Afonso, T.B.; Simões, L.C.; Lima, N. Effect of quorum sensing and quenching molecules on inter-kingdom biofilm formation by Penicillium expansum and bacteria. Biofouling 2020, 36, 965–976. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio) control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT 2020, 117, 108656. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- O’donnell, C.; Tiwari, B.; Bourke, P.; Cullen, P. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.; Kothakota, A.; Ramesh, S.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods–a review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Soliva-Fortuny, R.; Barbosa-Cánovas, G.V.; Martín-Belloso, O. Use of oscillating magnetic fields in food preservation. Nonthermal Process. Technol. Food 2011, 45, 222–243. [Google Scholar] [CrossRef]
- Siemer, C.; Toepfl, S.; Heinz, V. Inactivation of Bacillus subtilis spores by pulsed electric fields (PEF) in combination with thermal energy–I. Influence of process-and product parameters. Food Control 2014, 39, 163–171. [Google Scholar] [CrossRef]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by continuous and pulsed irradiation of ultraviolet light-emitting diodes (UV-LEDs). Chem. Eng. J. 2018, 343, 362–370. [Google Scholar] [CrossRef]
- Gavahian, M.; Meng-Jen, T.; Khaneghah, A.M. Emerging techniques in food science: The resistance of chlorpyrifos pesticide pollution against arc and dielectric barrier discharge plasma. Qual. Assur. Saf. Crops Foods 2020, 12, 9–17. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.H.; Sun, D.W. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Furtado, M.M.; Iwase, C.H.T.; Brondi-Mendes, J.Z.; Nascimento, M.d.S. Microbial resistance to sanitizers in the food industry. Crit. Rev. Food Sci. Nutr. 2022, 64, 654–669. [Google Scholar] [CrossRef]
- Afshari, R.; Hosseini, H. Non-thermal plasma as a new food preservation method, its present and future prospect. Arch. Adv. Biosci. 2014, 5, 116–120. [Google Scholar] [CrossRef]
- Asl, P.J.; Rajulapati, V.; Gavahian, M.; Kapusta, I.; Putnik, P.; Khaneghah, A.M.; Marszałek, K. Non-thermal plasma technique for preservation of fresh foods: A review. Food Control 2022, 134, 108560. [Google Scholar] [CrossRef]
- Patil, S.; Bourke, P.; Cullen, P. Principles of nonthermal plasma decontamination. In Cold Plasma in Food and Agriculture; Elsevier: Amsterdam, The Netherlands, 2016; pp. 143–177. [Google Scholar]
- Shen, J.; Cheng, C.; Xu, Z.; Lan, Y.; Ni, G.; Sui, S. Principles and characteristics of cold plasma at gas phase and gas-liquid phase. In Applications of Cold Plasma in Food Safety; Springer: Singapore, 2022; pp. 1–36. [Google Scholar]
- Cheng, J.-H.; Lv, X.; Pan, Y.; Sun, D.-W. Foodborne bacterial stress responses to exogenous reactive oxygen species (ROS) induced by cold plasma treatments. Trends Food Sci. Technol. 2020, 103, 239–247. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Ulrich, M.S.; Toushik, S.H.; Nahar, S.; Roy, P.K.; Mizan, F.R.; Park, S.H.; Ha, S.-D. Challenges and opportunities of non-conventional technologies concerning food safety. World’s Poult. Sci. J. 2023, 79, 3–26. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Fasciana, T.; Iozzo, P.; Raineri, S.M.; Gregoretti, C.; Giammanco, A.; Giarratano, A. What healthcare workers should know about environmental bacterial contamination in the intensive care unit. BioMed Res. Int. 2017, 2017, 6905450. [Google Scholar] [CrossRef]
- Fitzhenry, K.; Rowan, N.; del Rio, A.V.; Cremillieux, A.; Clifford, E. Inactivation efficiency of Bacillus endospores via modified flow-through PUV treatment with comparison to conventional LPUV treatment. J. Water Process Eng. 2019, 27, 67–76. [Google Scholar] [CrossRef]
- Otter, J.; Yezli, S.; Perl, T.; Barbut, F.; French, G. A guide to no-touch automated room disinfection (NTD) systems. In Decontamination in Hospitals and Healthcare; Elsevier: Amsterdam, The Netherlands, 2014; pp. 413–460. [Google Scholar]
- Farrell, H.; Garvey, M.; Rowan, N. Studies on the inactivation of medically important Candida species on agar surfaces using pulsed light. FEMS Yeast Res. 2009, 9, 956–966. [Google Scholar] [CrossRef]
- Garvey, M.; Rabbitt, D.; Stocca, A.; Rowan, N. Pulsed ultraviolet light inactivation of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Water Environ. J. 2015, 29, 36–42. [Google Scholar] [CrossRef]
- Anderson, J.G.; Rowan, N.J.; MacGregor, S.J.; Fouracre, R.A.; Farish, O. Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Trans. Plasma Sci. 2000, 28, 83–88. [Google Scholar] [CrossRef]
- Shikano, A.; Kuda, T.; Takahashi, H.; Kimura, B. Effect of quantity of food residues on resistance to desiccation, disinfectants, and UV-C irradiation of spoilage yeasts adhered to a stainless steel surface. LWT 2017, 80, 169–177. [Google Scholar] [CrossRef]
- Watson, I.; Kamble, P.; Shanks, C.; Khan, Z.; El Darra, N. Decontamination of chilli flakes in a fluidized bed using combined technologies: Infrared, UV and ozone. Innov. Food Sci. Emerg. Technol. 2020, 59, 102248. [Google Scholar] [CrossRef]
- Kulišová, M.; Maťátková, O.; Brányik, T.; Zelenka, J.; Drábová, L.; Kolouchová, I.J. Detection of microscopic filamentous fungal biofilms–Choosing the suitable methodology. J. Microbiol. Methods 2023, 205, 106676. [Google Scholar] [CrossRef]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; Romero-Peña, M. Conventional and non-conventional disinfection methods to prevent microbial contamination in minimally processed fruits and vegetables. LWT 2022, 165, 113714. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Gonçalves, M.P.M.; de Brito, F.A.E.; Barboza, G.R.; Rocha, L.d.O.; Silva, N.C.C. Dry surface biofilms in the food processing industry: An overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 688–713. [Google Scholar] [CrossRef]
- Cortegiani, A.; Russotto, V.; Maggiore, A.; Attanasio, M.; Naro, A.R.; Raineri, S.M.; Giarratano, A. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Hota, B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004, 39, 1182–1189. [Google Scholar] [CrossRef]
- Macaluso, J.N., Jr. Hospital, catheter, peritoneal dialysis acquired infections: Visible light as a new solution to reduce risk and incidence. Cureus 2023, 15, e43043. [Google Scholar] [CrossRef]
- Lu, J.; Hu, X.; Ren, L. Biofilm control strategies in food industry: Inhibition and utilization. Trends Food Sci. Technol. 2022, 123, 103–113. [Google Scholar] [CrossRef]
- Ashley, J.; Rasooly, J.A.; Tran, I.; Yost, L.E.; Chertow, G.M. Effect of UV light on disinfection of peritoneal dialysis catheter connections. Perit. Dial. Int. 2017, 37, 109–111. [Google Scholar] [CrossRef]
- Byun, K.-H.; Park, S.Y.; Lee, D.U.; Chun, H.S.; Ha, S.-D. Effect of UV-C irradiation on inactivation of Aspergillus flavus and Aspergillus parasiticus and quality parameters of roasted coffee bean (Coffea arabica L.). Food Addit. Contam. Part A 2020, 37, 507–518. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Nakonieczna, J.; Grinholc, M. Development of antimicrobial phototreatment tolerance: Why the methodology matters. Int. J. Mol. Sci. 2021, 22, 2224. [Google Scholar] [CrossRef]
- Goosen, N.; Moolenaar, G.F. Repair of UV damage in bacteria. DNA Repair 2008, 7, 353–379. [Google Scholar] [CrossRef]
- Coelho, L.M.; Aquino-Ferreira, R.; Maffei, C.M.L.; Martinez-Rossi, N.M. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J. Antimicrob. Chemother. 2008, 62, 758–761. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic drug resistant fungi: A review of mitigation strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Garvey, M.; Meade, E.; Rowan, N.J. Effectiveness of front line and emerging fungal disease prevention and control interventions and opportunities to address appropriate eco-sustainable solutions. Sci. Total Environ. 2022, 851, 158284. [Google Scholar] [CrossRef]
- Wuren, T.; Toyotome, T.; Yamaguchi, M.; Takahashi-Nakaguchi, A.; Muraosa, Y.; Yahiro, M.; Wang, D.-N.; Watanabe, A.; Taguchi, H.; Kamei, K. Effect of serum components on biofilm formation by Aspergillus fumigatus and other Aspergillus species. Jpn. J. Infect. Dis. 2014, 67, 172–179. [Google Scholar] [CrossRef]
- Rayón-López, G.; Carapia-Minero, N.; Medina-Canales, M.G.; García-Pérez, B.E.; Reséndiz-Sánchez, J.; Pérez, N.O.; Rodríguez-Tovar, A.V.; Ramírez-Granillo, A. Lipid-like biofilm from a clinical brain isolate of Aspergillus terreus: Quantification, structural characterization and stages of the formation cycle. Mycopathologia 2023, 188, 35–49. [Google Scholar] [CrossRef]
- Goldman, R.P.; Travisano, M. Experimental evolution of ultraviolet radiation resistance in Escherichia coli. Evolution 2011, 65, 3486–3498. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M. Revisiting non-thermal food processing and preservation methods—Action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Nayi, P.; Phimolsiripol, Y. Cold plasma for microbial safety: Principle, mechanism, and factors responsible. J. Food Process. Preserv. 2022, 46, e16850. [Google Scholar] [CrossRef]
- Soušková, H.; Scholtz, V.; Julák, J.; Savická, D. The fungal spores survival under the low-temperature plasma. In Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Dordrecht, The Netherlands, 2012; pp. 57–66. [Google Scholar]
- Nakpan, W.; Yermakov, M.; Indugula, R.; Reponen, T.; Grinshpun, S.A. Inactivation of bacterial and fungal spores by UV irradiation and gaseous iodine treatment applied to air handling filters. Sci. Total Environ. 2019, 671, 59–65. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Maeda, K.; Toyokawa, Y.; Shimizu, N.; Imanishi, Y.; Sakudo, A. Inactivation of Salmonella by nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 2015, 52, 54–59. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Hoppanová, L.; Kryštofová, S. Nonthermal plasma effects on fungi: Applications, fungal responses, and future perspectives. Int. J. Mol. Sci. 2022, 23, 11592. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Lakatoš, B.; Kryštofová, S.; Hoppanová, L.; Palušková, V.; Hudecová, D.; Ďurina, P. Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2018, 102, 6647–6658. [Google Scholar] [CrossRef]
- Nowinski, D.; Czapka, T.; Maliszewska, I. Effect of multiple nonthermal plasma treatments of filamentous fungi on cellular phenotypic changes and phytopathogenicity. Int. J. Food Microbiol. 2024, 408, 110428. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulišová, M.; Rabochová, M.; Lorinčík, J.; Brányik, T.; Hrudka, J.; Scholtz, V.; Jarošová Kolouchová, I. Exploring Non-Thermal Plasma and UV Radiation as Biofilm Control Strategies against Foodborne Filamentous Fungal Contaminants. Foods 2024, 13, 1054. https://doi.org/10.3390/foods13071054
Kulišová M, Rabochová M, Lorinčík J, Brányik T, Hrudka J, Scholtz V, Jarošová Kolouchová I. Exploring Non-Thermal Plasma and UV Radiation as Biofilm Control Strategies against Foodborne Filamentous Fungal Contaminants. Foods. 2024; 13(7):1054. https://doi.org/10.3390/foods13071054
Chicago/Turabian StyleKulišová, Markéta, Michaela Rabochová, Jan Lorinčík, Tomáš Brányik, Jan Hrudka, Vladimír Scholtz, and Irena Jarošová Kolouchová. 2024. "Exploring Non-Thermal Plasma and UV Radiation as Biofilm Control Strategies against Foodborne Filamentous Fungal Contaminants" Foods 13, no. 7: 1054. https://doi.org/10.3390/foods13071054
APA StyleKulišová, M., Rabochová, M., Lorinčík, J., Brányik, T., Hrudka, J., Scholtz, V., & Jarošová Kolouchová, I. (2024). Exploring Non-Thermal Plasma and UV Radiation as Biofilm Control Strategies against Foodborne Filamentous Fungal Contaminants. Foods, 13(7), 1054. https://doi.org/10.3390/foods13071054






