Inflammatory Indices and Preterm Delivery: A New Horizon in Obstetric Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Inflammatory Indices
2.2.1. Systemic Inflammatory Response Index (SIRI)
2.2.2. Systemic Immune-Inflammation Index (SII)
2.3. Neutrophil/Lymphocyte Ratio (NLR)
2.4. Platelet/Lymphocyte Ratio (PLR)
2.5. Monocyte/Lymphocyte Ratio (MLR)
2.6. Statistical Analysis
2.7. Ethics Committee Approval
3. Results
3.1. Comparison of Preterm Delivery Group and Term Group
3.2. Comparison of Early Preterm, Middle Preterm, and Late Preterm Delivery Groups
3.3. Factors Affecting the Risk of Preterm Delivery
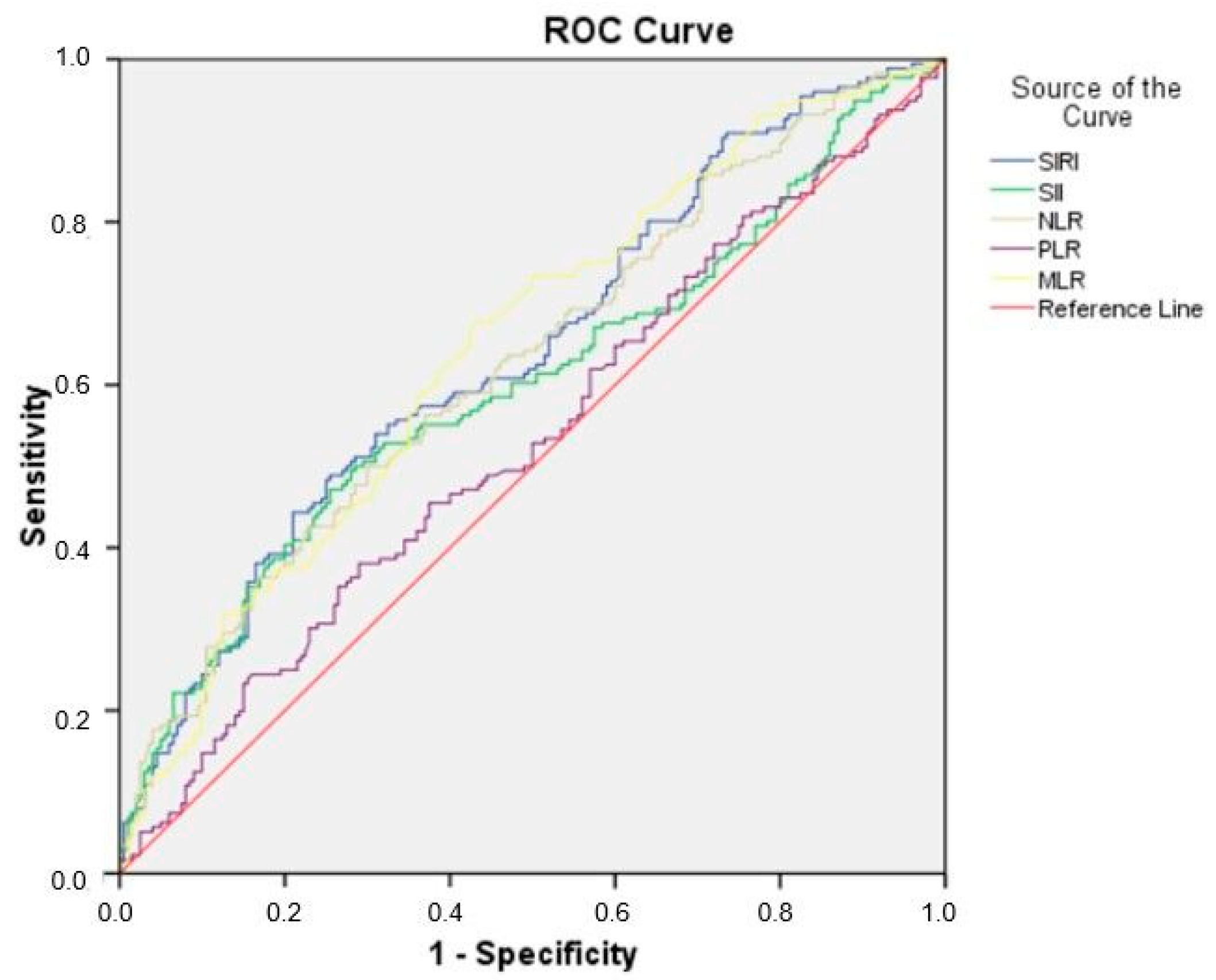
3.4. Receiver Operating Characteristic (ROC) Curve for SIRI, SII, NLR, PLR, and MLR in Predicting Preterm Delivery
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | C-Reactive Protein |
| FMF | Familial Mediterranean Fever |
| GDM | Gestational Diabetes Mellitus |
| IL-6 | Interleukin-6 |
| MLR | Monocyte/Lymphocyte Ratio |
| MMP-9 | Matrix Metalloproteinase-9 |
| NICU | Neonatal Intensive Care Unit |
| NLR | Neutrophil/Lymphocyte Ratio |
| PLR | Platelet/Lymphocyte Ratio |
| PPROM | Preterm Premature Rupture of Membranes |
| RDS | Respiratory Distress Syndrome |
| ROC | Receiver Operating Characteristic |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammatory Response Index |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Hrubaru, I.; Motoc, A.; Moise, M.L.; Miutescu, B.; Citu, I.M.; Pingilati, R.A.; Popescu, D.E.; Dumitru, C.; Gorun, F.; Olaru, F.; et al. The predictive role of maternal biological markers and inflammatory scores NLR, PLR, MLR, SII, and SIRI for the risk of preterm delivery. J. Clin. Med. 2022, 11, 6982. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Shima, Y.; Kato, M.; Ichikawa, T.; Ino, H.; Horii, Y.; Suzuki, S.; Morita, R. Inflammation in preterm birth: Novel mechanism of preterm birth associated with innate and acquired immunity. J. Reprod. Immunol. 2022, 154, 103748. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, Z.; Chi, P.; Alifu, X.; Zhuang, Y.; Si, S.; Qiu, Y.; Zhou, H.; Peng, Z.; Yu, Y. Association of systemic chronic inflammation during pregnancy in different periods and its trajectories with preterm birth. Am. J. Reprod. Immunol. 2024, 91, e13848. [Google Scholar] [CrossRef]
- Areia, A.L.; Mota-Pinto, A. Inflammation and preterm birth: A systematic review. Reprod. Med. 2022, 3, 101–111. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Wang, Y. Correlation of amniotic fluid inflammatory markers with preterm birth: A meta-analysis. J. Obstet. Gynaecol. 2024, 44, 2368764. [Google Scholar] [CrossRef]
- Matyas, M. Preterm Birth and Inflammation. In Current Topics in Caesarean Section; IntechOpen Limited: London, UK, 2021; p. 207. [Google Scholar] [CrossRef]
- Cakir, U.; Tayman, C. Evaluation of systemic inflammatory indices in the diagnosis of early onset neonatal sepsis in very low birth weight infants. J. Neonatal-Perinat. Med. 2024, 17, 169–176. [Google Scholar] [CrossRef]
- Farzaneh, F.; Ghobadi, S.; Absalan, A. The accuracy of selected hematology and inflammatory indices for predicting preterm labor; a cross-sectional study. Immunopathol. Persa 2019, 5, e05. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef]
- Dong, J.; Xue, H.; An, F.; Liu, Y.; Deng, W.; Gao, Q. Correlation between the neutrophil-to-lymphocyte ratio and clinicopathological parameters in epithelial ovarian cancer patients and its effect on prognosis—A retrospective cohort study. Gland. Surg. 2022, 11, 1367. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Kaplan, Z.S. Platelets are not just for clots. Transfus. Med. Rev. 2015, 29, 110–119. [Google Scholar] [CrossRef]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based Functional Taxonomy of the Preterm Birth Syndrome. Clin. Perinatol. 2024, 51, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Candiani, M.; Farina, A. Spontaneous Preterm Birth Phenotyping Based on Cervical Length and Immune-Mediated Factors. JAMA Netw. Open 2024, 7, e244559. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Salmeri, N.; Syngelaki, A.; Tan, M.Y.; Nicolaides, K.H. First trimester risk of preeclampsia and rate of spontaneous birth in patients without preeclampsia. Am. J. Obstet. Gynecol. 2024, 231, 452.e1–452.e7. [Google Scholar] [CrossRef] [PubMed]
- Küçükbaş, G.N.; Yavuz, A. Systemic immune inflammation indices: Novel predictors for preterm premature rupture of membranes and associated complications. J. Med. Palliat. Care 2023, 4, 516–523. [Google Scholar] [CrossRef]
- Akin, M.S.; Akyol, O.; Okman, E.; Yazici, A.; Sari, F.N.; Dizdar, E.A. Systemic Inflammatory Indices as Predictors of Lung Maturation in Preterm Infants Born Before 32 Weeks of Gestation. J. Pediatr. Intensive Care 2024. [Google Scholar] [CrossRef]
- Sahin, R.; Tanacan, A.; Serbetci, H.; Karagoz, B.; Agaoglu, Z.; Kara, O.; Sahin, D. First-trimester neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), and systemic immune-response index (SIRI) as predictors of composite adverse outcomes in pregnant women with Familial Mediterranean fever. Z. Geburtshilfe Neonatologie 2024, 228, 156–160. [Google Scholar] [CrossRef]
- Yildiz, E.G.; Tanacan, A.; Okutucu, G.; Bastemur, A.G.; Ipek, G.; Sahin, D. Can System Inflammation Response Index or Systemic Immune Inflammation Index predict gestational diabetes mellitus in the first trimester? A prospective observational study. Int. J. Gynecol. Obstet. 2024, 166, 837–843. [Google Scholar] [CrossRef]
- Ercan, A.; Firat, A. Impact of Complete Blood Count (CBC) Parameters in Preterm Birth Prediction in Cases with Threatened Preterm Labour (TPL). Clin. Exp. Obstet. Gynecol. 2024, 51, 180. [Google Scholar] [CrossRef]
- Demirdağ, E.; Arık, S.; Safarova, S.; Erdem, M.; Bozkurt, N.; Erdem, A. Is the First-Trimester Systemic Immune-Inflammation Index Associated with Preeclampsia? Cureus 2023, 15, e44063. [Google Scholar] [CrossRef]
- Yuce, E. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) can predict spontaneous preterm birth? J. Inflamm. Res. 2023, 16, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Ozel, A.; Alici Davutoglu, E.; Yurtkal, A.; Madazli, R. How do platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio change in women with preterm premature rupture of membranes, and threaten preterm labour? J. Obstet. Gynaecol. 2020, 40, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Balciuniene, G.; Kvederaite-Budre, G.; Gulbiniene, V.; Dumalakiene, I.; Viliene, R.; Pilypiene, I.; Drasutiene, G.S.; Ramasauskaite, D. Neutrophil–lymphocyte ratio for the prediction of histological chorioamnionitis in cases of preterm premature rupture of membranes: A case-control study. BMC Pregnancy Childbirth 2021, 21, 656. [Google Scholar] [CrossRef] [PubMed]
- Chandavar, D.S.M.; Minal, J.; Rashmi, K. Evaluation of the Predictive Role of Neutrophil Lymphocyte Ratio and Mean Platelet Volume in Preterm Deliveries: A Cross-sectional Study. J. Clin. Diagn. Res. 2024, 18, EC29–EC33. [Google Scholar] [CrossRef]
- Xu, R.; Wu, B.; Chen, J.; Yan, J. Changes in platelet and leukocyte parameters during pregnancy in women with spontaneous pre-term delivery. Investig. Clín. 2020, 61, 28–38. [Google Scholar] [CrossRef]
- Izzah, M.N.; Sumawan, H.; Achmad, E.D. Neutrophil-Lymphocyte Ratio (NLR) and Platelet-Lymphocyte Ratio (PLR) as Inflammatory Markers in Preterm Birth. Indones. J. Obstet. Gynecol. Sci. 2024, 7, 182–188. [Google Scholar] [CrossRef]
- Sultana, N.; Karim, F.; Rahman, M.K. Prediction of preterm premature membrane rupture by the platelet-lymphocyte ratio. Int. J. Reprod. Contracept. Obstet. Gynecol. 2023, 12, 3353–3358. [Google Scholar] [CrossRef]
- Seyhanli, Z.; Bayraktar, B.; Cakir, B.T.; Bucak, M.; Karabay, G.; Aktemur, G.; Yigit, A.; Yucel, K.Y.; Yılmaz, Z.V. The Efficacy of C-Reactive Protein (CRP) to Albumin Ratio (CAR) and Fibrinogen to CRP Ratio (FCR) in Predicting the Latent Period of Preterm Labor. Am. J. Reprod. Immunol. 2024, 92, e13899. [Google Scholar] [CrossRef]
- Khatoon, F.; Gupta, H.; Sinha, P.; Tiwari, K.; Singh, A. Prediction of preterm birth on the basis of complete blood count parameters. J. S. Asian Fed. Obstet. Gynaecol. 2020, 12, 289. [Google Scholar] [CrossRef]
- Eriç Horasanlı, J.; Alp, E.C.; Bülbül, R. Evaluation of complete blood cell count parameters in the diagnosis of threatened preterm labor and premature rupture of membranes. Dubai Med. J. 2022, 5, 157–162. [Google Scholar] [CrossRef]
- Baki Yıldırım, S.; Bezirganoglu Altuntas, N.; Bayoglu Tekin, Y. Monocyte-to-lymphocyte ratio in the early second trimester is a predictor of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2024, 37, 2371979. [Google Scholar] [CrossRef]
- Perelli, F.; Vidiri, A.; Palomba, G.; Franco, R.; Gallitelli, V.; Parasiliti, M.; Bisanti, M.; Spanò, A.; Silvagni, A.; Lopez, A.; et al. Preterm Birth and SARS-CoV-2: Does a Correlation Exist? Biomedicines 2025, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Riva, A.; Coldebella, D.; Cavaliere, A.F.; Signore, F.; Buzzaccarini, G.; Spagnol, G.; Laganà, A.S.; et al. Congenital Zika Syndrome: Genetic Avenues for Diagnosis and Therapy, Possible Management and Long-Term Outcomes. J. Clin. Med. 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Maranto, M.; Zaami, S.; Restivo, V.; Termini, D.; Gangemi, A.; Tumminello, M.; Culmone, S.; Billone, V.; Cucinella, G.; Gullo, G. Symptomatic COVID-19 in Pregnancy: Hospital Cohort Data between May 2020 and April 2021, Risk Factors and Medicolegal Implications. Diagnostics 2023, 13, 1009. [Google Scholar] [CrossRef]
- Gullo, G.; Lopez, A.; Loreto, C.; Cucinella, G.; La Verde, M.; Andrisani, A.; Burgio, S.; Carotenuto, R.; Ganduscio, S.; Baglio, G.; et al. COVID-19 and Female Fertility: An Observational Prospective Multicenter Cohort Study: Upholding Reproductive Rights in Emergency Circumstances. Diagnostics 2024, 14, 2118. [Google Scholar] [CrossRef] [PubMed]
| Preterm Group (n = 528) | Term Group (n = 600) | p | |
|---|---|---|---|
| Age (years) (Median (Min-Max)) | 33.5 (19–47) | 33.5 (23–53) | 0.385 |
| Gravida (Median (Min-Max)) | 1 (1–6) | 2 (1–6) | 0.031 |
| Parity (Median (Min-Max)) | 0 (0–4) | 1 (0–4) | <0.001 |
| Miscarriage (Median (Min-Max)) | 0 (0–3) | 0 (0–3) | <0.001 |
| Smoking (n, %) | 195 (%36.9) | 81 (%13.5) | <0.001 |
| Employment Status (n, %) | 195 (%36.9) | 210 (%35) | 0.500 |
| Education (n, %) | |||
| Primary school | 144 (%27.3) | 69 (%11.5) | <0.001 |
| High school | 315 (%59.7) | 480 (%80) | <0.001 |
| College/University | 69 (%13.1) | 51 (%8.5) | <0.001 |
| Yearly income level (n, %) | |||
| Low | 27 (%5.1) | 42 (%7) | <0.001 |
| Medium | 132 (%25) | 480 (%80) | <0.001 |
| High | 369 (%69.9) | 78 (%13) | <0.001 |
| Hospitalization Week (Median (Min-Max) | 34 (27–36) | 39.5 (37–42) | <0.001 |
| 1st minute APGAR score (Median (Min-Max)) | 6 (2–7) | 8 (7–9) | <0.001 |
| 5th minute APGAR score (Median (Min-Max)) | 8 (5–9) | 9 (8–10) | <0.001 |
| NICU (n, %) | 189 (%35.8) | 24 (%4) | <0.001 |
| Duration of NICU Admission (Median (Min-Max)) | 0 (0–28) | 0 (0–3) | <0.001 |
| SIRI (Median (Min-Max)) | |||
| 1st trimester | 1.42 (0–16) | 1.44 (0.10–26.58) | 0.078 |
| Hospitalization Time | 2.15 (0–16.45) | 1.62 (0.01–20.38) | <0.001 |
| SII (Median (Min-Max)) | |||
| 1st trimester | 898.65 (73.31–5067) | 786.25 (17.5–4398.7) | 0.001 |
| Hospitalization Time | 917.09 (86.18–3990) | 732.17 (15.91–2944) | <0.001 |
| NLR (Median (Min-Max)) | |||
| 1st trimester | 4.15 (0.47–21.45) | 3.58 (0.78–24.85) | <0.001 |
| Hospitalization Time | 3.83 (0.49–15.06) | 3.19 (0.08–12.29) | <0.001 |
| PLR (Median (Min-Max)) | |||
| 1st trimester | 130.19 (28.97–523.3) | 140 (4.5–655.5) | 0.019 |
| Hospitalization Time | 126.28 (31.56–417.5) | 125.37 (8.26–328.07) | 0.057 |
| MLR (Median (Min-Max)) | |||
| 1st trimester | 0.22 (0–1) | 0.22 (0.03–2.19) | 0.076 |
| Hospitalization Time | 0.307 (0–1) | 0.245 (0.06–1.64) | <0.001 |
| Early Preterm (n = 78) | Middle Preterm (n = 111) | Late Preterm (n = 339) | p | |
|---|---|---|---|---|
| Age (years) (Median (Min-Max)) | 34 (24–44) | 34 (23–46) | 33 (19–47) | 0.667 |
| Gravida (Median (Min-Max)) | 1 (1–4) | 1 (1–5) | 1 (1–6) | 0.597 |
| Parity (Median (Min-Max)) | 0 (0–3) | 0 (0–3) | 0 (0–4) | 0.935 |
| Miscarriage (Median (Min-Max)) | 0 (0–1) | 0 (0–3) | 0 (0–2) | 0.655 |
| Smoking (n, %) | 21 (%26.9) | 36 (%32.4) | 138 (%40.7) | 0.041 |
| Employment Status (n, %) | 24 (%30.8) | 27 (%24.3) | 144 (%42.5) | 0.001 |
| Education (n, %) | ||||
| Primary school | 18 (%23.1) | 12 (%10.8) | 114 (%33.6) | <0.001 |
| High school | 51 (%65.4) | 75 (%67.6) | 189 (%55.8) | <0.001 |
| College/University | 9 (%11.5) | 24 (%21.6) | 36 (%10.6) | <0.001 |
| Yearly income level (n, %) | ||||
| Low | 0 (%0) | 12 (%10.8) | 15 (%4.4) | <0.001 |
| Medium | 15 (%19.2) | 9 (%8.1) | 108 (%31.9) | <0.001 |
| High | 63 (%80.8) | 90 (%81.1) | 216 (%63.7) | <0.001 |
| Hospitalization Week (Median (Min-Max)) | 30 (27–31) | 32 (32–33) | 35 (34–36) | <0.001 |
| 1st minute APGAR score (Median (Min-Max)) | 2 (2–3) | 5 (4–6) | 6 (6–7) | <0.001 |
| 5th minute APGAR score (Median (Min-Max)) | 6 (5–7) | 7 (6–8) | 8 (8–9) | <0.001 |
| NICU (n, %) | 78 (%100) | 90 (%81.1) | 21 (%6.2) | <0.001 |
| Duration of NICU Admission (Median (Min-Max)) | 15 (7–28) | 7 (0–7) | 0 (0–10) | <0.001 |
| SIRI (Median (Min-Max)) | ||||
| 1st trimester | 5.72 (4.07–16) | 2.76 (1.87–4.39) | 0.94 (0–2.3) | <0.001 |
| Hospitalization Time | 6.89 (5.24–16.45) | 3.5 (3.01–4.93) | 1.45 (0–2.96) | <0.001 |
| SII (Median (Min-Max)) | ||||
| 1st trimester | 2035 (808–4089) | 1212 (640–3456) | 625.9 (73.3–5067) | <0.001 |
| Hospitalization Time | 1930 (855–3262) | 1225 (680–3217) | 641 (86–3990) | <0.001 |
| NLR (Median (Min-Max)) | ||||
| 1st trimester | 10.2 (4.57–21.45) | 5.94 (3.02–10.85) | 3.37 (0.479–20.6) | <0.001 |
| Hospitalization Time | 9.1 (4.37–15.06) | 5.20 (2.92–8.67) | 3.11 (0.49–15) | <0.001 |
| PLR (Median (Min-Max)) | ||||
| 1st trimester | 171.8 (63.6–426) | 140 (61.53–320) | 121.8 (28.9–523.3) | <0.001 |
| Hospitalization Time | 158 (65.7–332.85) | 134.46 (64.16–292) | 118.5 (31.5–417.5) | <0.001 |
| MLR (Median (Min-Max)) | ||||
| 1st trimester | 0.54 (0.29–1) | 0.32 (0.17–0.69) | 0.16 (0–0.38) | <0.001 |
| Hospitalization Time | 0.59 (0.34–1) | 0.40 (0.26–0.75) | 0.25 (0–0.46) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| aOR | 95% C.I | p | aOR | 95% C.I | p | |
| Age (years) (Median (Min-Max)) | 1.009 | 0.989–1.030 | 0.388 | 1.015 | 0.990–1.039 | 0.241 |
| Gravida (Median (Min-Max)) | 0.941 | 0.843–1.050 | 0.276 | 1.566 | 1.043–2.351 | 0.031 |
| Parity (Median (Min-Max)) | 0.795 | 0.700–0.903 | <0.001 | 0.553 | 0.365–0.837 | 0.005 |
| Miscarriage (Median (Min-Max)) | 1.586 | 1.166–2.158 | 0.003 | 1.439 | 0.858–2.415 | 0.168 |
| Smoking (n, %) | 0.267 | 0.199–0.357 | <0.001 | 0.246 | 0.177–0.342 | <0.001 |
| Employment Status (n, %) | 1.088 | 0.852–1.388 | 0.500 | 0.951 | 0.714–1.268 | 0.733 |
| 1st trimester (Median (Min-Max)) | ||||||
| SIRI | 1.057 | 1.003–1.114 | 0.038 | 1.345 | 1.059–1.708 | 0.015 |
| SII | 1.0 | 1.000–1.001 | <0.001 | 1.0 | 0.999–1.001 | 0.776 |
| NLR | 1.119 | 1.077–1.163 | <0.001 | 1.383 | 1.249–1.532 | <0.001 |
| PLR | 0.998 | 0.997–1.0 | 0.066 | 0.993 | 0.989–0.997 | 0.002 |
| MLR | 0.586 | 0.309–1.112 | 0.102 | 0.001 | 0.000–0.008 | <0.001 |
| Variables | AUC | S.E. | p | OR (95% CI) | Cutt-Off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| SIRI | 0.639 | 0.016 | <0.001 | 0.607–0.671 | 1.78 | 59.1% | 59% |
| SII | 0.599 | 0.017 | <0.001 | 0.566–0.633 | 783.48 | 56.8% | 57% |
| NLR | 0.628 | 0.017 | <0.001 | 0.596–0.660 | 3.44 | 58.5% | 58.5% |
| PLR | 0.533 | 0.017 | 0.057 | 0.499–0.567 | 126.02 | 50% | 50% |
| MLR | 0.645 | 0.016 | <0.001 | 0.613–0.677 | 0.27 | 61.4% | 61.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kırat, S. Inflammatory Indices and Preterm Delivery: A New Horizon in Obstetric Risk Assessment. Diagnostics 2025, 15, 1188. https://doi.org/10.3390/diagnostics15101188
Kırat S. Inflammatory Indices and Preterm Delivery: A New Horizon in Obstetric Risk Assessment. Diagnostics. 2025; 15(10):1188. https://doi.org/10.3390/diagnostics15101188
Chicago/Turabian StyleKırat, Samet. 2025. "Inflammatory Indices and Preterm Delivery: A New Horizon in Obstetric Risk Assessment" Diagnostics 15, no. 10: 1188. https://doi.org/10.3390/diagnostics15101188
APA StyleKırat, S. (2025). Inflammatory Indices and Preterm Delivery: A New Horizon in Obstetric Risk Assessment. Diagnostics, 15(10), 1188. https://doi.org/10.3390/diagnostics15101188





