Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes
Abstract
:1. Introduction
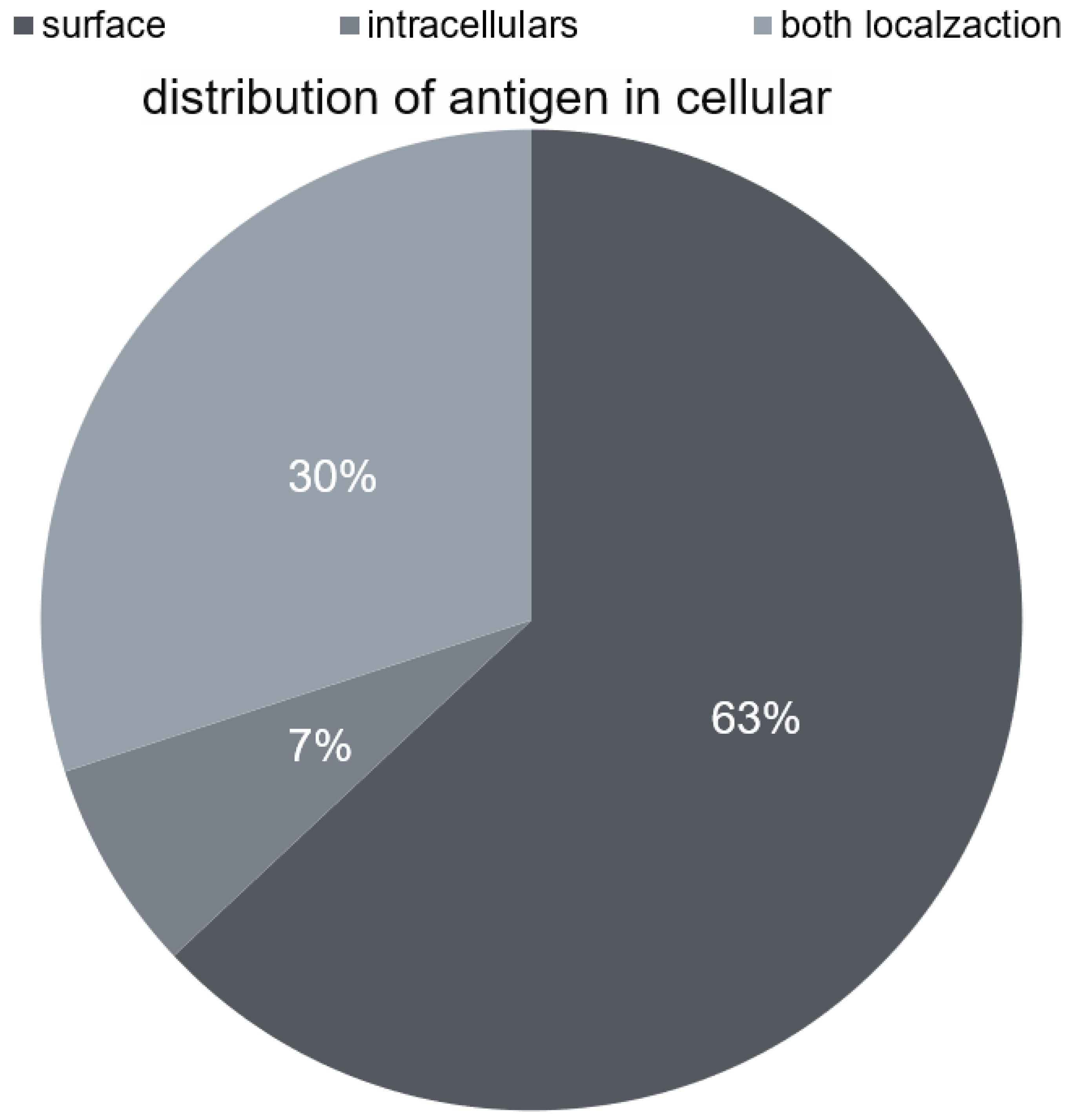
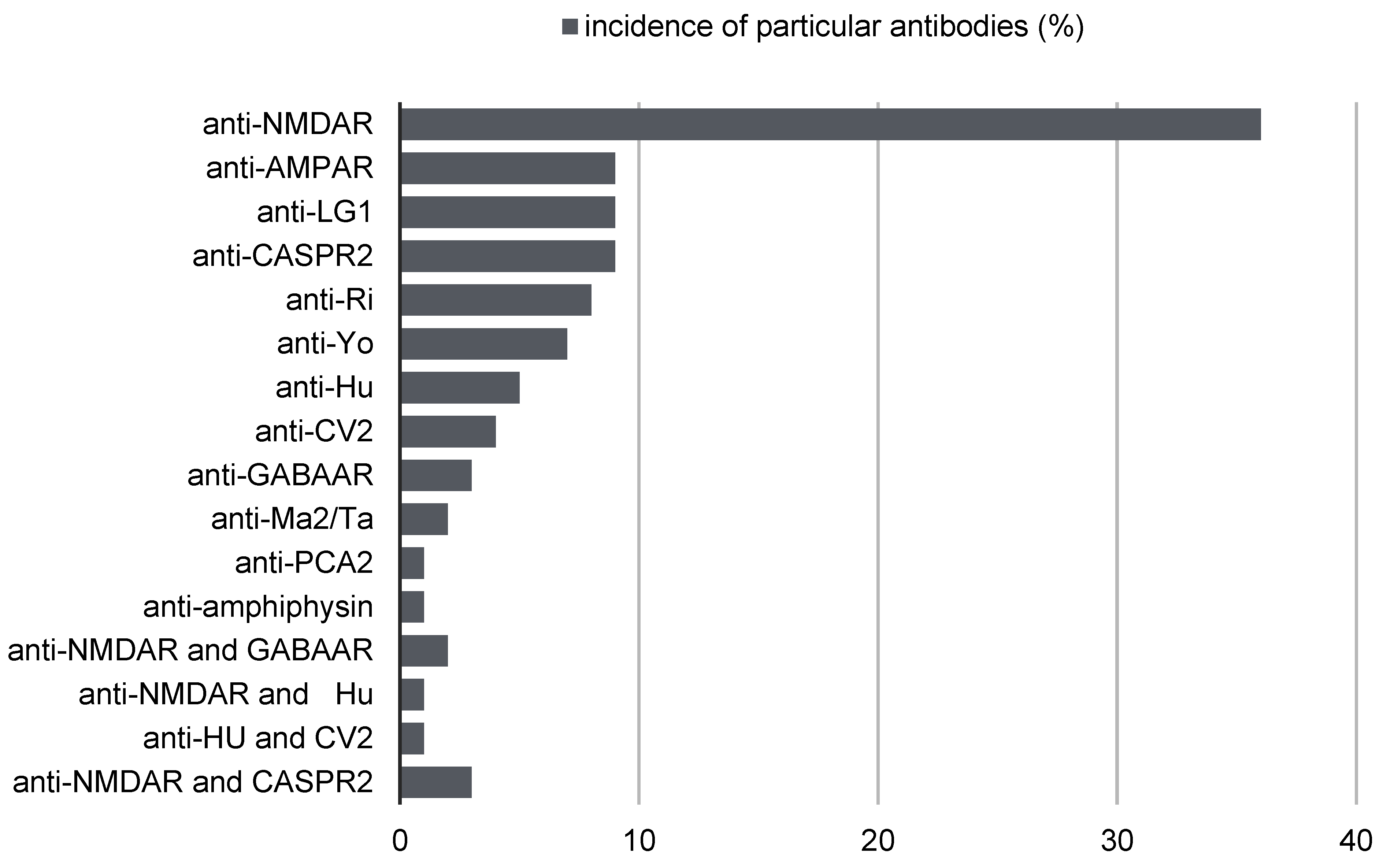
2. Antibodies in the Course of Autoimmune Encephalitis
2.1. Autoimmune Encephalitis with Anti-NMDAR Antibodies
2.2. Autoimmune Encephalitis with Anti-AMPAR Antibodies
2.3. Autoimmune Encephalitis with Anti-GQ1b, Anti-DPPX, Anti-CASPR2, and Anti-LGI1 Antibodies
3. Autoimmune Encephalitis and Psychosis
4. Relationship between Autoimmune Encephalitis and Neoplastic Diseases and Paraneoplastic Syndromes
- Lung cancer, with an incidence of 50% of patients with autoimmune encephalitis, of which 40% are small cell lung cancer, 10% are non-small cell lung cancer;
- Testicular tumors, with an incidence of 20% of patients with autoimmune encephalitis;
- Breast cancer, with an incidence of 8% of patients with autoimmune encephalitis;
- Ovarian teratoma, with an incidence of 4% of patients with autoimmune encephalitis;
- Hodgkin’s disease, with an incidence of 4% of patients with autoimmune encephalitis;
- Thymus, with an incidence of 2% of patients with autoimmune encephalitis;
- Other cancers, with an incidence of 8% of patients with autoimmune encephalitis.
5. Diagnostics of Autoimmune Encephalitis in Adults and Children
6. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations:
| MRI | magnetic resonance imaging |
| EEG | electroencephalography |
| FDG-PET | fluorodeoxyglucose-positron emission tomography |
| CT | computed tomography |
References
- Gałecki, P.; Szulc, A. Psychiatry; Edra Urban & Partner: Wroclaw, Poland, 2018; p. 159. [Google Scholar]
- Gałecki, P. Mental State Examination, ICD-11 Criteria; Edra Urban & Partner: Wroclaw, Poland, 2022; pp. 63–88. [Google Scholar]
- Granerod, J.; Ambrose, H.E.; Davies, N.W.; Clewley, J.P.; Walsh, A.L.; Morgan, D.; Cunningham, R.; Zuckerman, M.; Mutton, K.J.; Solomon, T.; et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010, 10, 835–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alloza, C.; Blesa-Cábez, M.; Bastin, M.E.; Madole, J.W.; Buchanan, C.R.; Janssen, J.; Gibson, J.; Deary, I.J.; Tucker-Drob, E.M.; Whalley, H.C.; et al. Psychotic-like experiences, polygenic risk scores for schizophrenia, and structural properties of the salience, default mode, and central-executive networks in healthy participants from UK Biobank. Transl. Psychiatry 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Julayanont, P.; Suryadevara, U. Psychosis. Contin. (Minneap. Minn.) 2021, 27, 1682–1711. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Graus, F. Antibody-Mediated encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Zuliani, L.; Graus, F.; Giometto, B.; Bien, C.; Vincent, A. Central nervous system neuronal surface antibody associated syndromes: Review and guidelines for recognition. J. Neurol. Neurosurg. Psychiatry 2012, 83, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Newman, M.P.; Blum, S.; Wong, R.C.; Scott, J.G.; Prain, K.; Wilson, R.J.; Gillis, D. Autoimmune encephalitis. Intern. Med. J. 2016, 46, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, S.; Al-Diwani, A.; Waters, P.; Irani, S.R. The autoantibody-mediated encephalitides: From clinical observations to molecular pathogenesis. J. Neurol. 2021, 268, 1689–1707. [Google Scholar] [CrossRef] [Green Version]
- Wandinger, K.P.; Saschenbrecker, S.; Stoecker, W.; Dalmau, J. Anti-NMDA-receptor encephalitis: A severe, multistage, treatable disorder presenting with psychosis. J. Neuroimmunol. 2011, 231, 86–91. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Tüzün, E.; Wu, H.Y.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Lancaster, E.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011, 10, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.; Hughes, E.G.; Peng, X.; Zhou, L.; Gleichman, A.J.; Shu, H.; Matà, S.; Kremens, D.; Vitaliani, R.; Geschwind, M.D.; et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 2009, 65, 424–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Xie, Y.; Hu, Z.; Tang, X. Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Res. 2020, 1727, 146549. [Google Scholar] [CrossRef] [PubMed]
- Al-Diwani, A.; Handel, A.; Townsend, L.; Pollak, T.; Leite, M.I.; Harrison, P.J.; Lennox, B.R.; Okai, D.; Manohar, S.G.; Irani, S.R. The psychopathology of NMDAR-antibody encephalitis in adults: A systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry 2019, 6, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varley, J.A.; Andersson, M.; Grant, E.; Berretta, A.; Zandi, M.S.; Bondet, V.; Duffy, D.; Hunt, D.; Piehl, F.; Waters, P.; et al. Absence of neuronal autoantibodies in neuropsychiatric systemic lupus erythematosus. Ann. Neurol. 2020, 88, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Tseng, J.R.; Wu, C.L.; Su, F.C.; Weng, W.C.; Hsu, C.C.; Chang, K.H.; Wu, C.F.; Hsiao, I.T.; Lin, C.P. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: Case report and literature review. Brain Behav. 2020, 10, e01540. [Google Scholar] [CrossRef] [Green Version]
- Omi, T.; Kinoshita, M.; Nishikawa, A.; Tomioka, T.; Ohmori, K.; Fukada, K.; Matsunaga, H. Clinical relapse of anti-AMPAR encephalitis associated with recurrence of thymoma. Intern. Med. 2018, 57, 1011–1013. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Li, K.; Li, J.; Liu, X.; Wu, X.; Xu, X.; Xiong, K.; Chen, X.; Zhang, Y. Thymoma-associated autoimmune encephalitis: Analysis of factors determining prognosis. CNS Neurosci. Ther. 2023, 29, 1213–1221. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Cai, M.T.; Zheng, Y.; Lai, Q.L.; Shen, C.H.; Qiao, S.; Zhang, Y.X. Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor encephalitis: A review. Front. Immunol. 2021, 12, 652820. [Google Scholar] [CrossRef]
- McCombe, J.A.; Zivelonghi, C.; Vorasoot, N.; Majed, M.; Flanagan, E.P.; Dubey, D.; Pittock, S.J.; McKeon, A.; Zekeridou, A. AMPAR autoimmunity: Neurological and oncological accompaniments and co-existing neural autoantibodies. J. Neuroimmunol. 2023, 375, 578012. [Google Scholar] [CrossRef]
- Li, X.; Mao, Y.T.; Wu, J.J.; Li, L.X.; Chen, X.J. Anti-AMPA receptor encephalitis associated with thymomatous myasthenia gravis. J. Neuroimmunol. 2015, 281, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.; Kerschen, P.; Zekeridou, A.; Desestret, V.; Rogemond, V.; Chaffois, M.O.; Ducray, F.; Larrue, V.; Daubail, B.; Idbaih, A.; et al. Clinical Spectrum of Encephalitis Associated with Antibodies Against the α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor: Case Series and Review of the Literature. JAMA Neurol. 2015, 72, 1163–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spatola, M.; Stojanova, V.; Prior, J.O.; Dalmau, J.; Rossetti, A.O. Serial brain 18FDG-PET in anti-AMPA receptor limbic encephalitis. J. Neuroimmunol. 2014, 271, 53–55. [Google Scholar] [CrossRef]
- Shahrizaila, N.; Yuki, N. Bickerstaff brainstem encephalitis and Fisher syndrome: Anti-GQ1b antibody syndrome. J Neurol. Neurosurg. Psychiatry 2013, 84, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Kuwahara, M.; Morikawa, M.; Kusunoki, S. Bickerstaff brainstem encephalitis with or without anti-GQ1b antibody. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e889. [Google Scholar] [CrossRef]
- Piepgras, J.; Höltje, M.; Michel, K.; Li, Q.; Otto, C.; Drenckhahn, C.; Probst, C.; Schemann, M.; Jarius, S.; Stöcker, W.; et al. Anti-DPPX encephalitis: Pathogenic effects of antibodies on gut and brain neurons. Neurology 2015, 85, 890–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Fu, P.C.; Li, Z.J. Clinical and imaging analysis to evaluate the response of patients with anti-DPPX encephalitis to immunotherapy. BMC Neurol. 2022, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Seery, N.; Butzkueven, H.; O’Brien, T.J.; Monif, M. Rare antibody-mediated and seronegative autoimmune encephalitis: An update. Autoimmun. Rev. 2022, 21, 103118. [Google Scholar] [CrossRef]
- Seery, N.; Butzkueven, H.; O’Brien, T.J.; Monif, M. Contemporary advances in antibody-mediated encephalitis: Anti-LGI1 and anti-Caspr2 antibody (Ab)-mediated encephalitides. Autoimmun. Rev. 2022, 21, 103074. [Google Scholar] [CrossRef]
- Ghimire, P.; Khanal, U.P.; Gajurel, B.P.; Karn, R.; Rajbhandari, R.; Paudel, S.; Gautam, N.; Ojha, R. Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: A systematic review. Brain Behav. 2020, 10, e01793. [Google Scholar] [CrossRef]
- Guo, Y.P.; Li, X.Y.; Liu, H.F.; Zhang, M.; Shi, L.; Zhao, X.J.; Li, J.Z.; Liu, X.Y.; Cui, J. Clinical analysis of 7 cases with anti-Caspr2 antibody-associated autoimmune encephalitis. Zhonghua Yi Xue Za Zhi 2020, 100, 513–515. [Google Scholar]
- Van Sonderen, A.; Petit-Pedrol, M.; Dalmau, J.; Titulaer, M.J. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 2017, 13, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Leypoldt, F.; Bechter, K.; Hasan, A.; Steiner, J.; Domschke, K.; Wandinger, K.P.; Falkai, P.; Arolt, V.; Stich, O.; et al. Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: Clinical symptomatology, pathophysiology, dignostic ap- proach, and therapeutic considerations. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 803–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, M.; Goodfellow, J.; Oto, M.; Krishnadas, R. Anti-NMDAR encephalitis for psychiatrists: The essentials. BJPsych Bull. 2021, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, S.H.; Rosenfeld, M.R.; Voltz, R.; Eichen, J.; Posner, J.B.; Dalmau, J. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000, 123, 1481–1494. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, B.; Arvidsson, A.; Bergkvist, M.; Nilsson, P. Limbic encephalitis in a neuroscientist: CASPR 2 antibody-associated disease after antigen exposure. J. Neuroimmunol. 2020, 343, 577231. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Armangué, T.; Glaser, C.; Iizuka, T.; Honig, L.S.; Benseler, S.M.; Kawachi, I.; Martinez-Hernandez, E.; et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013, 12, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Kayser, M.S.; Dalmau, J. Anti-NMDA Receptor Encephalitis in Psychiatry. Curr. Psychiatry Rev. 2011, 7, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef] [Green Version]
- Kayser, M.S.; Titulaer, M.J.; Gresa-Arribas, N.; Dalmau, J. Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013, 70, 1133–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejuste, F.; Thomas, L.; Picard, G.; Desestret, V.; Ducray, F.; Rogemond, V.; Psimaras, D.; Antoine, J.C.; Delattre, J.Y.; Groc, L.; et al. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol. Neuroimmunol Neuroinflamm. 2016, 3, e280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruijn, M.A.A.M.; van Sonderen, A.; van Coevorden-Hameete, M.H.; Bastiaansen, A.E.M.; Schreurs, M.W.J.; Rouhl, R.P.W.; van Donselaar, C.A.; Majoie, M.H.J.M.; Neuteboom, R.F.; Sillevis Smitt, P.A.E.; et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology 2019, 92, e2185–e2196. [Google Scholar] [CrossRef] [Green Version]
- Sansing, L.H.; Tüzün, E.; Ko, M.W.; Baccon, J.; Lynch, D.R.; Dalmau, J. A patient with encephalitis associated with NMDA receptor antibodies. Nat. Clin. Pract. Neurol. 2007, 3, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Hernandez, E.; Horvath, J.; Shiloh-Malawsky, Y.; Sangha, N.; Martinez-Lage, M.; Dalmau, J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology 2011, 77, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Fujita, H.; Shioda, M.; Suzuki, K. Anti-LGI1 encephalitis recurring 3 years after the first episode: A case report. BMC Neurol. 2022, 22, 148. [Google Scholar] [CrossRef]
- Irani, S.R.; Bera, K.; Waters, P.; Zuliani, L.; Maxwell, S.; Zandi, M.S.; Friese, M.A.; Galea, I.; Kullmann, D.M.; Beeson, D.; et al. N-methyl-D-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010, 133 Pt 6, 1655–1667. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, T.; Yoshii, S.; Kan, S.; Hamada, J.; Dalmau, J.; Sakai, F.; Mochizuki, H. Reversible brain atrophy in anti-NMDA receptor encephalitis: A long-term observational study. J. Neurol. 2010, 257, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Mocellin, R.; Walterfang, M.; Velakoulis, D. Hashimoto’s encephalopathy: Epidemiology, pathogenesis and management. CNS Drugs 2007, 21, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P. An update on the pathogenesis of Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef]
- Tzakas, P.; Sit, S.W. Progressive impairment of cognition and motor function: Hashimoto encephalopathy. CMAJ 2011, 183, E495–E497. [Google Scholar] [CrossRef]
- Kishitani, T.; Matsunaga, A.; Ikawa, M.; Hayashi, K.; Yamamura, O.; Hamano, T.; Watanabe, O.; Watanabe, O.; Nakamoto, Y.; Yoneda, M. Limbic encephalitis associated with anti-NH2-terminal of α-enolase antibodies: A clinical subtype of Hashimoto encephalopathy. Medicine 2017, 96, e6181. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Ejma, M. Hashimoto Encephalopathy-Still More Questions than Answers. Cells 2022, 11, 2873. [Google Scholar] [CrossRef]
- Abboud, H. Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Cellucci, T. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knurowska, A.; Zawadzka, M.; Anuszkiewicz, K.; Stogowski, P.; Mazurkiewicz-Beldzińska, M. Autoimmune encephalitis with anti-NMDA antibodies in the pediatric population—Literature review and case report. Pol. Przegl. Neurol. 2020, 16, 239–243. [Google Scholar]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.C.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1014. [Google Scholar] [CrossRef]
- Bacchi, S.; Franke, K.; Wewegama, D.; Needham, E.; Patel, S.; Menon, D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: A systematic review. J. Clin. Neurosci. 2018, 52, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Suh-Lailam, B.B.; Haven, T.R.; Copple, S.S.; Knapp, D.; Jaskowski, T.D.; Tebo, A.E. Anti-NMDA-receptor antibody encephalitis: Performance evaluation and laboratory experience with the anti-NMDA-receptor IgG assay. Clin. Chim. Acta 2013, 421, 1–6. [Google Scholar] [CrossRef]
- Armangue, T.; Leypoldt, F.; Málaga, I.; Raspall-Chaure, M.; Marti, I.; Nichter, C.; Pugh, J.; Vicente-Rasoamalala, M.; Lafuente-Hidalgo, M.; Macaya, A.; et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann. Neurol. 2014, 75, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, F.; Prüss, H.; Mengel, A.M.; Kohler, S.; Nümann, A.; Köhnlein, M.; Ruprecht, K.; Alexander, T.; Hiepe, F.; Meisel, A. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology 2017, 88, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Gable, M.S.; Sheriff, H.; Dalmau, J.; Tilley, D.H.; Glaser, C.A. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin. Infect. Dis. 2012, 54, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmau, J.; Armangué, T.; Planagumà, J.; Radosevic, M.; Mannara, F.; Leypoldt, F.; Geis, C.; Lancaster, E.; Titulaer, M.J.; Rosenfeld, M.R.; et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braczkowski, M.; Soszyński, D.; Sierakowska, A.; Braczkowski, R.; Kufel, K.; Łabuz-Roszak, B. Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes. Diagnostics 2023, 13, 2589. https://doi.org/10.3390/diagnostics13152589
Braczkowski M, Soszyński D, Sierakowska A, Braczkowski R, Kufel K, Łabuz-Roszak B. Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes. Diagnostics. 2023; 13(15):2589. https://doi.org/10.3390/diagnostics13152589
Chicago/Turabian StyleBraczkowski, Michał, Dariusz Soszyński, Alicja Sierakowska, Ryszard Braczkowski, Klaudia Kufel, and Beata Łabuz-Roszak. 2023. "Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes" Diagnostics 13, no. 15: 2589. https://doi.org/10.3390/diagnostics13152589
APA StyleBraczkowski, M., Soszyński, D., Sierakowska, A., Braczkowski, R., Kufel, K., & Łabuz-Roszak, B. (2023). Autoimmune Encephalitis with Antibodies: Anti-NMDAR, Anti-AMPAR, Anti-GQ1b, Anti-DPPX, Anti-CASPR2, Anti-LGI1, Anti-RI, Anti-Yo, Anti-Hu, Anti-CV2 and Anti-GABAAR, in the Course of Psychoses, Neoplastic Diseases, and Paraneoplastic Syndromes. Diagnostics, 13(15), 2589. https://doi.org/10.3390/diagnostics13152589






