Immunohistochemical and Ultrastructural Study of the Degenerative Processes of the Hip Joint Capsule and Acetabular Labrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Tissue Sample Collection
2.4. Histological and Immunohistochemical Analysis
- −
- 0 = negative;
- −
- 1 = weak/low expression;
- −
- 2 = moderate expression;
- −
- 3 = strong/high expression.
- −
- Score 0: No detectable staining or <5% positive cells, regardless of intensity (negative).
- −
- Score 1: Weak staining intensity and/or 5–25% positive cells (low expression).
- −
- Score 2: Moderate intensity and/or 26–50% positive cells (moderate expression).
- −
- Score 3: Strong staining intensity and/or >50% positive cells (high expression).
2.5. Surface Electron Microscopy (SEM) Analysis
2.6. Statistical Analysis
- −
- Score 0—organized, parallel collagen fibers with intact surface topography;
- −
- Score 1—mild disruption with initial signs of fiber fragmentation or waviness;
- −
- Score 2—moderate disorganization, loss of parallel architecture, irregular surfaces;
- −
- Score 3—severe disintegration, collapsed or amorphous fiber structures, highly irregular topography.
2.7. Ethical Considerations
3. Results
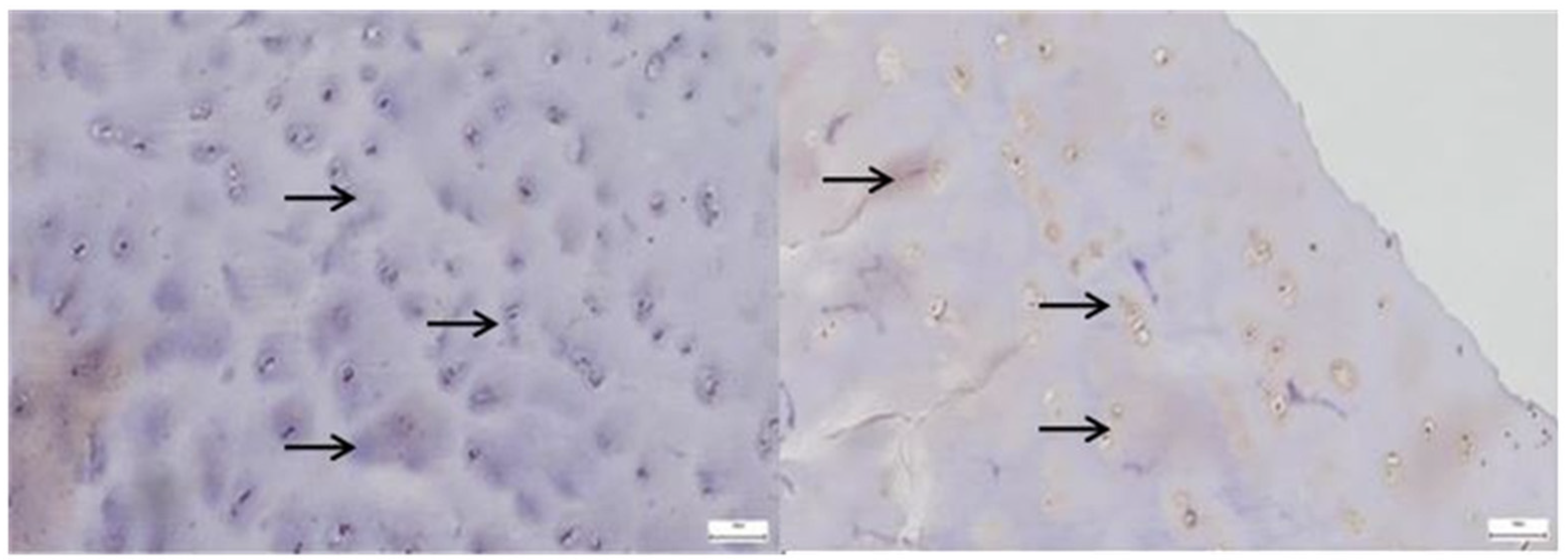
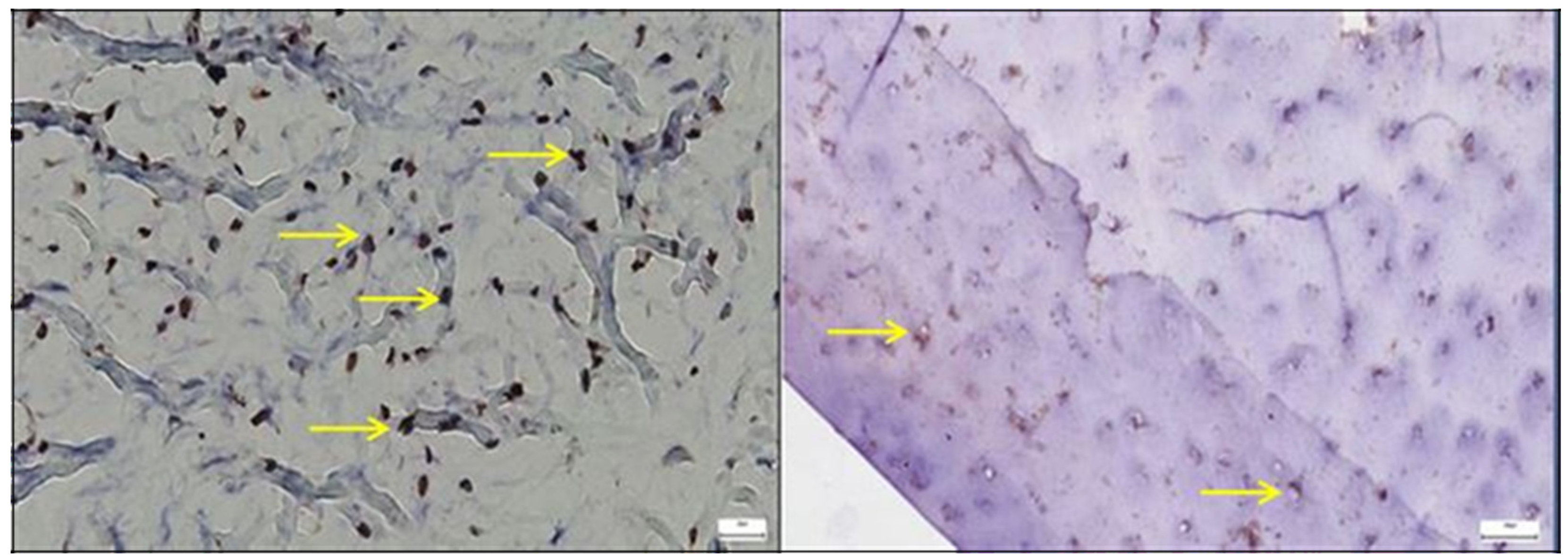
3.1. Results of the IHC Study
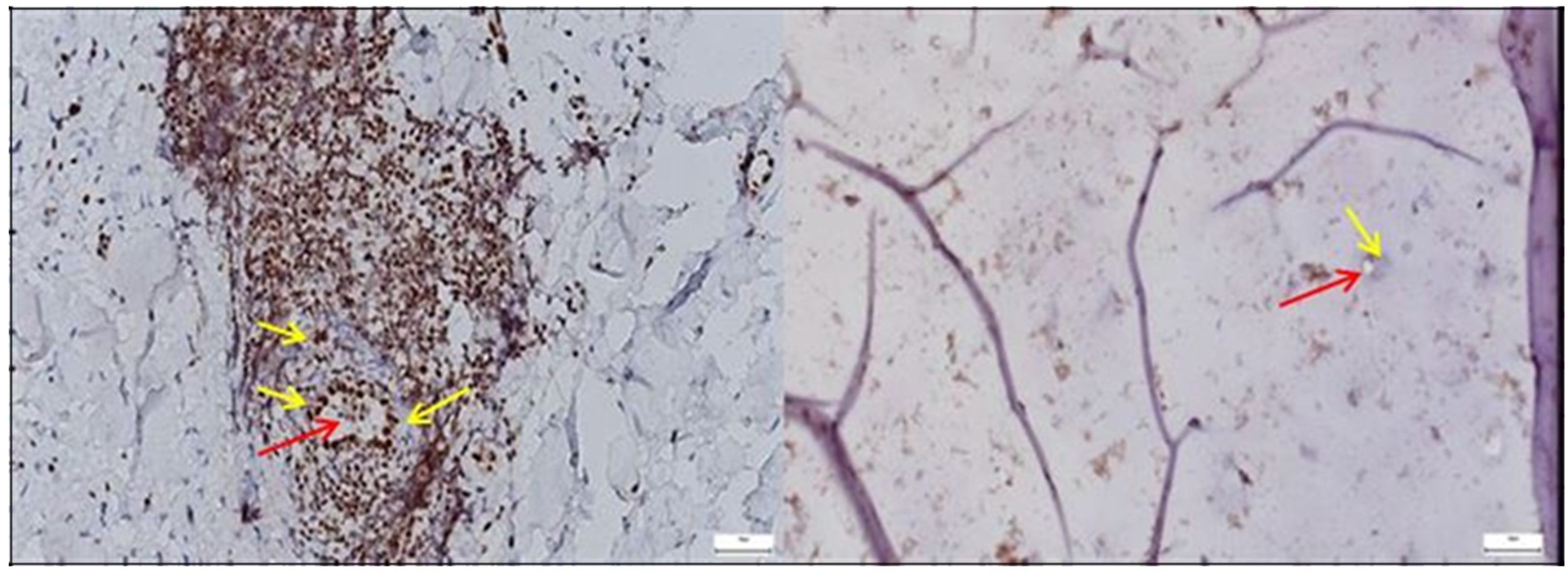
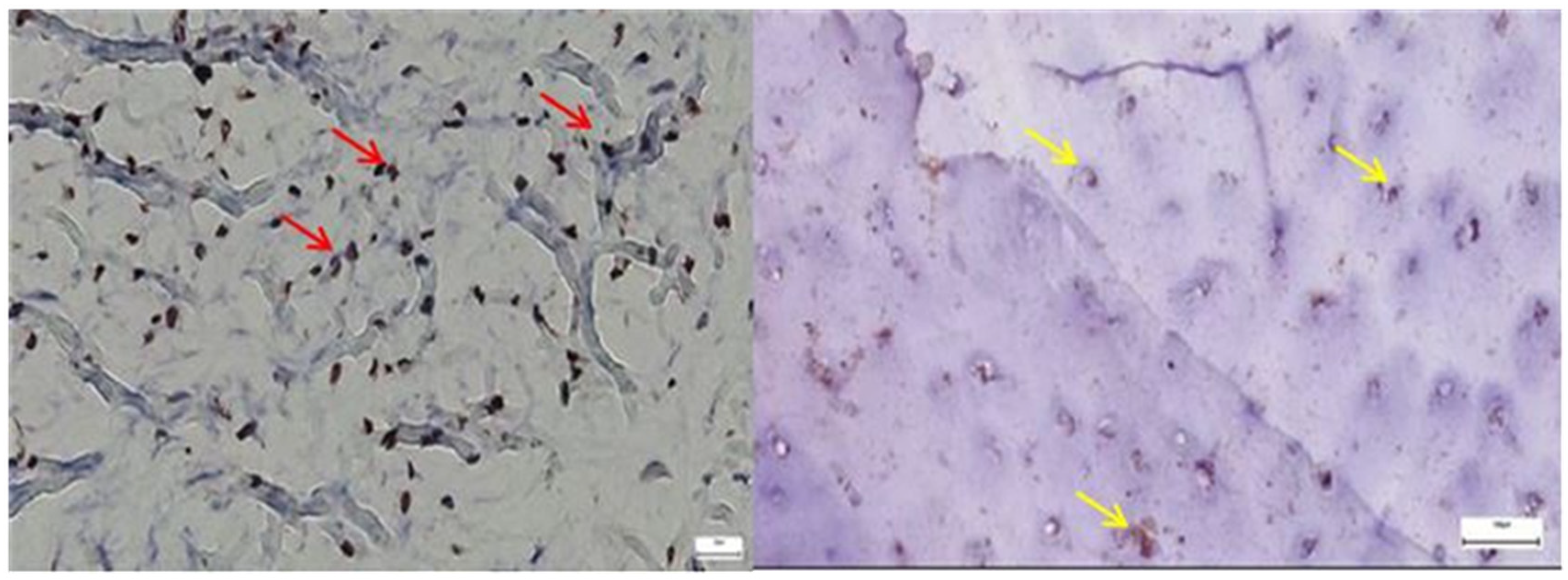
3.1.1. Results of the IHC Study on Human Hip Joint Capsule
3.1.2. Results of the IHC Study on Human Hip Joint Acetabular Labrum
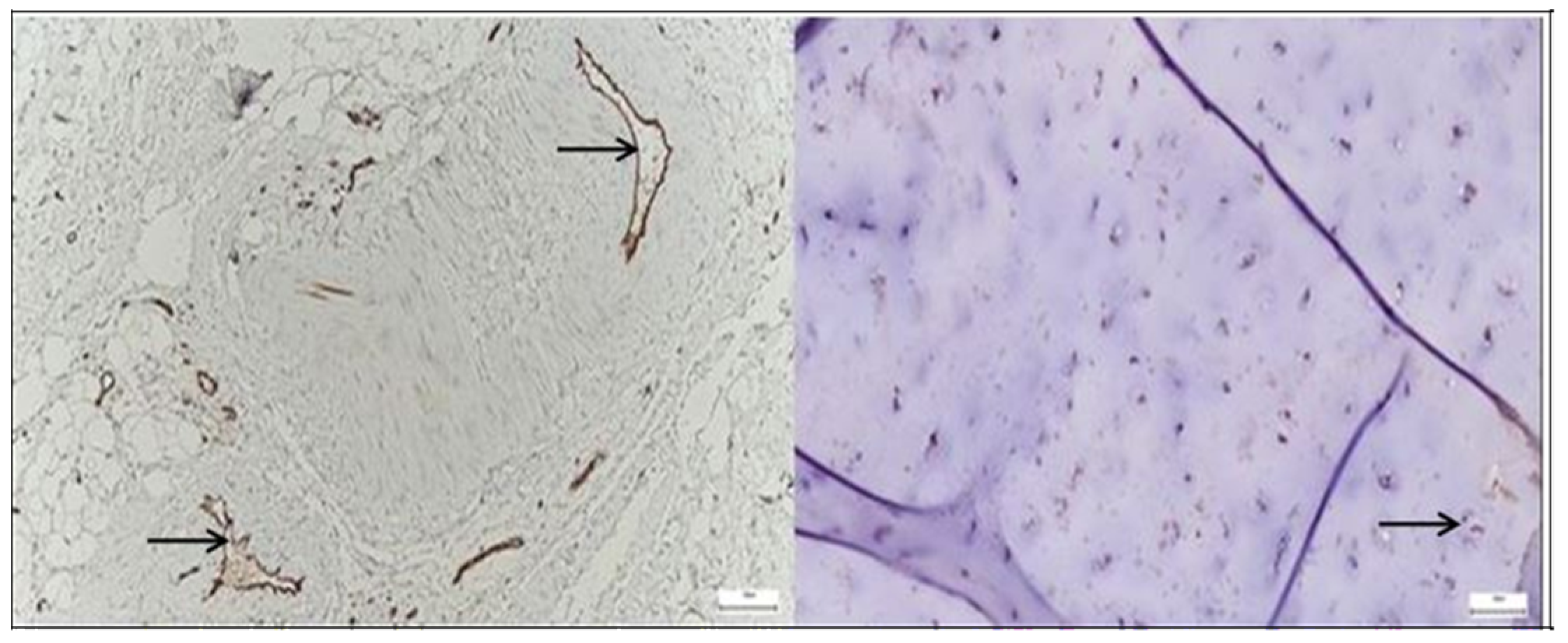
3.2. Results of the SEM Study
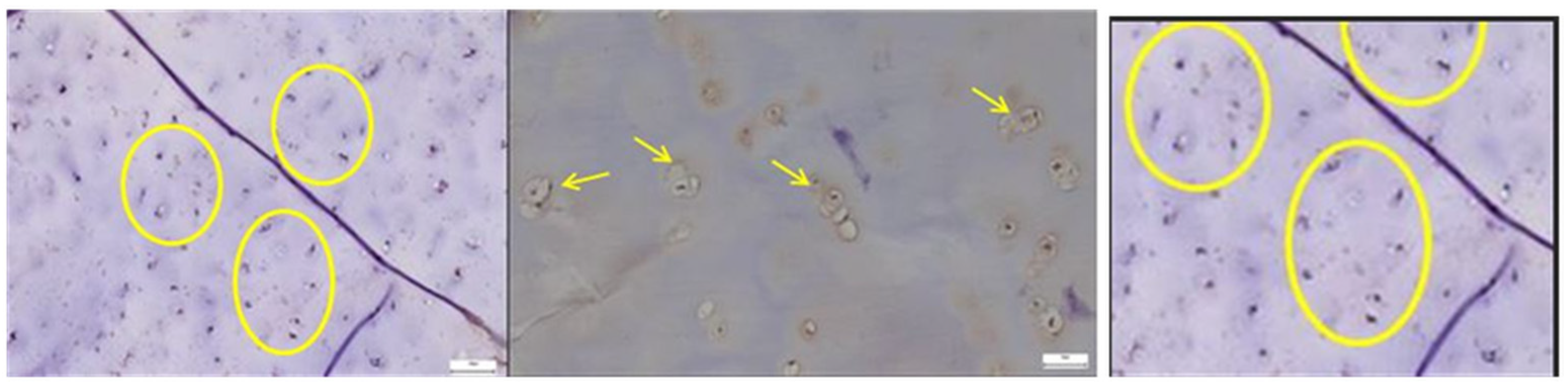
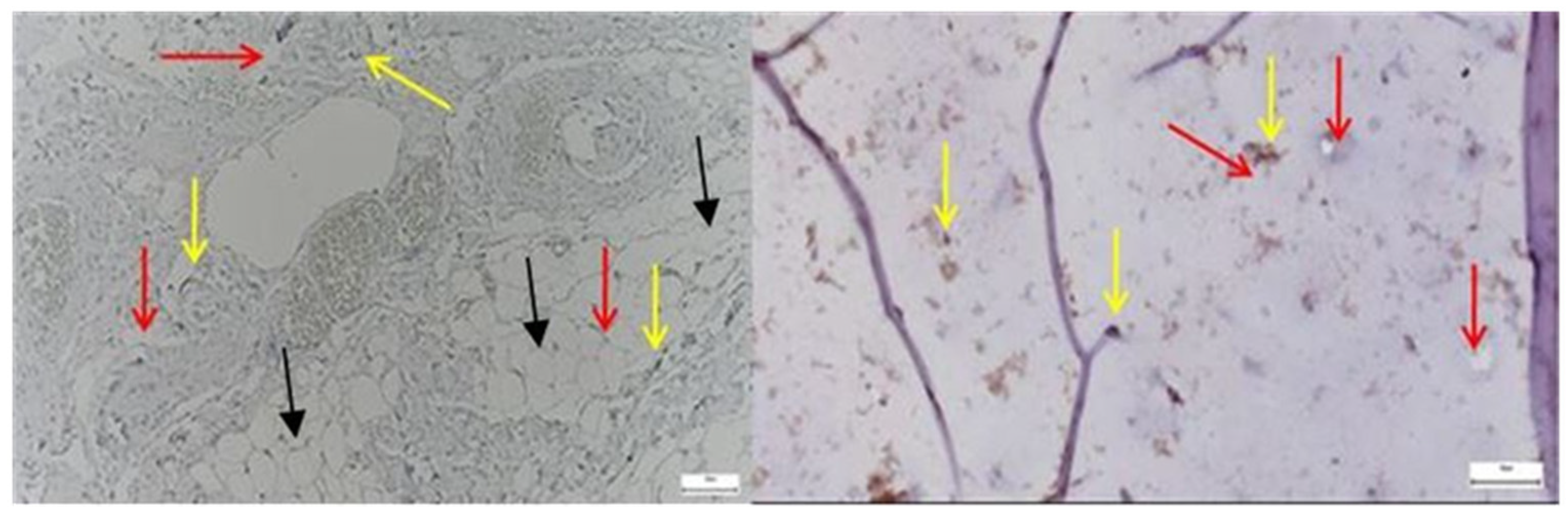
3.2.1. Results of the SEM Study on Human Hip Joint Capsule
3.2.2. Results of the SEM Study on Human Hip Joint Acetabular Labrum
3.3. Statistical Analisis Results
3.3.1. IHC Results of Statistical Study
| Patient ID | Group | Ki67 | CD68 | CD31 | SOX9 | ERG | Lubricin |
|---|---|---|---|---|---|---|---|
| 1 | Pathological | 3 | 3 | 3 | 3 | 2 | 1 |
| 2 | Pathological | 3 | 3 | 3 | 3 | 2 | 1 |
| 3 | Pathological | 3 | 2 | 3 | 3 | 2 | 0 |
| 4 | Pathological | 2 | 2 | 3 | 3 | 3 | 0 |
| 5 | Pathological | 3 | 2 | 3 | 3 | 3 | 0 |
| 6 | Pathological | 3 | 2 | 2 | 3 | 2 | 0 |
| 7 | Pathological | 2 | 2 | 3 | 3 | 3 | 1 |
| 8 | Pathological | 3 | 2 | 3 | 3 | 2 | 1 |
| 9 | Pathological | 3 | 3 | 3 | 3 | 3 | 1 |
| 10 | Pathological | 3 | 3 | 2 | 3 | 2 | 1 |
| 11 | Pathological | 3 | 2 | 3 | 2 | 2 | 1 |
| 12 | Pathological | 3 | 3 | 3 | 3 | 3 | 1 |
| 13 | Pathological | 2 | 2 | 3 | 3 | 2 | 1 |
| 14 | Pathological | 3 | 2 | 3 | 3 | 3 | 1 |
| 15 | Pathological | 3 | 2 | 2 | 2 | 3 | 0 |
| 16 | Control | 1 | 0 | 1 | 0 | 0 | 2 |
| 17 | Control | 0 | 1 | 1 | 1 | 1 | 3 |
| 18 | Control | 0 | 0 | 1 | 1 | 1 | 2 |
| 19 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 20 | Control | 1 | 1 | 0 | 0 | 1 | 2 |
| 21 | Control | 1 | 1 | 1 | 0 | 1 | 2 |
| 22 | Control | 1 | 0 | 1 | 0 | 1 | 3 |
| 23 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 24 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 25 | Control | 1 | 1 | 1 | 1 | 0 | 3 |
| 26 | Control | 0 | 0 | 0 | 0 | 1 | 2 |
- Ki67: Nuclear protein expressed during cell proliferation; high scores indicate increased mitotic activity.
- CD68: Marker of macrophage infiltration; elevated values suggest an active inflammatory response.
- CD31: Endothelial marker indicating vascular density and neovascularization processes.
- SOX9: Transcription factor essential for chondrocyte differentiation and cartilage matrix maintenance.
- ERG: Nuclear transcription factor associated with endothelial integrity and angiogenesis.
- Lubricin: Glycoprotein responsible for joint lubrication; reduced levels are indicative of mechanical stress and cartilage deterioration.
- 0—absent or minimal expression,
- 1—low expression,
- 2—moderate expression,
- 3—high expression.
| Patient ID | Group | Ki67 | CD68 | CD31 | SOX9 | ERG | Lubricin |
|---|---|---|---|---|---|---|---|
| 1 | Pathological | 2 | 3 | 3 | 3 | 3 | 0 |
| 2 | Pathological | 2 | 2 | 2 | 3 | 2 | 1 |
| 3 | Pathological | 3 | 2 | 3 | 3 | 3 | 0 |
| 4 | Pathological | 2 | 3 | 3 | 3 | 3 | 0 |
| 5 | Pathological | 3 | 3 | 2 | 3 | 3 | 0 |
| 6 | Pathological | 2 | 2 | 2 | 2 | 2 | 0 |
| 7 | Pathological | 2 | 3 | 3 | 3 | 3 | 1 |
| 8 | Pathological | 2 | 3 | 2 | 3 | 2 | 1 |
| 9 | Pathological | 2 | 3 | 3 | 3 | 3 | 0 |
| 10 | Pathological | 2 | 2 | 2 | 3 | 3 | 1 |
| 11 | Pathological | 2 | 3 | 3 | 2 | 2 | 1 |
| 12 | Pathological | 2 | 3 | 2 | 3 | 3 | 0 |
| 13 | Pathological | 3 | 3 | 3 | 3 | 3 | 1 |
| 14 | Pathological | 3 | 2 | 3 | 3 | 3 | 1 |
| 15 | Pathological | 2 | 3 | 3 | 3 | 3 | 0 |
| 16 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 17 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 18 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 19 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 20 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 21 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
| 22 | Control | 0 | 1 | 0 | 1 | 1 | 3 |
| 23 | Control | 1 | 0 | 1 | 1 | 1 | 2 |
| 24 | Control | 0 | 1 | 1 | 1 | 1 | 2 |
| 25 | Control | 1 | 1 | 1 | 1 | 0 | 2 |
| 26 | Control | 1 | 1 | 1 | 1 | 1 | 2 |
- Ki67: Nuclear protein expressed during cell proliferation; high scores indicate increased mitotic activity.
- CD68: Marker of macrophage infiltration; elevated values suggest an active inflammatory response.
- CD31: Endothelial marker indicating vascular density and neovascularization processes.
- SOX9: Transcription factor essential for chondrocyte differentiation and cartilage matrix maintenance.
- ERG: Nuclear transcription factor associated with endothelial integrity and angiogenesis.
- Lubricin: Glycoprotein responsible for joint lubrication; reduced levels are indicative of mechanical stress and cartilage deterioration.
- 0—absent or minimal expression,
- 1—low expression,
- 2—moderate expression,
- 3—high expression.
3.3.2. SEM Results of the Statistical Study
- Structural continuity/integrity: 0 = intact structure, 3 = severely affected.
- Presence of collagen/fibrillar organization: 0 = no collagen, 3 = well-organized matrix.
- Vascularization/visible capillaries: 0 = absent, 3 = extensive vascular network.
SEM Score Distribution
IHC Score Distribution
Correlation Between SEM and IHC
4. Discussion
4.1. Proliferative and Inflammatory Markers
4.2. Vascular and Endothelial Changes
4.3. Transcriptional and Matrix-Related Findings
4.4. Ultrastructural Alterations (SEM)
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porta, M.; Pau, M.; Leban, B.; Deidda, M.; Sorrentino, M.; Arippa, F.; Marongiu, G. Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Steingrebe, H.; Spancken, S.; Sell, S.; Stein, T. Effects of hip osteoarthritis on lower body joint kinematics during locomotion tasks: A systematic review and meta-analysis. Front. Sports Act. Living 2023, 5, 1197883. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.G.; Salahoru, P.; Hinganu, M.V.; Hinganu, D.; Cozma, L.C.D.; Patrascu, A.; Grigorescu, C. Reducing the Duration and Improving Hospitalisation Time by Using New Surgical Tehniques and Psychotherapy. Rev. Chim. 2019, 70, 143–146. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, X.; Guo, Z.; Zhao, R.; Feng, T.; Xiang, C. Comparison of Walking Quality Variables between End-Stage Osteonecrosis of Femoral Head Patients and Healthy Subjects by a Footscan Plantar Pressure System. Medicina 2022, 59, 59. [Google Scholar] [CrossRef]
- Nakajima, A.; Sakai, R.; Inoue, E.; Harigai, M. Prevalence of patients with rheumatoid arthritis and age-stratified trends in clinical characteristics and treatment, based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int. J. Rheum. Dis. 2020, 23, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Haubruck, P.; Pinto, M.M.; Moradi, B.; Little, C.B.; Gentek, R. Monocytes, Macrophages, and Their Potential Niches in Synovial Joints—Therapeutic Targets in Post-Traumatic Osteoarthritis? Front. Immunol. 2021, 12, 763702. [Google Scholar] [CrossRef]
- Smith, M.H.; Berman, J.R. What Is Rheumatoid Arthritis? JAMA 2022, 327, 1194. [Google Scholar] [CrossRef]
- Cush, J.J. Rheumatoid Arthritis: Early Diagnosis and Treatment. Med. Clin. N. Am. 2021, 105, 355–365. [Google Scholar] [CrossRef]
- Takanashi, S.; Kaneko, Y.; Takeuchi, T. CDAI and DAS28 in the management of rheumatoid arthritis in clinical practice. Ann. Rheum. Dis. 2020, 79, 671–674. [Google Scholar] [CrossRef]
- Lin, Y.J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Johannsen, A.M.; Ejnisman, L.; Behn, A.W.; Shibata, K.; Thio, T.; Safran, M.R. Contributions of the Capsule and Labrum to Hip Mechanics in the Context of Hip Microinstability. Orthop. J. Sports Med. 2019, 7, 2325967119890846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Bai, L.K.; Su, Y.Z.; Wang, X.X.; Bai, B.; Zhang, C.Q.; Zhang, L.Y.; Zhang, G.L. Synovial Macrophages: Past Life, Current Situation, and Application in Inflammatory Arthritis. Front. Immunol. 2022, 13, 905356. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Padyukov, L. Genetics of rheumatoid arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, H.Y.; Luo, S.F.; Lai, J.H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 686. [Google Scholar] [CrossRef]
- Ryan, D.T.; Hanley, M.; White, A.; Hynes, J.P.; Long, N.M.; Eustace, S.J.; Kavanagh, E.C. Comparison of 3T MR arthrography and 3T MRI in intra-articular hip pathology: A cost-analysis. Ir. J. Med. Sci. 2024, 193, 2515–2523. [Google Scholar] [CrossRef]
- Ben-Artzi, A.; Horowitz, D.L.; Mandelin, A.M., II; Tabechian, D. Best practices for ultrasound-guided synovial biopsy in the United States. Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101834. [Google Scholar] [CrossRef]
- Tu, J.; Wang, X.; Gong, X.; Hong, W.; Han, D.; Fang, Y.; Guo, Y.; Wei, W. Synovial Macrophages in Rheumatoid Arthritis: The Past, Present, and Future. Mediat. Inflamm. 2020, 2020, 1583647. [Google Scholar] [CrossRef]
- Maeda, K.; Yoshida, K.; Nishizawa, T.; Otani, K.; Yamashita, Y.; Okabe, H.; Hadano, Y.; Kayama, T.; Kurosaka, D.; Saito, M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022, 23, 2871. [Google Scholar] [CrossRef]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 922. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, K.; Kang, K.; Kim, H.; Lee, M.; Oh, B.; Kaneko, K.; Ma, S.; Choi, J.H.; Kwak, H.; et al. RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts. Cell. Mol. Immunol. 2023, 20, 94–109. [Google Scholar] [CrossRef]
- Mortensen, A.J.; Featherall, J.; Metz, A.K.; Rosenthal, R.M.; O’Neill, D.C.; Froerer, D.L.; Khalil, A.Z.; Tomasevich, K.M.; Aoki, S.K. The Role of the Hip Capsule in Restoring Stability in the Initial Phase of Hip Distraction: An In Vivo Analysis. Orthop. J. Sports Med. 2024, 12, 23259671241249719. [Google Scholar] [CrossRef]
- Matta, C.; Takács, R.; Dvir-Ginzberg, M.; Richardson, S.M.; Pelttari, K.; Pattappa, G.; Risbud, M.V.; Mobasheri, A. Insights into chondrocyte populations in cartilaginous tissues at the single-cell level. Nat. Rev. Rheumatol. 2025, 21, 465–477. [Google Scholar] [CrossRef]
- Curtis, D.M.; Pullen, W.M.; Murray, I.R.; Money, A.; Safran, M. The diagnosis of hip microinstability is correlated with ease of intra-operative hip distraction. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Schon, J.; Chahla, J.; Paudel, S.; Manandhar, L.; Feltham, T.; Huard, J.; Philippon, M.; Zhang, Z. Expression profile of matrix metalloproteinases in the labrum of femoroacetabular impingement. Bone Jt. Res. 2020, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Türker, M.; Kılıçoğlu, Ö.; Göksan, B.; Bilgiç, B. Vascularity and histology of fetal labrum and chondrolabral junction: Its relevance to chondrolabral detachment tears. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, S.; Gkantsinikoudis, N.; Dermon, A.; Kommata, V.; Papathanasiou, J.; Soukakos, P.; Dermon, C. Normal microscopic architecture of acetabular labrum of hip joint: A qualitative original study with clinical aspects. Muscles Ligaments Tendons J. 2017, 7, 279–285. [Google Scholar] [CrossRef]
- Webb, M.S.L.; Devitt, B.M.; O’Donnell, J.M. Preserving the chondrolabral junction reduces the rate of capsular adhesions. J. Hip Preserv. Surg. 2019, 6, 50–54. [Google Scholar] [CrossRef] [PubMed]
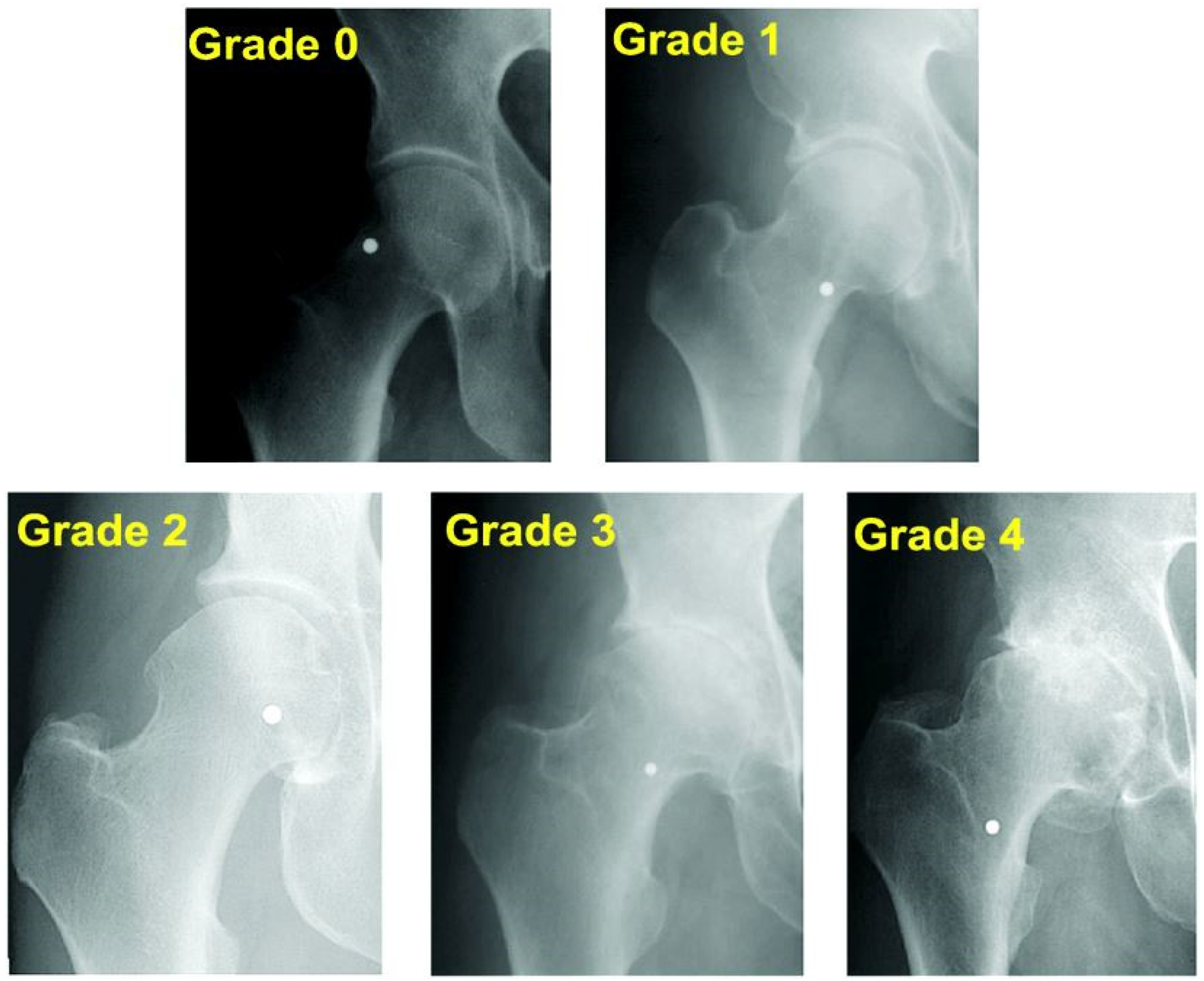
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Tanner, M.C.; Heller, R.A.; Grimm, A.; Zimmermann, S.; Pilz, M.; Jurytko, L.; Miska, M.; Helbig, L.; Schmidmaier, G.; Haubruck, P. The Influence of an Occult Infection on the Outcome of Autologous Bone Grafting During Surgical Bone Reconstruction: A Large Single-Center Case-Control Study. J. Inflamm. Res. 2021, 14, 995–1005. [Google Scholar] [CrossRef]
- Tavallaee, G.; Lively, S.; Rockel, J.S.; Ali, S.A.; Im, M.; Sarda, C.; Mitchell, G.M.; Rossomacha, E.; Nakamura, S.; Potla, P.; et al. Contribution of MicroRNA-27b-3p to Synovial Fibrotic Responses in Knee Osteoarthritis. Arthritis Rheumatol. 2022, 74, 1928–1942. [Google Scholar] [CrossRef]
- Gebre, R.K.; Hirvasniemi, J.; Lantto, I.; Saarakkala, S.; Leppilahti, J.; Jämsä, T. Discrimination of Low-Energy Acetabular Fractures from Controls Using Computed Tomography-Based Bone Characteristics. Ann. Biomed. Eng. 2021, 49, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Itoh, T.; Takaku, Y.; Suzuki, H.; Kosugi, I.; Meguro, S.; Iwashita, T.; Hariyama, T. The NanoSuit method: A novel histological approach for examining paraffin sections in a nondestructive manner by correlative light and electron microscopy. Lab. Investig. 2020, 100, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Suzuki, H.; Maekawa, M.; Hariyama, T. Combination of the NanoSuit method and gold/platinum particle-based lateral flow assay for quantitative and highly sensitive diagnosis using a desktop scanning electron microscope. J. Pharm. Biomed. Anal. 2021, 196, 113924. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Zhao, J.; Long, W.; Wang, B.; Wang, Y.; Chen, H.; Wu, R. Synovial macrophages drive severe joint destruction in established rheumatoid arthritis. Sci. Rep. 2025, 15, 12111. [Google Scholar] [CrossRef]
- Siddiq, B.S.; Mun, J.S.; Dean, M.C.; Gillinov, S.M.; Lee, J.S.; Dowley, K.S.; Cherian, N.J.; Martin, S.D. Os Acetabuli Do Not Portend Inferior 2-Year Functional Outcomes in Patients Undergoing Arthroscopic Acetabular Labral Reconstruction. Arthrosc. Sports Med. Rehabil. 2025, 7, 101026. [Google Scholar] [CrossRef]
- Caliogna, L.; Berni, M.; Torriani, C.; Mancuso, M.E.; Di Minno, M.N.D.; Brancato, A.M.; Jannelli, E.; Mosconi, M.; Pasta, G. Pathogenesis of osteoarthritis, rheumatoid arthritis, and hemophilic arthropathy: The role of angiogenesis. Haemophilia 2024, 30, 1256–1264. [Google Scholar] [CrossRef]
- Shah, A.V.; Birdsey, G.M.; Peghaire, C.; Pitulescu, M.E.; Dufton, N.P.; Yang, Y.; Weinberg, I.; Osuna Almagro, L.; Payne, L.; Mason, J.C.; et al. The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of Notch signalling and vascular stability. Nat. Commun. 2017, 8, 16002. [Google Scholar] [CrossRef]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Roggio, F.; Petrigna, L.; Trovato, B.; Di Rosa, M.; Musumeci, G. The Role of Lubricin, Irisin and Exercise in the Prevention and Treatment of Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 5126. [Google Scholar] [CrossRef]
- Trivanović, D.; Vujačić, M.; Arsić, A.; Kukolj, T.; Rajković, M.; Bogosavljević, N.; Baščarević, Z.; Maljković Ružičić, M.; Kovačević, J.; Jauković, A. Skeletal Site-Specific Lipid Profile and Hematopoietic Progenitors of Bone Marrow Adipose Tissue in Patients Undergoing Primary Hip Arthroplasty. Metabolites 2025, 15, 16. [Google Scholar] [CrossRef]
- Fuchioka, Y.; Endo, K.; Sakamaki, Y.; Tanimoto, T.; Ozeki, N.; Nakagawa, Y.; Koga, H.; Tomita, M.; Sekiya, I. Scanning electron microscopy analysis of synovial and adipose mesenchymal stem cells adhering to cartilage. Regen. Ther. 2024, 27, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.; Zhang, H.; Wu, W.; Xue, S.; Zhu, Z.; Ding, C. Inflammatory mechanisms underlying metabolic syndrome-associated and potential treatments. Osteoarthr. Cartil. Open 2025, 7, 100614. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.M.; Arnold, K.M.; Oviedo, M.; Westendorf, J.J.; Weaver, S.R. Inflammatory Processes Affecting Bone Health and Repair. Curr. Osteoporos. Rep. 2023, 21, 842–853. [Google Scholar] [CrossRef] [PubMed]










| Antibody | Reference | Source | Dilution |
|---|---|---|---|
| Ki67 | MM1 | Abcam, UK | 1:100 |
| CD68 | 514H12 | Abcam, UK | 1:300 |
| CD31 | 1A10 | Abcam, UK | 1:100 |
| ERG | EPR3864 | Abcam, UK | 1:1000 |
| SOX9 | EPR14335-78 | Abcam, UK | 1:1000 |
| Lubricin/MSF | ab28484 | Abcam, UK | 1:250 |
| A. Bone and Cartilage | ||||
| Group | Number of Cases | Structural Continuity/Integrity | Collagen/Fibrillar Organization | Vascularization |
| Control | 15 | 0 | 0 | 0 |
| Pathological | 11 | 18 | 23 | 19 |
| B. Capsule | ||||
| Group | Number of Cases | Structural Continuity/Integrity | Collagen/Fibrillar Organization | Vascularization |
| Control | 15 | 0 | 0 | 0 |
| Pathological | 11 | 15 | 21 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huzum, R.M.; Huzum, B.; Hînganu, M.V.; Lozneanu, L.; Lupu, F.C.; Hînganu, D. Immunohistochemical and Ultrastructural Study of the Degenerative Processes of the Hip Joint Capsule and Acetabular Labrum. Diagnostics 2025, 15, 1932. https://doi.org/10.3390/diagnostics15151932
Huzum RM, Huzum B, Hînganu MV, Lozneanu L, Lupu FC, Hînganu D. Immunohistochemical and Ultrastructural Study of the Degenerative Processes of the Hip Joint Capsule and Acetabular Labrum. Diagnostics. 2025; 15(15):1932. https://doi.org/10.3390/diagnostics15151932
Chicago/Turabian StyleHuzum, Riana Maria, Bogdan Huzum, Marius Valeriu Hînganu, Ludmila Lozneanu, Fabian Cezar Lupu, and Delia Hînganu. 2025. "Immunohistochemical and Ultrastructural Study of the Degenerative Processes of the Hip Joint Capsule and Acetabular Labrum" Diagnostics 15, no. 15: 1932. https://doi.org/10.3390/diagnostics15151932
APA StyleHuzum, R. M., Huzum, B., Hînganu, M. V., Lozneanu, L., Lupu, F. C., & Hînganu, D. (2025). Immunohistochemical and Ultrastructural Study of the Degenerative Processes of the Hip Joint Capsule and Acetabular Labrum. Diagnostics, 15(15), 1932. https://doi.org/10.3390/diagnostics15151932







