Longitudinal Analysis of P100 Wave Amplitude and Latency in Multiple Sclerosis: A 19-Year Retrospective VEP Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamm, C.P.; Uitdehaag, B.M.; Polman, C.H. Multiple Sclerosis: Current Knowledge and Future Outlook. Eur. Neurol. 2014, 72, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2018, 26, 27–40. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Horbrügger, M.; Loewe, K.; Kaufmann, J.; Opfer, R.; Wagner, M.; Al-Nosairy, K.O.; Meuth, S.G.; Hoffmann, M.B.; Schippling, S. MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e665. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L. Optic Neuritis. CONTINUUM Lifelong Learn. Neurol. 2019, 25, 1236–1264. [Google Scholar] [CrossRef]
- Redler, Y.; Levy, M. Rodent Models of Optic Neuritis. Front. Neurol. 2020, 11, 580951. [Google Scholar] [CrossRef]
- Donica, V.C.; Alexa, A.I.; Pavel, I.A.; Danielescu, C.; Ciapă, M.A.; Donica, A.L.; Bogdănici, C.M. The Evolvement of OCT and OCT-A in Identifying Multiple Sclerosis Biomarkers. Biomedicines 2023, 11, 3031. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Behbehani, R.; Ali, A.; Al-Omairah, H.; Rousseff, R.T. Optimization of spectral domain optical coherence tomography and visual evoked potentials to identify unilateral optic neuritis. Mult. Scler. Relat. Disord. 2020, 41, 101988. [Google Scholar] [CrossRef]
- Halliday, A.M.; Mcdonald, W.I.; Mushin, J. DELAYED VISUAL EVOKED RESPONSE IN OPTIC NEURITIS. Lancet 1972, 299, 982–985. [Google Scholar] [CrossRef]
- Berman, S.; Backner, Y.; Krupnik, R.; Paul, F.; Petrou, P.; Karussis, D.; Levin, N.; Mezer, A.A. Conduction delays in the visual pathways of progressive multiple sclerosis patients covary with brain structure. NeuroImage 2020, 221, 117204. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.; McClelland, C.; Lee, M.S. Critical review: Typical and atypical optic neuritis. Surv. Ophthalmol. 2019, 64, 770–779. [Google Scholar] [CrossRef]
- Luo, J.J.; Bumanlag, F.; Dun, N. Low-contrast visual evoked potential and early detection of optic demyelination. J. Neurol. Sci. 2019, 399, 108–110. [Google Scholar] [CrossRef]
- Yang, E.B.; Hood, D.C.; Rodarte, C.; Zhang, X.; Odel, J.G.; Behrens, M.M. Improvement in Conduction Velocity after Optic Neuritis Measured with the Multifocal VEP. Investig. Opthalmol. Vis. Sci. 2007, 48, 692. [Google Scholar] [CrossRef] [PubMed]
- Oertel, F.C.; Krämer, J.; Motamedi, S.; Keihani, A.; Zimmermann, H.G.; Dimitriou, N.G.; Condor-Montes, S.; Bereuter, C.; Cordano, C.; Abdelhak, A.; et al. Visually Evoked Potential as Prognostic Biomarker for Neuroaxonal Damage in Multiple Sclerosis From a Multicenter Longitudinal Cohort. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200092. [Google Scholar] [CrossRef] [PubMed]
- Amezcua, L.; Robers, M.V.; Soneji, D.; Manouvakhova, O.; Martinez, A.; Islam, T. Inclusion of optic neuritis in dissemination in space improves the performance of McDonald 2017 criteria in Hispanic people with suspected multiple sclerosis. Mult. Scler. J. 2023, 29, 1748–1754. [Google Scholar] [CrossRef]
- Vidal-Jordana, A.; Rovira, A.; Calderon, W.; Arrambide, G.; Castilló, J.; Moncho, D.; Rahnama, K.; Collorone, S.; Toosy, A.T.; Ciccarelli, O.; et al. Adding the Optic Nerve in Multiple Sclerosis Diagnostic Criteria: A Longitudinal, Prospective, Multicenter Study. Neurology 2024, 102, e200805. [Google Scholar] [CrossRef]
- Roldán, M.; Caballé, N.; Sainz, C.; Pérez-Rico, C.; Ayuso, L.; Blanco, R. Assessing the visual afferent pathway with the multifocal visual evoked potentials in the radiologically isolated syndrome. Sci. Rep. 2024, 14, 20169. [Google Scholar] [CrossRef]
- Vecchio, D.; Barbero, P.; Galli, G.; Virgilio, E.; Naldi, P.; Comi, C.; Cantello, R. Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis. J. Clin. Med. 2023, 12, 2382. [Google Scholar] [CrossRef]
- Nikolic, B.; Zaletel, I.; Ivancevic, N.; Rovcanin, B.; Pepic, A.; Samardzic, J.; Jancic, J. The usefulness of visual evoked potentials in the assessment of the pediatric multiple sclerosis. Eur. J. Paediatr. Neurol. 2022, 36, 130–136. [Google Scholar] [CrossRef]
- Filgueiras, T.G.; Oyamada, M.K.; Hokazono, K.; Cunha, L.P.; Apóstolos-Pereira, S.L.; Callegaro, D.; Monteiro, M.L.R. Comparison of Visual Evoked Potentials in Patients Affected by Optic Neuritis From Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder. J. Neuro-Ophthalmol. 2021, 42, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Ekayanti, M.S.; Mahama, C.N.; Ngantung, D.J. Normative values of visual evoked potential in adults. Indian J. Ophthalmol. 2021, 69, 2328–2332. [Google Scholar] [CrossRef]
- Donica, V.C.; Donica, A.L.; Pavel, I.A.; Danielescu, C.; Alexa, A.I.; Bogdănici, C.M. Variabilities in Retinal Hemodynamics Across the Menstrual Cycle in Healthy Women Identified Using Optical Coherence Tomography Angiography. Life 2024, 15, 22. [Google Scholar] [CrossRef]
- Fortepiani, L.; Foutch, B.K.; Wilson, M.R. The Effects of Sex, Oral Contraception, and Menstrual Cycle Phase on Intraocular Pressure, Central Corneal Thickness, and Foveal Thickness: A Descriptive Analysis. Vision 2021, 5, 48. [Google Scholar] [CrossRef]
- Vander Wall, R.; Basavarajappa, D.; Palanivel, V.; Sharma, S.; Gupta, V.; Klistoner, A.; Graham, S.; You, Y. VEP Latency Delay Reflects Demyelination Beyond the Optic Nerve in the Cuprizone Model. Investig. Ophthalmol. Vis. Sci. 2024, 65, 50. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Pfister, A.; Tsagkas, C.; Gaetano, L.; Sellathurai, S.; D’Souza, M.; Cerdá-Fuertes, N.; Gugleta, K.; Descoteaux, M.; Chakravarty, M.M.; et al. Visual evoked potentials in multiple sclerosis: P100 latency and visual pathway damage including the lateral geniculate nucleus. Clin. Neurophysiol. 2024, 161, 122–132. [Google Scholar] [CrossRef]
- Ciapă, M.A.; Șalaru, D.L.; Stătescu, C.; Sascău, R.A.; Bogdănici, C.M. Optic Neuritis in Multiple Sclerosis—A Review of Molecular Mechanisms Involved in the Degenerative Process. Curr. Issues Mol. Biol. 2022, 44, 3959–3979. [Google Scholar] [CrossRef] [PubMed]
- Schurz, N.; Sariaslani, L.; Altmann, P.; Leutmezer, F.; Mitsch, C.; Pemp, B.; Rommer, P.; Zrzavy, T.; Berger, T.; Bsteh, G. Evaluation of Retinal Layer Thickness Parameters as Biomarkers in a Real-World Multiple Sclerosis Cohort. Eye Brain 2021, 13, 59–69. [Google Scholar] [CrossRef]
- Tepavčević, V.; Lubetzki, C. Oligodendrocyte progenitor cell recruitment and remyelination in multiple sclerosis: The more, the merrier? Brain 2022, 145, 4178–4192. [Google Scholar] [CrossRef]
- Deschamps, R.; Shor, N.; Vignal, C.; Guillaume, J.; Boudot de la Motte, M.; Salviat, F.; Lecler, A.; Marignier, R.; Hage, R.; Coulette, S.; et al. Prospective longitudinal study on prognostic factors of visual recovery and structural change after a first episode of optic neuritis. Eur. J. Neurol. 2022, 29, 2781–2791. [Google Scholar] [CrossRef]
- Carroll, W.M.; Jennings, A.R.; Mastaglia, F.L. The origin of remyleinating oligodendrocytes in antiserum-mediated demyelinative optic neuropathy. Brain 1990, 113, 953–973. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Barnard, R.O.; Kwon, E.E.; Sharer, L.R.; Cho, E. Multiple sclerosis: Remyelination of nascent lesions: Remyelination of nascent lesions. Ann. Neurol. 1993, 33, 137–151. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Brück, W.; Rodriguez, M.; Lassmann, H. Distinct Patterns of Multiple Sclerosis Pathology Indicates Heterogeneity in Pathogenesis. Brain Pathol. 1996, 6, 259–274. [Google Scholar] [CrossRef]
- Hanafy, K.A.; Sloane, J.A. Regulation of remyelination in multiple sclerosis. FEBS Lett. 2011, 585, 3821–3828. [Google Scholar] [CrossRef]
- Wu, L.; Williams, A.; Delaney, A.; Sherman, D.; Brophy, P. Increasing Internodal Distance in Myelinated Nerves Accelerates Nerve Conduction to a Flat Maximum. Curr. Biol. 2012, 22, 1957–1961. [Google Scholar] [CrossRef]
- Cordano, C.; Sin, J.H.; Timmons, G.; Yiu, H.H.; Stebbins, K.; Guglielmetti, C.; Cruz-Herranz, A.; Xin, W.; Lorrain, D.; Chan, J.R.; et al. Validating visual evoked potentials as a preclinical, quantitative biomarker for remyelination efficacy. Brain 2022, 145, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Saridas, F.; Hojjati, F.; Alizada, S.; Lazrak, S.; Koc, E.R.; Turan, O.F. Effects of disease-modifying therapies on remyelination in multiple sclerosis; evaluation via visual evoked potential test. Mult. Scler. Relat. Disord. 2024, 91, 105850. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.L.; Garber, J.Y.; Klistorner, A.; Barnett, M.H. The electrophysiological assessment of visual function in Multiple Sclerosis. Clin. Neurophysiol. Pract. 2019, 4, 90–96. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Vidal-Jordana, A.; Rovira, A.; Arrambide, G.; Otero-Romero, S.; Río, J.; Comabella, M.; Nos, C.; Castilló, J.; Galan, I.; Cabello, S.; et al. Optic Nerve Topography in Multiple Sclerosis Diagnosis: The Utility of Visual Evoked Potentials. Neurology 2021, 96, e482–e490. [Google Scholar] [CrossRef] [PubMed]
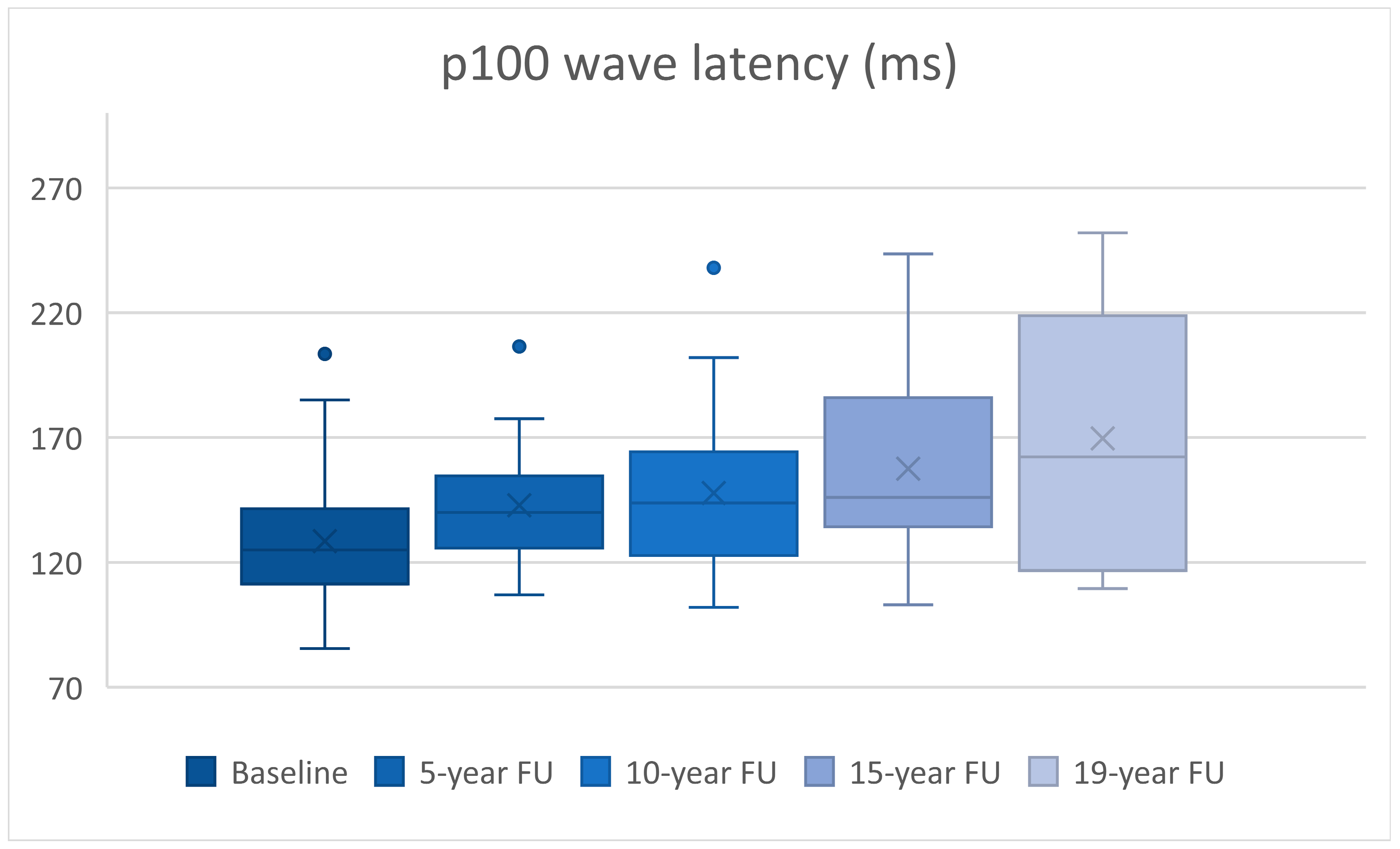

| n | Latency (ms) | p | Amplitude (mV) | p | |
|---|---|---|---|---|---|
| Baseline vs. 5-year FU | 30 | 14.35 ± 4.47 | p = 0.003 | 0.68 ± 0.43 | p = 0.123 |
| Baseline vs. 10-year FU | 30 | 19.26 ± 4.87 | p < 0.0005 | 2.29 ± 0.52 | p < 0.0005 |
| Baseline vs. 15-year FU | 24 | 31.39 ± 7.8 | p = 0.001 | 2.51 ± 0.6 | p < 0.0005 |
| Baseline vs. 19-year FU | 10 | 53.45 ± 18.42 | p = 0.018 | 4.06 ± 1.32 | p = 0.014 |
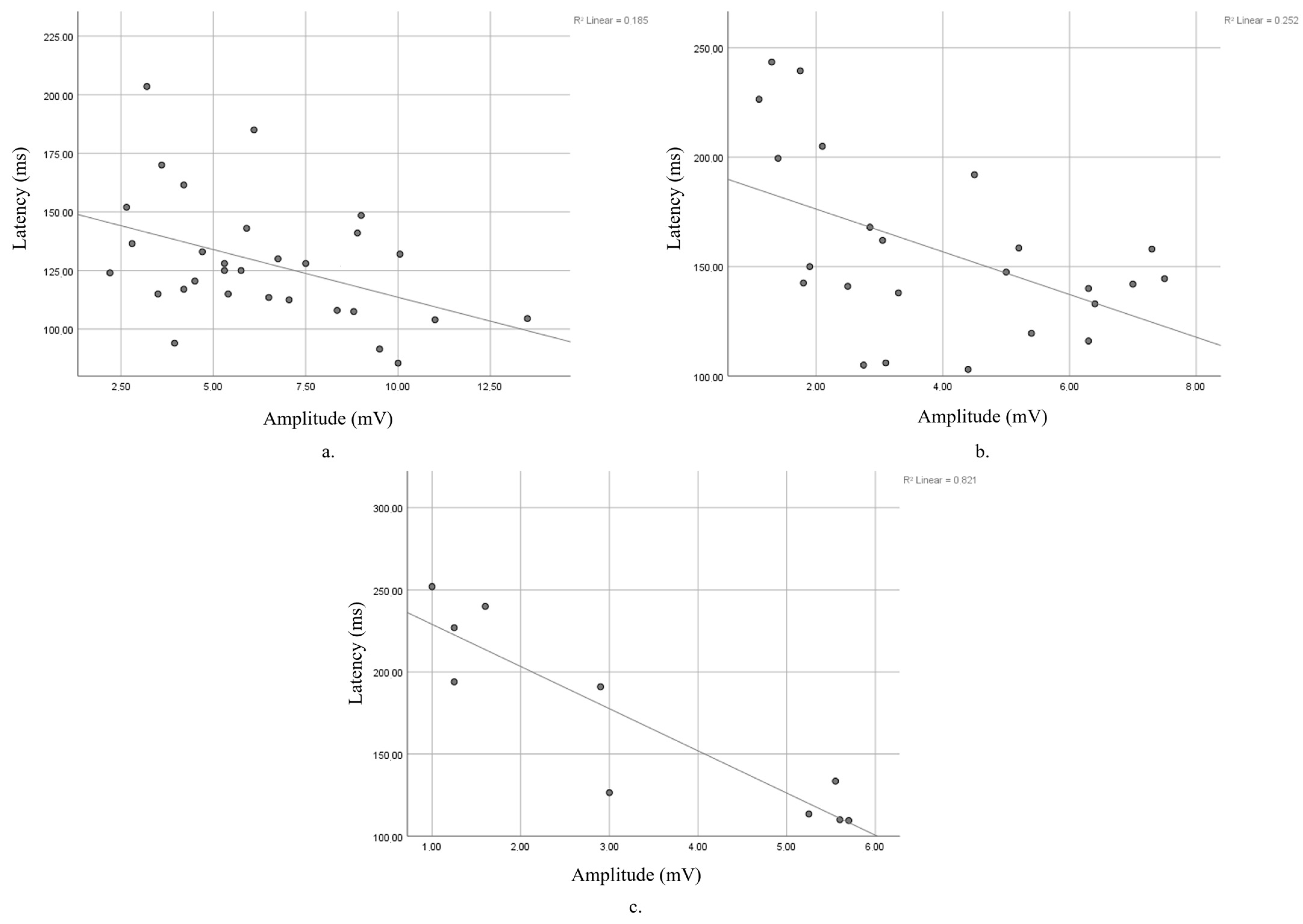
| n | r | p | |
|---|---|---|---|
| Baseline | 30 | −0.43 | p = 0.018 |
| 5-year FU | 30 | −0.225 | p = 0.232 |
| 10-year FU | 30 | −0.251 | p = 0.181 |
| 15-year FU | 24 | −0.502 | p = 0.012 |
| 19-year FU | 10 | −0.906 | p < 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciapă, M.A.; Donica, V.C.; Costea, C.F.; Bogdănici, C.M. Longitudinal Analysis of P100 Wave Amplitude and Latency in Multiple Sclerosis: A 19-Year Retrospective VEP Study. Diagnostics 2025, 15, 1189. https://doi.org/10.3390/diagnostics15101189
Ciapă MA, Donica VC, Costea CF, Bogdănici CM. Longitudinal Analysis of P100 Wave Amplitude and Latency in Multiple Sclerosis: A 19-Year Retrospective VEP Study. Diagnostics. 2025; 15(10):1189. https://doi.org/10.3390/diagnostics15101189
Chicago/Turabian StyleCiapă, Manuela Andreea, Vlad Constantin Donica, Claudia Florida Costea, and Camelia Margareta Bogdănici. 2025. "Longitudinal Analysis of P100 Wave Amplitude and Latency in Multiple Sclerosis: A 19-Year Retrospective VEP Study" Diagnostics 15, no. 10: 1189. https://doi.org/10.3390/diagnostics15101189
APA StyleCiapă, M. A., Donica, V. C., Costea, C. F., & Bogdănici, C. M. (2025). Longitudinal Analysis of P100 Wave Amplitude and Latency in Multiple Sclerosis: A 19-Year Retrospective VEP Study. Diagnostics, 15(10), 1189. https://doi.org/10.3390/diagnostics15101189







