Limited Diagnostic Yield of Routine Gastroscopy in FIT-Positive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Statistical Analysis
3. Results
3.1. FIT Patient Demographics and Population Characteristics
3.1.1. Age and Gender Distribution
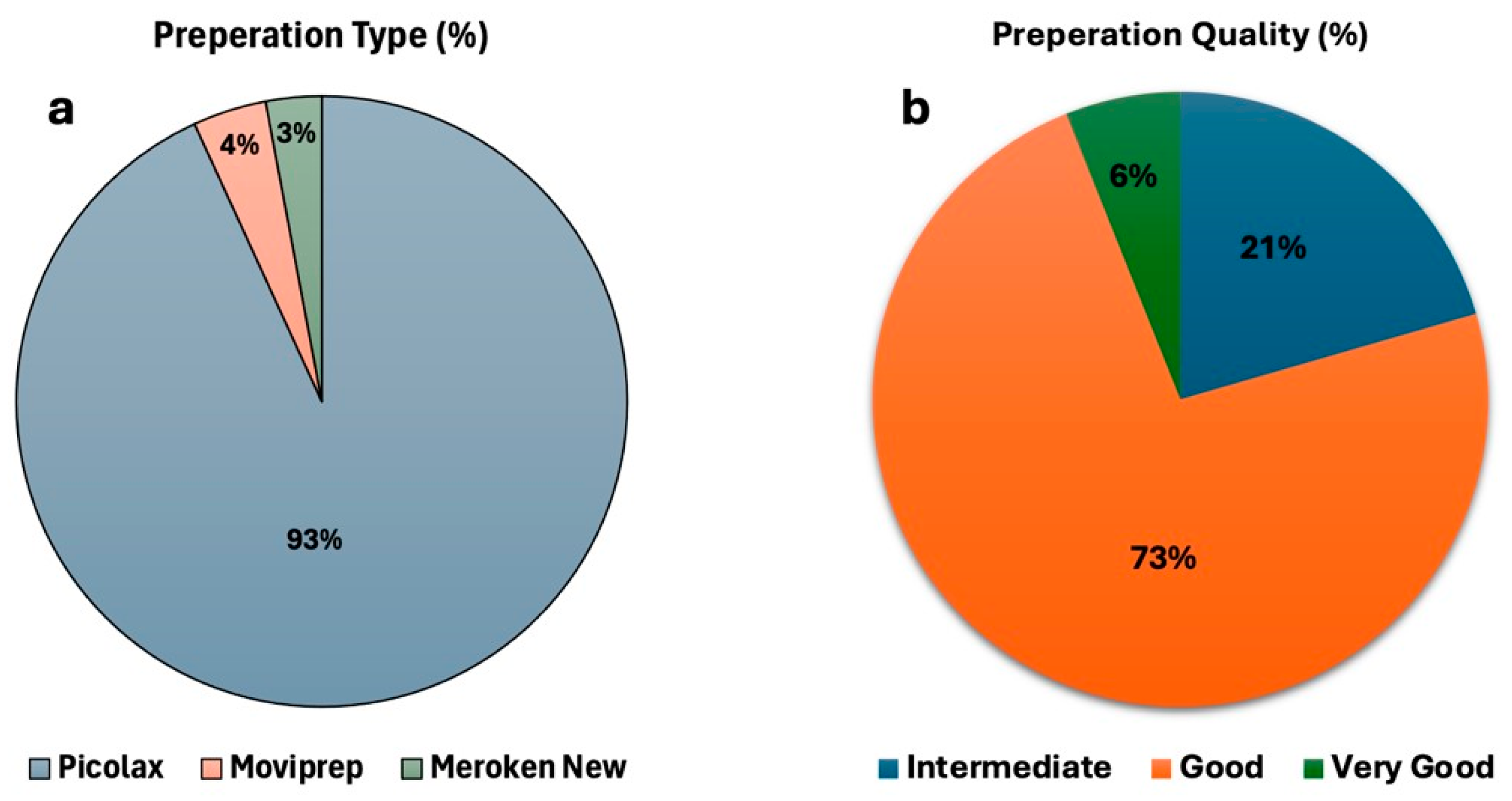
3.1.2. Bowel Preparation Quality and Effectiveness
3.1.3. Adenoma Detection and Distribution
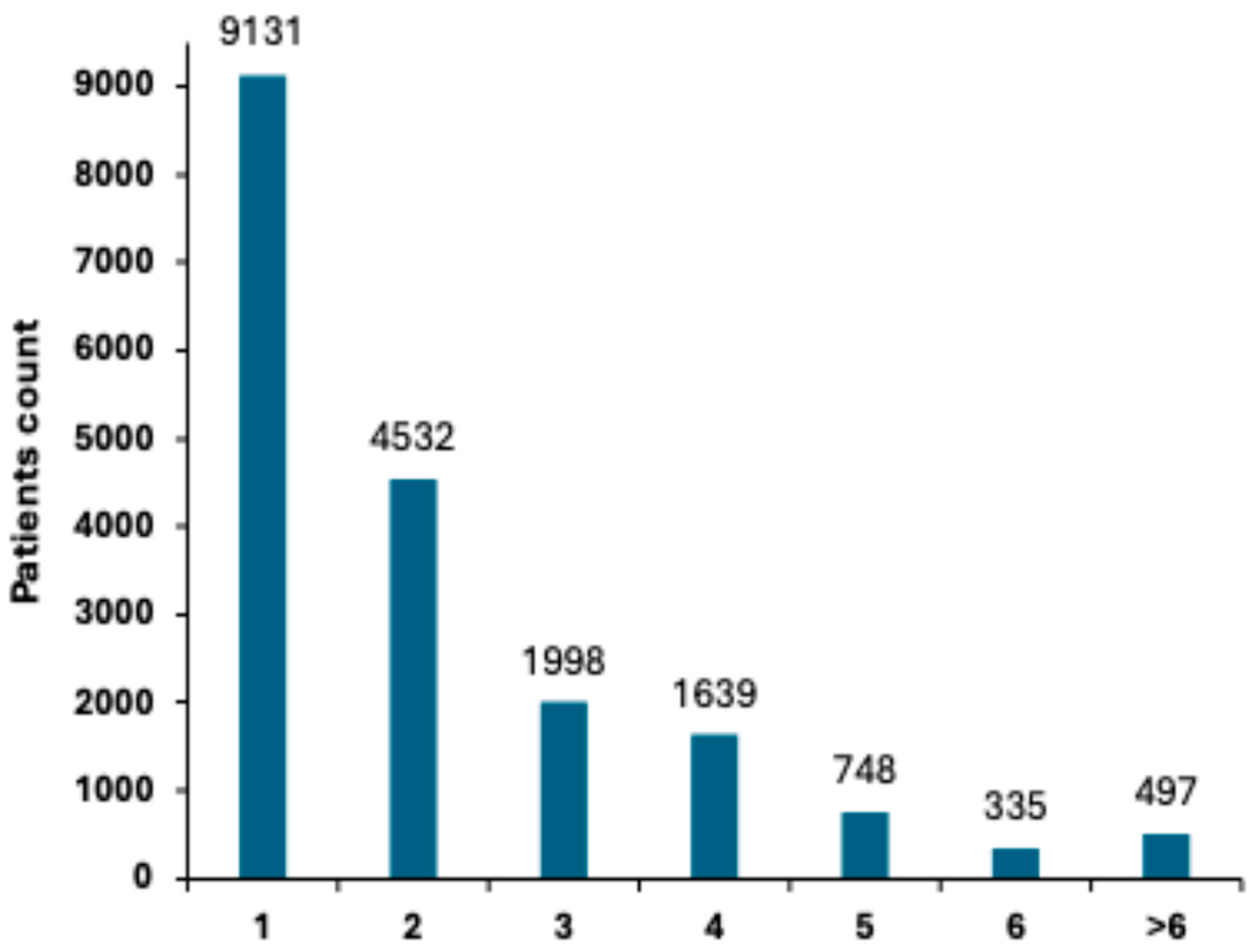
3.1.4. Polyp Detection Rate (PDR) and Distribution
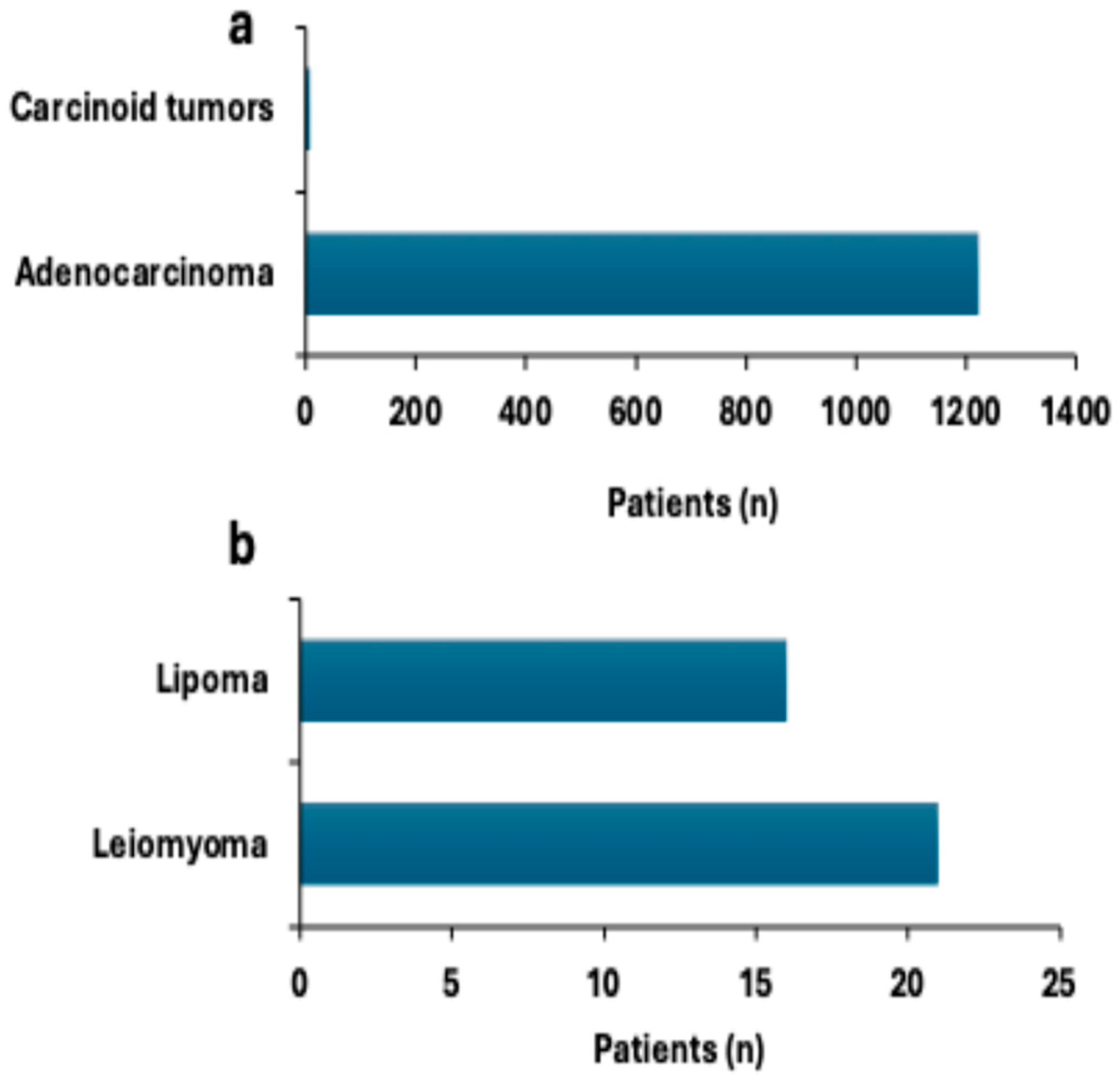
3.1.5. Colorectal Neoplasms and Tumor Distribution
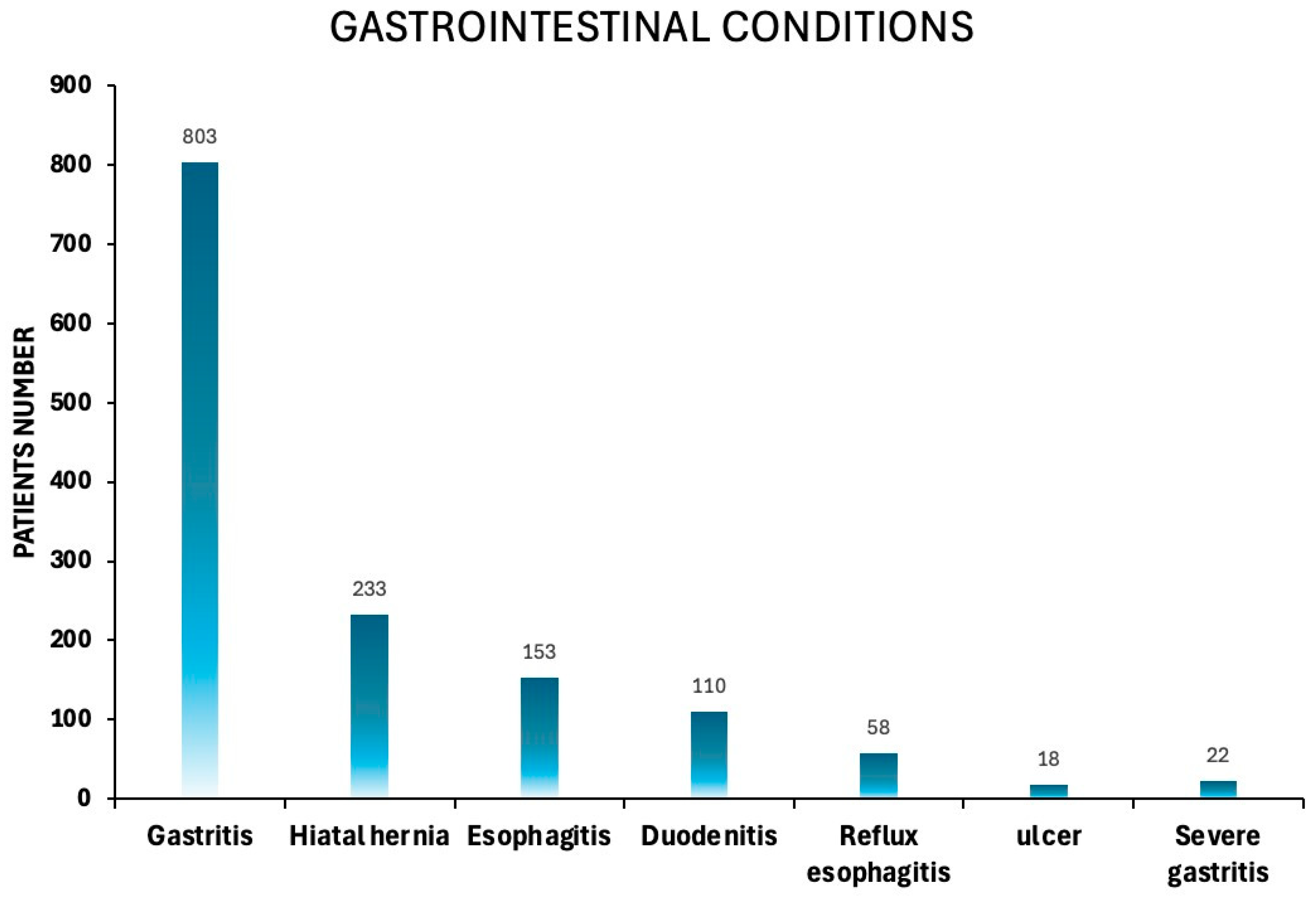
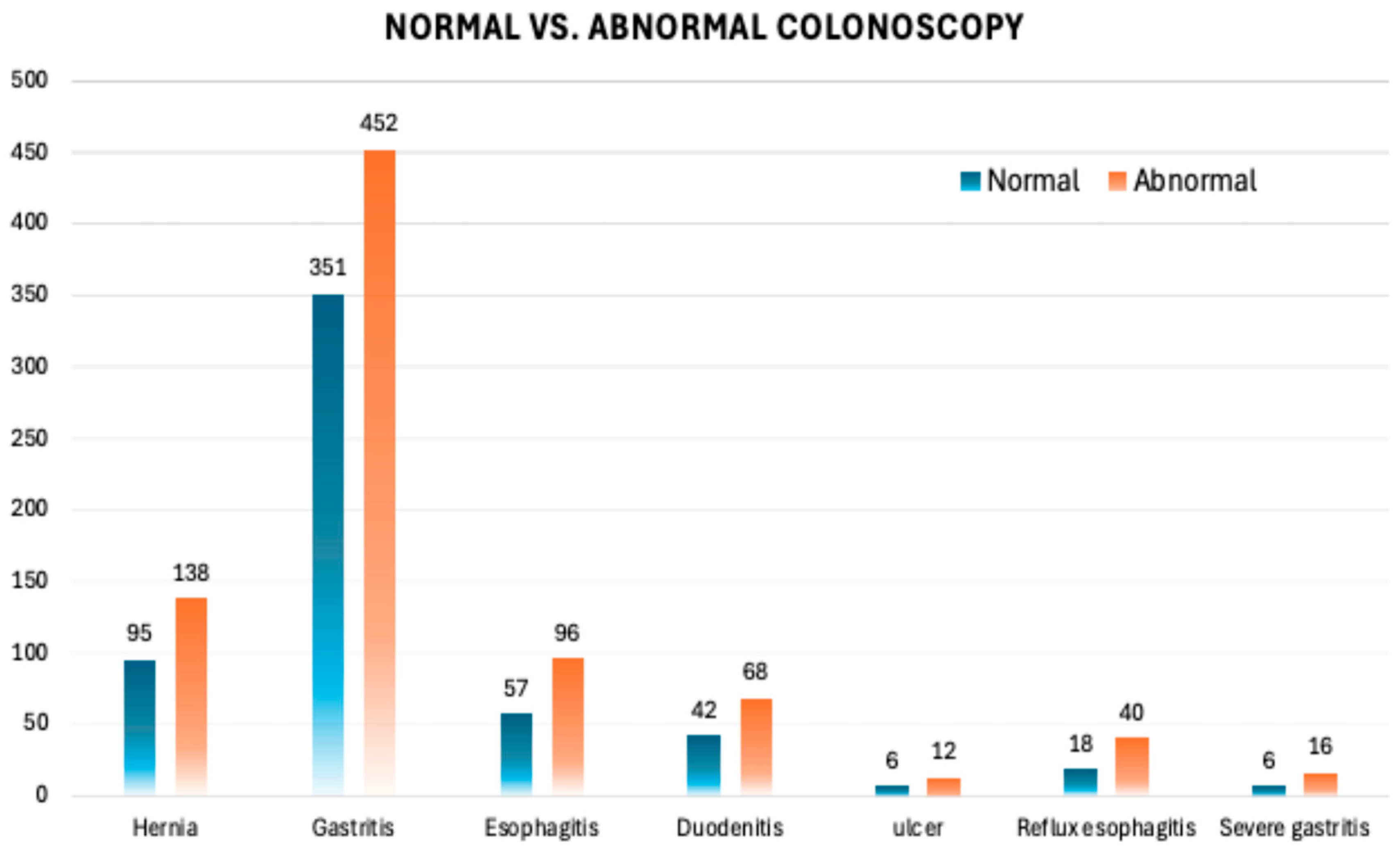
3.1.6. Prevalence of Gastrointestinal Disorders
3.1.7. Gastric Outcomes in FIT-Positive Patients Undergoing Both Colonoscopy and Gastroscopy
3.1.8. Gastroscopy as a Key Diagnostic Tool in FIT-Positive Patients with Normal Colonoscopy Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gastric Finding | p-Value |
|---|---|
| Hernia | 0.92377 |
| Gastritis | 0.040289 * |
| Esophagitis | 0.327184 |
| Duodenitis | 0.559483 |
| Ulcer | 0.654623 |
| Reflux esophagitis | 0.138902 |
| Severe gastritis | 0.260343 |
References
- Li, X.; Xiao, X.; Wu, Z.; Li, A.; Wang, W.; Lin, R. Global, regional, and national burden of early-onset colorectal cancer and projection to 2050: An analysis based on the Global Burden of Disease Study 2021. Public Health 2025, 238, 245–253. [Google Scholar] [CrossRef]
- Klimeck, L.; Heisser, T.; Hoffmeister, M.; Brenner, H. Colorectal cancer: A health and economic problem. Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101839. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.P.; Ruan, W.J.; Wang, W. Colorectal cancer screening: The value of early detection and modern challenges. World J. Gastroenterol. 2024, 30, 2726–2730. [Google Scholar] [CrossRef]
- Álvarez-Delgado, A.; García, M.L.P.; García-González, J.M.; de Sena, H.I.; Chamorro, A.J.; Gómez, M.F.L.; Marcos, M.; Mirón-Canelo, J.A. Improvements in the Effectiveness of Early Detection in Colorectal Cancer with Open-Label Randomised Study. J. Clin. Med. 2021, 10, 5072. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Li, Y.M. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J. Gastrointest. Oncol. 2016, 8, 793–800. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Corley, D.A.; Jensen, C.D.; Levin, T.R.; Ghai, N.R.; Cannavale, K.; Zhao, W.K.; Selby, K.; Buckner-Petty, S.; Zauber, A.G.; et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw. Open 2024, 7, e2423671. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Rex, D.K.; Ciani, O.; Drummond, M.F. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology 2024, 166, 758–771. [Google Scholar] [CrossRef]
- Ding, H.; Lin, J.; Xu, Z.; Wang, H.H.X.; Huang, L.; Huang, J.; Wong, M.C.S. The association between organised colorectal cancer screening strategies and reduction of its related mortality: A systematic review and meta-analysis. BMC Cancer 2024, 24, 365. [Google Scholar] [CrossRef] [PubMed]
- Núñez Rodríguez, M.H.; Díez Redondo, P.; Riu Pons, F.; Cimavilla, M.; Loza, A.; Perez-Miranda, M. Findings in the distal and proximal colon in colonoscopy screening after positive FIT and related pre-procedure factors. Rev. Esp. Enfermedades Dig. 2022, 114, 719–724. [Google Scholar] [CrossRef]
- Fritzell, K.; Forsberg, A.; Wangmar, J.; Wengström, Y.; Bottai, M.; Hultcrantz, R. Gender, having a positive FIT and type of hospital are important factors for colonoscopy experience in colorectal cancer screening—Findings from the SCREESCO study. Scand. J. Gastroenterol. 2020, 55, 1354–1362. [Google Scholar] [CrossRef]
- Krigel, A.; Wan, D.W. Colonoscopy after a Positive Stool-based Test for Colon Cancer Screening: Moving Toward a Better Understanding of What to Expect. Cancer Prev. Res. 2022, 15, 417–418. [Google Scholar] [CrossRef]
- Choe, L.; Lau, J.; Yip, L.T.; Kim, G.; Tan, K.K. Gastroscopy after positive screening for faecal immunochemical tests and colonoscopy: A systematic review. PLoS ONE 2023, 18, e0281557. [Google Scholar] [CrossRef]
- Shah, A.; Eqbal, A.; Moy, N.; Koloski, N.; Messmann, H.; Kendall, B.J.; Sharma, P.; Dulleck, U.; Jones, M.P.; Holtmann, G.J. Upper GI endoscopy in subjects with positive fecal occult blood test undergoing colonoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2023, 97, 1005–1015.e30. [Google Scholar] [CrossRef]
- Elsafi, S.H.; Alqahtani, N.I.; Zakary, N.Y.; Al Zahrani, E.M. The sensitivity, specificity, predictive values, and likelihood ratios of fecal occult blood test for the detection of colorectal cancer in hospital settings. Clin. Exp. Gastroenterol. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiu, H.M.; Chiang, T.H.; Yen, A.M.; Chiu, S.Y.; Chen, S.L.; Fann, J.C.; Yeh, Y.P.; Liao, C.S.; Hu, T.H.; et al. Accuracy of faecal occult blood test and Helicobacter pylori stool antigen test for detection of upper gastrointestinal lesions. BMJ Open 2013, 3, e003989. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, R.; Hoilat, G.J.; Ross, A.B. Esophagogastroduodenoscopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532268/ (accessed on 1 June 2025).
- Park, J.Y. Image-enhanced endoscopy in upper gastrointestinal disease: Focusing on texture and color enhancement imaging and red dichromatic imaging. Clin. Endosc. 2025, 58, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Planade, O.; Dessomme, B.; Chapelle, N.; Verdier, M.; Duchalais, E.; Queneherve, L.; Le Rhun, M.; Coron, E.; Mosnier, J.F.; Matysiak-Budnik, T.; et al. Systematic upper endoscopy concomitant with colonoscopy performed within the colorectal cancer screening program: Impact on the patients’ management. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101501. [Google Scholar] [CrossRef] [PubMed]
- Triadafilopoulos, G. Prevalence of abnormalities at tandem endoscopy in patients referred for colorectal cancer screening/surveillance colonoscopy. Cancers 2024, 16, 3998. [Google Scholar] [CrossRef]
- Nemakallu, S.R.; Daoud, N.; Ali, Y.; Ridout, N.P.; Cheung, D. A Rare Case of Gastric Submucosal Lipoma Presenting with Upper GI bleed. Am. J. Gastroenterol. 2024, 119, S2401–S2402. [Google Scholar] [CrossRef]
- Burri, E.; Manz, M.; Schroeder, P.; Froehlich, F.; Rossi, L.; Beglinger, C.; Lehmann, F.S. Diagnostic yield of endoscopy in patients with abdominal complaints: Incremental value of faecal calprotectin on guidelines of appropriateness. BMC Gastroenterol. 2014, 14, 57. [Google Scholar] [CrossRef]
- Ishtiaq, R.; Zulfiqar, L.; Gangwani, M.K.; Aziz, M. Adenoma detection rate vs. adenoma per colonoscopy as quality indicators for colon cancer screening. Transl. Gastroenterol. Hepatol. 2023, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Stray, N.; Weberg, R. A prospective study of same day bi-directional endoscopy in the evaluation of patients with occult gastrointestinal bleeding. Scand. J. Gastroenterol. 2006, 41, 844–850. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, M.T.; Telford, J.J. Positive occult blood and negative colonoscopy--should we perform gastroscopy? Can. J. Gastroenterol. 2007, 21, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Cosby, R.; Del Giudice, M.E.; Irvine, E.J.; Morgan, D.; Tinmouth, J. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: Systematic review and guideline. Can. J. Gastroenterol. 2010, 24, 113–120. [Google Scholar] [CrossRef]
- Cai, C.; Xu, Z.; Ye, B. Lower adenoma detection rate in anesthesia assisted colonoscopy: A retrospective study. Front. Oncol. 2025, 15, 1571387. [Google Scholar] [CrossRef]
- Kay, C.L.; Bader, G.A.; Miller, C.B. Traditional and Novel Colonoscopy Quality Metrics: What’s Important in 2025. Curr. Gastroenterol. Rep. 2025, 27, 58. [Google Scholar] [CrossRef]
- Ng, J.Y.; Chan, D.K.H.; Tan, K.K. Is gastroscopy for fecal immunochemical test positive patients worthwhile? Int. J. Color. Dis. 2017, 32, 697–702. [Google Scholar] [CrossRef]




| Patients with Adenoma (n) | ADR (%) |
|---|---|
| 13,954 | 36.34 |
| Age Group (Years) | Number of Cases with Adenoma | (%) |
|---|---|---|
| <50 | 1395 | 9.9 |
| 50–59 | 3488 | 24.9 |
| 60–69 | 4883 | 34.9 |
| 70–79 | 2790 | 19.9 |
| ≥80 | 1398 | 10 |
| Gender | Number of Cases with Adenoma | ADR (%) |
|---|---|---|
| Male (n = 20,078) | 7674 | 38.2 |
| Female (n = 18,314) | 6280 | 34.3 |
| Patients with Polyps (n) | PDR (%) |
|---|---|
| 18,880 | 49.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khader, M.; Abu Baker, F.; Delgado, J.-S.; Yitzhak, A.; Guterman, R.; Elhayany, R.; Bakshi, O.; Klaitman, V.; Braun, T.; Abu-Freha, N.; et al. Limited Diagnostic Yield of Routine Gastroscopy in FIT-Positive Patients. Diagnostics 2025, 15, 2781. https://doi.org/10.3390/diagnostics15212781
Khader M, Abu Baker F, Delgado J-S, Yitzhak A, Guterman R, Elhayany R, Bakshi O, Klaitman V, Braun T, Abu-Freha N, et al. Limited Diagnostic Yield of Routine Gastroscopy in FIT-Positive Patients. Diagnostics. 2025; 15(21):2781. https://doi.org/10.3390/diagnostics15212781
Chicago/Turabian StyleKhader, Majd, Fadi Abu Baker, Jorge-Shmuel Delgado, Avraham Yitzhak, Revital Guterman, Ruhama Elhayany, Or Bakshi, Vered Klaitman, Tali Braun, Naim Abu-Freha, and et al. 2025. "Limited Diagnostic Yield of Routine Gastroscopy in FIT-Positive Patients" Diagnostics 15, no. 21: 2781. https://doi.org/10.3390/diagnostics15212781
APA StyleKhader, M., Abu Baker, F., Delgado, J.-S., Yitzhak, A., Guterman, R., Elhayany, R., Bakshi, O., Klaitman, V., Braun, T., Abu-Freha, N., & Artoul, R. (2025). Limited Diagnostic Yield of Routine Gastroscopy in FIT-Positive Patients. Diagnostics, 15(21), 2781. https://doi.org/10.3390/diagnostics15212781





