MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review
Abstract
1. Introduction
2. Article Search Process
3. Results
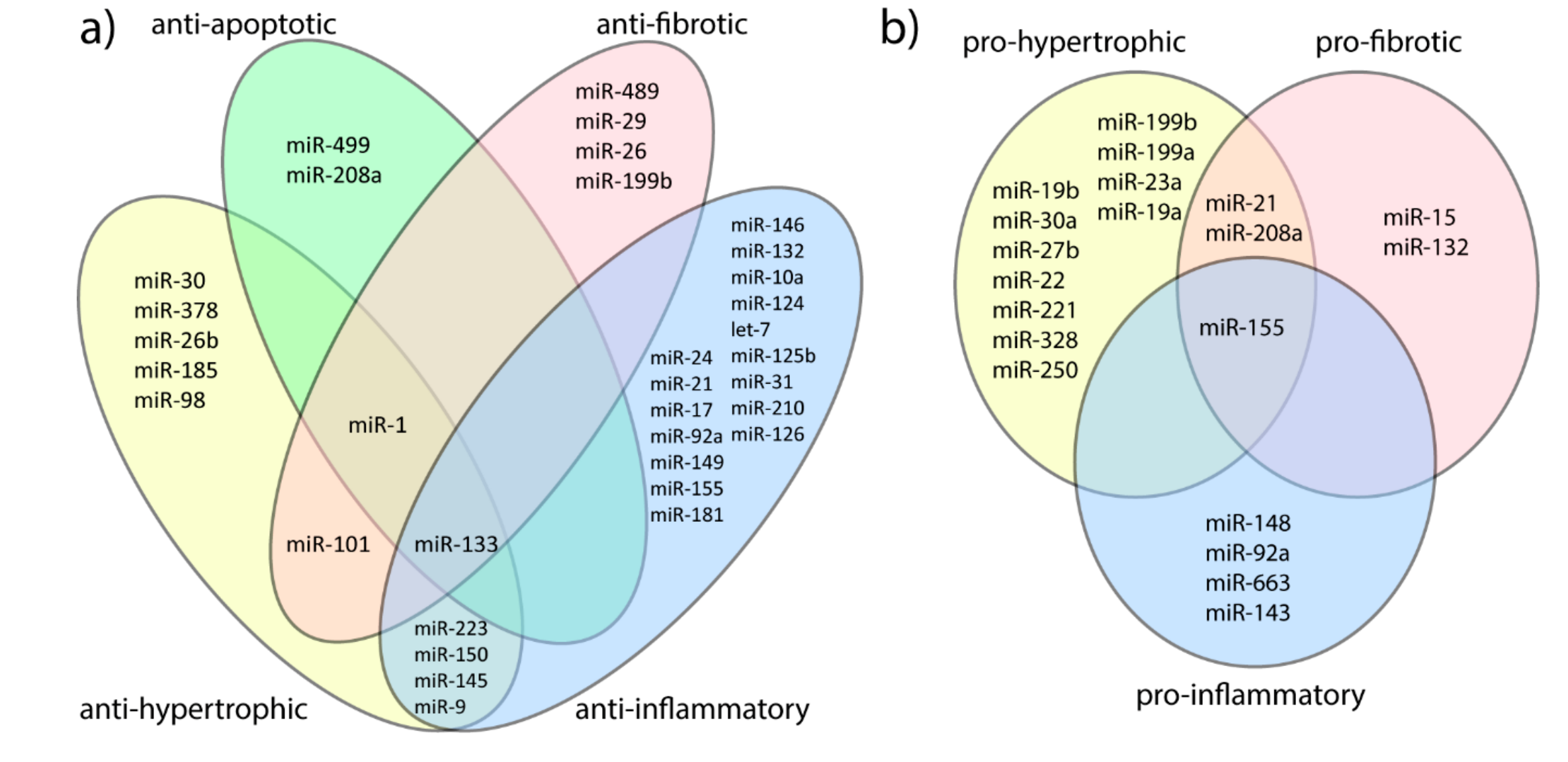
3.1. MicroRNAs and Adaptive Cardiac Hypertrophy Versus Hypertrophic Cardiomyopathy
| First Author | miRNAs | Material | Exercise | Training Protocol and Samples Collection | Methodology | Subjects | Results (p < 0.05) |
|---|---|---|---|---|---|---|---|
| Alack 2019 [31] | miR-24, miR-27a, miR-21, miR-15a, miR-23a, miR-221, miR-125b | Leukocytes | - | At rest | qRT-PCR, miRNAeasy Mini kit, TaqTM Universal SYBR Green Supermix | 13 trained triathletes and marathon runners (VO2max > 59 mL/kg × min) and 12 untrained healthy controls (VO2max < 45 mL/kg × min) | Endurance athletes: downregulated: miR-21, miR-23a |
| Backes 2014 [32] | 1205 different miRNAs | Whole blood | Cycling | Before and after exhaustive exercise on cyclic ergometer in each group | Microarray, qRT-PCR miScript SYBR Green | 12 elite endurance athletes (6 males, 6 females; 10 triathletes, 2 cyclists) and 12 age- and sex-matched controls; included 8 athletes and 8 controls | Endurance/control after exercise miR-181a, miR-320b were decreased in athletes |
| Baggish 2011 [16] | miR-20a, miR-210, miR-221, miR-222, miR-328, miR-21, miR-146a, miR-21, miR-133a, miR-21, miR-146a, and miR-210 | Blood (plasma) | Cycling | At rest and during acute exhaustive exercise testing on upright cycle ergometer, before and after a 90-day period of aerobic exercise training | qRT-PCR | 10 competitive male rowers (n = 10, age = 19.1 ± 0.6 years) | Elevated by acute exercise before and after sustained training: miR-146a, miR-222 elevated by acute exercise before but not after sustained training: miR-21, miR-221 elevated after sustained training: miR-20a nonresponsive: miR-133a, miR-210, miR-328 |
| Baggish 2014 [12] | miR-1, miR-133a, miR-499-5p, miR-208a, miR-126, miR-146a | Blood (plasma) | Running | At rest, immediately after marathon and 24 h after | qRT-PCR, TaqMan miRNA | 21 healthy male marathon runners | Upregulated after the race: miR-126, miR-1, miR-133a, miR-499-5p, miR-208a, miR-146a |
| Bye 2013 [33] | miR-210, miR-21, miR-125a, miR-652, miR-151, miR-29a, Let-7d, miR-222 | Blood (plasma) | VO2max test | Before the start of the exercise test | qRT-PCR | Screening cohort: 12- high VO2max, 12- low VO2max validation cohort: 38- high VO2max, 38- low VO2max | Low VO2max group: upregulated: miR-210, miR-222, miR-21 (with only males) |
| Danese 2018 [34] | miR-133a, miR-206 | Blood (plasma) | Half-marathon | Before and immediately after the half-marathon—21.1 km | qRT-PCR, TaqMan MicroRNA assay | 28 middle-aged, recreation athletes (11 women and 17 men; mean age, 46 years) | Elevated after the half-marathon run: miR-133a and miR-206 |
| Fernandez-Sanjurjo 2020 [22] | Global miRNA screening (752 miRs) | Blood (plasma) | Running | Before and immediately after: 10 km race, half-marathon, and marathon | qRT-PCR | 9 runners | After 10 km run Upregulated: miR-199b-5p, miR-424-3p, miR-33a-5p, miR-551a, miR-1537, miR-223-5p, miR-1260q, let-7b-3p, miR-150-5p, miR-423-5p, miR-223-3p, miR-345-5p, miR-505-3p Downregulated: miR-346 After half-marathon: Upregulated: miR-425-3p, miR-33a-5p, miR-338-3p, miR-339-5p, miR-106b-3p, miR-502-3p, miR-27a-3p, miR-660-5p, miR-505-3p, miR-100-5p, miR-22-3p, miR-30e-5p, miR-497-5p After marathon: Upregulated:miR-1972, miR- 940, miR-424-3p, miR-130b-5p, miR-223-5p, miR-145-3p, miR-181c-30, miR-501-3p, miR1260a, miR675-3p, miR345-5p, miR-424-5p, miR-1-3p, miR-34a-5p, miR-629-5p, miR-30a-5p, miR-148a-3p, miR-596, miR-10b-5p, miR-30d-5p, miR-320d Downregulated: miR-192-5p, miR-20b-5p, miR-103a-3p, miR-106b-5p, miR144-3p, miR-665, miR-486-3p |
| Gomes 2014 [13] | miR-1, miR-133a, miR-206 | Blood (plasma) | Half-marathon | Before warm-up and up to 10 min after the run | qRT-PCRTaq Man miRNA | 5 male recreational runners | Upregulated: miR-1, miR-133a, miR-206 |
| Gonzalo-Calvo 2018 [30] | Panel of 74 c-miRNAs | Blood (plasma) | 10 km, half-marathon, marathon | Before and after (0 h, 24 h, 72 h): 10-km, half-marathon, and marathon separated by one-month break | qRT-PCR miScript SYBR Green | 9 healthy, highly trained middle-aged amateur subjects | 10 km run: immediately after – increased miRNAs: miR-132-3p, miR-150-5p, decreased miRNAs: miR-103a-3p and miR-139-5p 24 h after – decreased miRNA: miR-590-5p Marathon run: immediately after – increased miRNAs: miR-21-5p, miR-27a-3p, miR-29a-3p, miR-30a-5p, miR-34a-5p, miR-126-3p, miR-142-5p, miR-143-3p, miR-195-5p, miR-199a-3p 24 h after– decreased miRNAs: miR-25-3p, miR-29b-3p, miR-30b-5p, miR-106b-5p, miR-107, miR-497-5p downregulated immediately after and remained downregulated for 24 h: miR-103a-3p and miR-375-5p |
| Denham 2016 [18] | miR-1, miR-133a, miR-181a, miR-486, and miR-494 | Whole blood | Running-sprint | Before and after 4 weeks (thrice weekly) of sprint interval training and a single bout of maximal aerobic treadmill exercise | qRT-PCR, TaqMan miRNA | 67 endurance athletes and 61 healthy controls; 19 young men—acute exercise trial | Endurance athletes, increased: miR-1, miR-486, and miR-494 after endurance training Healthy, young men decreased: miR-1, miR-133a, and miR-486 immediately after maximal aerobic exercise |
| Kern 2020 [35] | Global miRNA | Blood (plasma) | Running | Before, after 8 weeks of endurance training, after 8 weeks of wash-out phase, and after another 8 weeks of endurance training | Microarray | 23 healthy untrained volunteers | Most important miRNA associated with VO2max Cluster 1:miR-4465, miR-5581-5p, miR-6879-5p, miR-6869-5p Cluster 2: miR-7975 Cluster 6: miR-326-5p, miR-502-5p, miR-502-3p, miR-340-5p |
| Kravinen 2019 [36] | miR-21, miR-26, miR-126, miR-146, miR-221, miR-222 | Blood (serum and extracellular vesicles, EV) and sweat (EV) | Cycling | Sweat collection during, blood collection before and after each protocol: (1) maximal aerobic capacity test (2) anaerobic threshold, and (3) aerobic threshold (AerT) tests. Sauna—control | qRT-PCR, miRNAeasy Mini kit, miScript II RT Kit | 8 healthy trained subjects (all protocols) | Elevated In sweat: All endurance exercise: miR-26 Protocol 3 vs. control: miR-21 In serum EVs: Protocol 2 vs. control: miR-21, miR-222 |
| Mooren 2013 [11] | miR-1, miR-133, miR- 206, miR-499, miR-208b, miR- 21, and miR-155 | Blood (plasma) | Marathon | 2 days before in the morning, directly after, and 24 h after a public marathon run | qRT-PCR TaqMan miRNA | 14 male endurance athletes | Increased after race: miR-1, miR-133a, miR-206, miR-208b, miR-499 Elevated 24 h after race: miR-1, miR-133a, miR-206 |
| Nielsen 2013 [37] | global miRNA (742 miRNA) | Blood (plasma) | Cycling | Before (at rest) and immediately after 1 h, post 1 h, post 3 h an acute exercise training (60 min cycle ergometer exercise at 65% of Pmax) and following 12 weeks of endurance training (cycle ergometer with frequency of 5 times per week for 12 weeks) | Microarray, RT-PCR, miScript SYBR green and ROX, Exiqons miRNome panel V.2, ViiA7 Sequence Detection | 13 healthy men—acute exercise training, 7 healthy men—endurance training | Immediately after: all downregulated: miR-106a, miR-221, miR-30b, miR-151-5p, Let7i, miR-146a, miR-652, miR-151-3p upregulated 1 h–3 h: after 1 h: miR-330-3p, miR-223, miR-139-5p, miR-143, miR-145, miR-424 after 3 h: miR-1, miR-424, miR-133a, miR-133b after 12-week training: a) upregulated: miR-103, miR-107 b) downregulated: miR-342-3p, Let-7d, miR-766, miR-25, miR-148a, miR-185, miR-21, miR-148b, miR-133a, miR- 92a, miR-29b |
| Ramos 2017 [17] | miR-21, miR-210, miR-24, miR-146, miR-1, miR-133, miR-222 | Blood (plasma) | Running | Two studies: 1) controlled intensity 1-week intervals at 3 intensities (6,7,8 miles/h) and final 5-mile test 2) duration test speed 7 miles/h, 30,60, 90 min duration, final 5-mile treadmill run. Blood samples collected immediately after treadmill running | qRT-PCR, TaqMan miRNA | 26 healthy young men—12 in intensity trial and 14 in duration trial | Elevated in both groups and not intensity- or duration-dependent: miR-24, miR-146a Elevated and intensity-responsive: miR-1 Elevated and duration-responsive: miR-133, miR-222 |
| Uhlemann 2014 [38] | miR-126, miR-133 | Blood (plasma) | Three studies regarding endurance exercise: Study 1: maximal symptom–limited exercise test, Study 2: bicycling for 4 h, Study 3: running a marathon | qRT-PCR, TaqMan miRNA | Study 1: 13 healthy participants, Study 2: 12 healthy well-trained men, Study 3: 22 male middle-aged marathon runners with no history of coronary artery disease | Study 1: increased miR-126 at maximum power Study 2: increased miR-126 Study 3: increased miR-126 and miR-133 | |
| Yin 2020 [39] | miR-1-3p, miR-133a-3p, miR-133b, miR-206 | Blood (plasma) | Running | Before, immediately after, and 24 h after 8 km run | qRT-PCR | 18 healthy trained young men | Immediately after run elevated: miR-1–3p, miR-133a-3p, and miR-133b 24 h after run: elevated: miR-133a-3p |
3.2. MicroRNAs and Cardiomyocytes Damage
3.3. MicroRNAs and Fibrosis
3.4. MicroRNAs and Inflammatory Response
3.5. MicroRNAs and VO2max
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| CAD | coronary artery disease |
| c-miRNA | circulating microRNA |
| CRP | c-reactive proteins |
| CTGF | connective tissue growth factor |
| CVD | cardiovascular disease |
| GDF1 | growth differentiation factor 1 |
| HCM | hypertrophic cardiomyopathy |
| hs-cTnT | high-sensitivity cardiac troponin T |
| ISH | ischemic heart disease |
| LA | left atrium |
| LV | left ventricle |
| MACE | major adverse cardiac events |
| min/week | minutes per week |
| miR | microRNA |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| NT-pro-BNP | n-terminal b-type natriuretic peptide |
| RNA | ribonucleotide acid |
| SCD | sudden cardiac death |
| TIMP | tissue inhibitor of metalloproteinase |
| TLR | toll-like receptors |
| WHO | World Health Organization |
References
- World Health Organization (WHO). World Health Statistics 2019: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Booth, F.W.; Gordon, S.E.; Carlson, C.J.; Hamilton, M.T. Waging war on modern chronic diseases: Primary prevention through exercise biology. J. Appl. Physiol. 1985 2000, 88, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Back, M.; Borjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Bacopoulou, F.; Apostolaki, D.; Chrousos, G.P. Sudden cardiac death in athletes and the value of cardiovascular magnetic resonance. Eur. J. Clin. Investig. 2018, 48, e12955. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Osiak, A.; Kozar-Kamińska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Romaine, S.P.R.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Mooren, F.C.; Viereck, J.; Krüger, K.; Thum, T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am. J. Physiol. Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef]
- Baggish, A.L.; Park, J.; Min, P.K.; Isaacs, S.; Parker, B.A.; Thompson, P.D.; Troyanos, C.; D’Hemecourt, P.; Dyer, S.; Thiel, M.; et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J. Appl. Physiol. 1985 2014, 116, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.D.C.; Oliveira, G.P., Jr.; Madrid, B.; Almeida, J.A.; Franco, O.L.; Pereira, R.W. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers 2014, 19, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. Compr. Physiol. 2015, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.T. Overview of MicroRNAs in Cardiac Hypertrophy, Fibrosis, and Apoptosis. Int. J. Mol. Sci. 2016, 17, 749. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hale, A.; Weiner, R.B.; Lewis, G.D.; Systrom, D.; Wang, F.; Wang, T.J.; Chan, S.Y. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 2011, 589, 3983–3994. [Google Scholar] [CrossRef]
- Ramos, A.E.; Lo, C.; Estephan, L.E.; Tai, Y.Y.; Tang, Y.; Zhao, J.; Sugahara, M.; Gorcsan, J., III; Brown, M.G.; Lieberman, D.E.; et al. Specific circulating microRNAs display dose-dependent responses to variable intensity and duration of endurance exercise. Am. J. Physiol. Circ. Physiol. 2018, 315, H273–H283. [Google Scholar] [CrossRef]
- Denham, J.; Prestes, P.R. Muscle-Enriched MicroRNAs Isolated from Whole Blood Are Regulated by Exercise and Are Potential Biomarkers of Cardiorespiratory Fitness. Front. Genet. 2016, 7, 196. [Google Scholar] [CrossRef]
- Zilahi, E.; Adamecz, Z.; Bodoki, L.; Griger, Z.; Póliska, S.; Nagy-Vincze, M.; Dankó, K. Dysregulated expression profile of myomiRs in the skeletal muscle of patients with polymyositis. EJIFCC 2019, 30, 237–245. [Google Scholar]
- Fochi, S.; Giuriato, G.; De Simone, T.; Gomez-Lira, M.; Tamburin, S.; Del Piccolo, L.; Schena, F.; Venturelli, M.; Romanelli, M.G. Regulation of microRNAs in Satellite Cell Renewal, Muscle Function, Sarcopenia and the Role of Exercise. Inallt. J. Mol. Sci. 2020, 21, 6732. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Fernández-Sanjurjo, M.; Úbeda, N.; Fernández-García, B.; Del Valle, M.; De Molina, A.R.; Crespo, M.C.; Martin-Hernández, R.; Casas-Agustench, P.; Martínez-Camblor, P.; De Gonzalo-Calvo, D.; et al. Exercise dose affects the circulating microRNA profile in response to acute endurance exercise in male amateur runners. Scand. J. Med. Sci. Sports 2020, 30, 1896–1907. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Li, Y.; Xu, C.; Li, X.; Zhu, D.; Zhang, Y.; Xing, S.; Wang, H.; Zhang, Z.; et al. Tanshinone IIA Improves miR-133 Expression Through MAPK ERK1/2 Pathway in Hypoxic Cardiac Myocytes. Cell. Physiol. Biochem. 2012, 30, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wen, Y.; Bi, P.; Lai, X.; Liu, X.S.; Liu, X.; Kuang, S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 2012, 139, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.K.; Kumar, R.M.; Farkhondeh, M.; Baskerville, S.; Lodish, H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 2006, 103, 8721–8726. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.; Reguero, J.R.; Martín, M.; Molina, B.D.; Moris, C.; Alvarez, V.; Coto, E. Profile of MicroRNAs Differentially Produced in Hearts from Patients with Hypertrophic Cardiomyopathy and Sarcomeric Mutations. Clin. Chem. 2011, 57, 1614–1616. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, R.; Anselmi, C.V.; Losi, M.A.; Papa, L.; Cavarretta, E.; Martins, P.D.C.; Contaldi, C.; Jotti, G.S.; Franzone, A.; Galastri, L.; et al. Circulating miR-29a, Among Other Up-Regulated MicroRNAs, Is the Only Biomarker for Both Hypertrophy and Fibrosis in Patients With Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef]
- Karlsen, A.; Soendenbroe, C.; Malmgaard-Clausen, N.M.; Wagener, F.; Moeller, C.E.; Senhaji, Z.; Damberg, K.; Andersen, J.L.; Schjerling, P.; Kjaer, M.; et al. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J. 2020, 34, 6418–6436. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Dávalos, A.; Fernández-Sanjurjo, M.; Amado-Rodriguez, L.; Diaz-Coto, S.; Tomás-Zapico, C.; Montero, A.; García-González, Á.; Llorente-Cortés, V.; Heras, M.E.; et al. Circulating microRNAs as emerging cardiac biomarkers responsive to acute exercise. Int. J. Cardiol. 2018, 264, 130–136. [Google Scholar] [CrossRef]
- Alack, K.; Krüger, K.; Weiss, A.; Schermuly, R.; Frech, T.; Eggert, M.; Mooren, F.C. Aerobic endurance training status affects lymphocyte apoptosis sensitivity by induction of molecular genetic adaptations. Brain Behav. Immun. 2019, 75, 251–257. [Google Scholar] [CrossRef]
- Backes, C.; Leidinger, P.; Keller, A.; Hart, M.; Meyer, T.; Meese, E.; Hecksteden, A. Blood Born miRNAs Signatures that Can Serve as Disease Specific Biomarkers Are Not Significantly Affected by Overall Fitness and Exercise. PLoS ONE 2014, 9, e102183. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Røsjø, H.; Aspenes, S.T.; Condorelli, G.; Omland, T.; Wisloff, U. Circulating MicroRNAs and Aerobic Fitness—The HUNT-Study. PLoS ONE 2013, 8, e57496. [Google Scholar] [CrossRef]
- Danese, E.; Benati, M.; Sanchis-Gomar, F.; Tarperi, C.; Salvagno, G.L.; Paviati, E.; Montagnana, M.; Schena, F.; Lippi, G. Influence of middle-distance running on muscular micro RNAs. Scand. J. Clin. Lab. Investig. 2018, 78, 165–170. [Google Scholar] [CrossRef]
- Kern, F.; Ludwig, N.; Backes, C.; Maldener, E.; Fehlmann, T.; Suleymanov, A.; Meese, E.; Hecksteden, A.; Keller, A.; Meyer, T. Systematic Assessment of Blood-Borne MicroRNAs Highlights Molecular Profiles of Endurance Sport and Carbohydrate Uptake. Cells 2019, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, S.; Sievänen, T.; Karppinen, J.E.; Hautasaari, P.; Bart, G.; Samoylenko, A.; Vainio, S.J.; Ahtiainen, J.P.; Laakkonen, E.K.; Kujala, U.M. MicroRNAs in Extracellular Vesicles in Sweat Change in Response to Endurance Exercise. Front. Physiol. 2020, 11, 676. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Akerstrom, T.; Nielsen, A.R.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA Plasma Signature in Response to Acute Aerobic Exercise and Endurance Training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, M.; Möbius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Möhlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef]
- Yin, X.; Cui, S.; Li, X.; Li, W.; Lu, Q.J.; Jiang, X.H.; Wang, H.; Chen, X.; Ma, J.Z. Regulation of Circulatory Muscle-specific MicroRNA during 8 km Run. Int. J. Sports Med. 2020, 41, 582–588. [Google Scholar] [CrossRef]
- Patil, H.R.; O’Keefe, J.H.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Cardiovascular Damage Resulting from Chronic Excessive Endurance Exercise. Mo. Med. 2012, 109, 312–321. [Google Scholar]
- Scherr, J.; Braun, S.; Schuster, T.; Hartmann, C.; Moehlenkamp, S.; Wolfarth, B.; Pressler, A.; Halle, M. 72-h Kinetics of High-Sensitive Troponin T and Inflammatory Markers after Marathon. Med. Sci. Sports Exerc. 2011, 43, 1819–1827. [Google Scholar] [CrossRef]
- Fernandes, T.; Barauna, V.G.; Negrao, C.E.; Phillips, M.I.; De Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Liu, X.; Yang, P. Ghrelin Alleviates Angiotensin II-Induced H9c2 Apoptosis: Impact of the miR-208 Family. Med. Sci. Monit. 2018, 24, 6707–6716. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Dong, Y.H.; Du, W.; Shi, C.Y.; Wang, K.; Akram, J.; Wang, J.; Li, P.F. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int. J. Mol. Sci. 2017, 18, 745. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, F.; Liu, Q.; Xu, D. Regulatory non-coding RNAs in acute myocardial infarction. J. Cell. Mol. Med. 2017, 21, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L. Focal Fibrosis in the Endurance Athlete’s Heart. JACC Cardiovasc. Imaging 2018, 11, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.L.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Kiriazis, H.; Gao, X.M.; Du, X.J. Cardiac Fibrosis and Arrhythmogenesis. Compr. Physiol. 2017, 7, 1009–1049. [Google Scholar] [CrossRef]
- Creemers, E.E.; van Rooij, E. Function and Therapeutic Potential of Noncoding RNAs in Cardiac Fibrosis. Circ. Res. 2016, 118, 108–118. [Google Scholar] [CrossRef]
- Thum, T. Noncoding RNAs and myocardial fibrosis. Nat. Rev. Cardiol. 2014, 11, 655–663. [Google Scholar] [CrossRef]
- Clauss, S.; Wakili, R.; Hildebrand, B.; Kaab, S.; Hoster, E.; Klier, I.; Martens, E.; Hanley, A.; Hanssen, H.; Halle, M.; et al. MicroRNAs as Biomarkers for Acute Atrial Remodeling in Marathon Runners (The miRathon Study—A Sub-Study of the Munich Marathon Study). PLoS ONE 2016, 11, e148599. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Lucía, A. Pathophysiology of atrial fibrillation in endurance athletes: An overview of recent findings. CMAJ 2016, 188, E433–E435. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Rolfes, F.; Schelleckes, K.; Mewes, M.; Thorwesten, L.; Krüger, M.; Klose, A.; Brand, S.-M. Longer Work/Rest Intervals During High-Intensity Interval Training (HIIT) Lead to Elevated Levels of miR-222 and miR-29c. Front. Physiol. 2018, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.R.; Wei, L.H.; Chung, A.C.; Yu, C.M.; Lan, H.Y. miR-29b as a Therapeutic Agent for Angiotensin II-induced Cardiac Fibrosis by Targeting TGF-β/Smad3 signaling. Mol. Ther. 2014, 22, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Katsiari, C.G.; Bogdanos, D.P.; Sakkas, L. Inflammation and cardiovascular disease. World J. Transl. Med. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Barros, E.S.; Nascimento, D.C.; Prestes, J.; Nóbrega, O.T.; Córdova, C.; Sousa, F.; Boullosa, D.A. Acute and Chronic Effects of Endurance Running on Inflammatory Markers: A Systematic Review. Front. Physiol. 2017, 8, 779. [Google Scholar] [CrossRef]
- Tahamtan, A.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front. Immunol. 2018, 9, 1377. [Google Scholar] [CrossRef]
- Grabarek, B.O.; Wcisło-Dziadecka, D.; Gola, J. In silico analysis of CpG islands and miRNAs potentially regulating the JAK-STAT signalling pathway. Adv. Dermatol. Allergol. 2020, 37, 513–519. [Google Scholar] [CrossRef]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic High-Intensity Intervals Improve VO2max More Than Moderate Training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Brawner, C.A.; Savage, P.D.; Ehrman, J.K.; Schairer, J.; Divine, G.; Aldred, H.; Ophaug, K.; Ades, P.A. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am. Heart J. 2008, 156, 292–300. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soplinska, A.; Zareba, L.; Wicik, Z.; Eyileten, C.; Jakubik, D.; Siller-Matula, J.M.; De Rosa, S.; Malek, L.A.; Postula, M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review. Diagnostics 2020, 10, 813. https://doi.org/10.3390/diagnostics10100813
Soplinska A, Zareba L, Wicik Z, Eyileten C, Jakubik D, Siller-Matula JM, De Rosa S, Malek LA, Postula M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review. Diagnostics. 2020; 10(10):813. https://doi.org/10.3390/diagnostics10100813
Chicago/Turabian StyleSoplinska, Aleksandra, Lukasz Zareba, Zofia Wicik, Ceren Eyileten, Daniel Jakubik, Jolanta M. Siller-Matula, Salvatore De Rosa, Lukasz A. Malek, and Marek Postula. 2020. "MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review" Diagnostics 10, no. 10: 813. https://doi.org/10.3390/diagnostics10100813
APA StyleSoplinska, A., Zareba, L., Wicik, Z., Eyileten, C., Jakubik, D., Siller-Matula, J. M., De Rosa, S., Malek, L. A., & Postula, M. (2020). MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review. Diagnostics, 10(10), 813. https://doi.org/10.3390/diagnostics10100813







