Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Macrophage Differentiation and Polarization
2.4. Macrophage and Breast Cancer Cell Culture System
2.5. RNA Purification and Quantitative Reverse-Transcription Real-Time PCR
2.6. Cytokine Array
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Western-Blotting Analysis
2.9. Flow Cytometry
2.10. Phagocytosis Assay
2.11. Cytotoxicity Assays
2.12. Statistical Analysis
3. Results
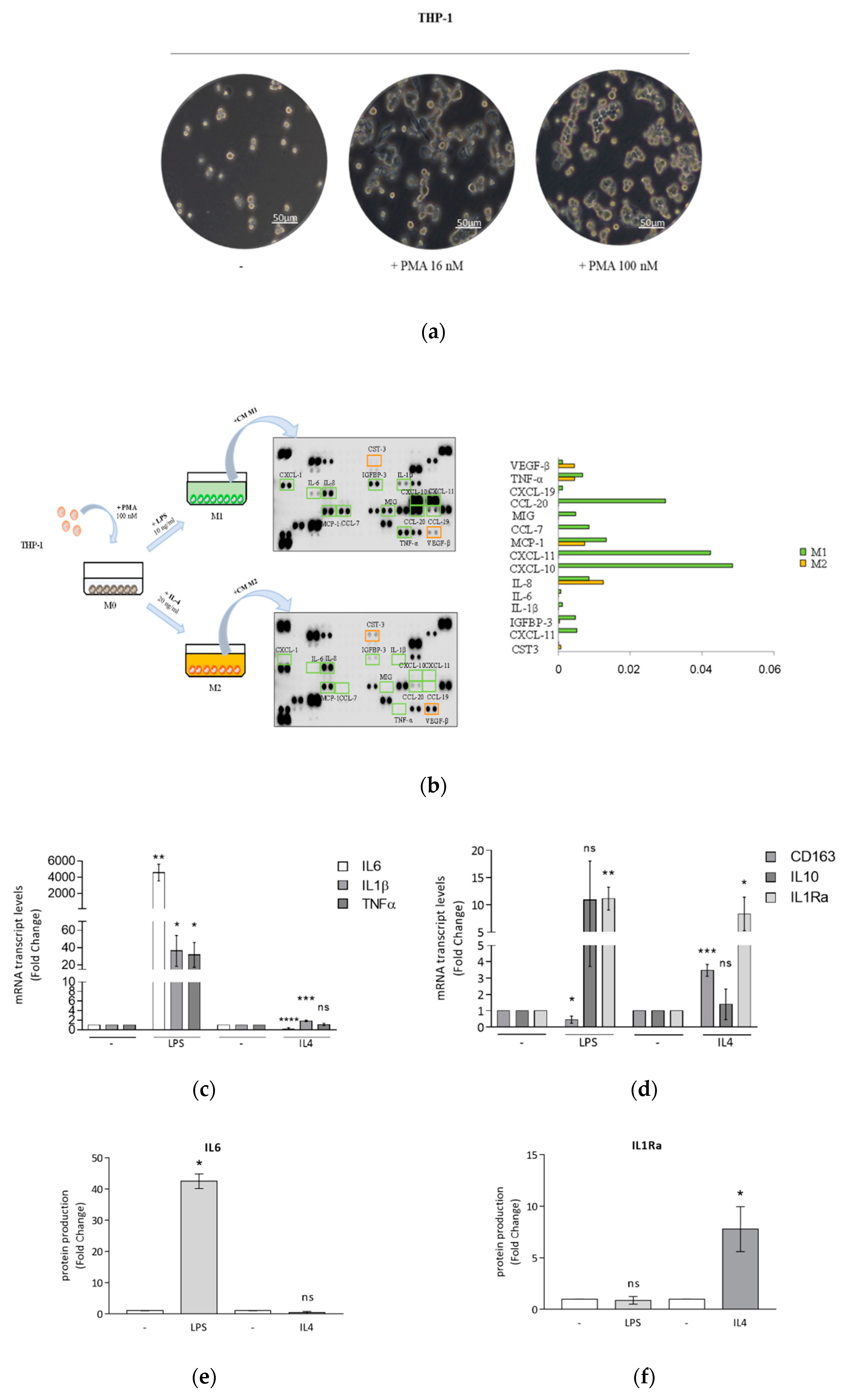
3.1. Optimized Technical Conditions for Human THP-1 Macrophage Polarization
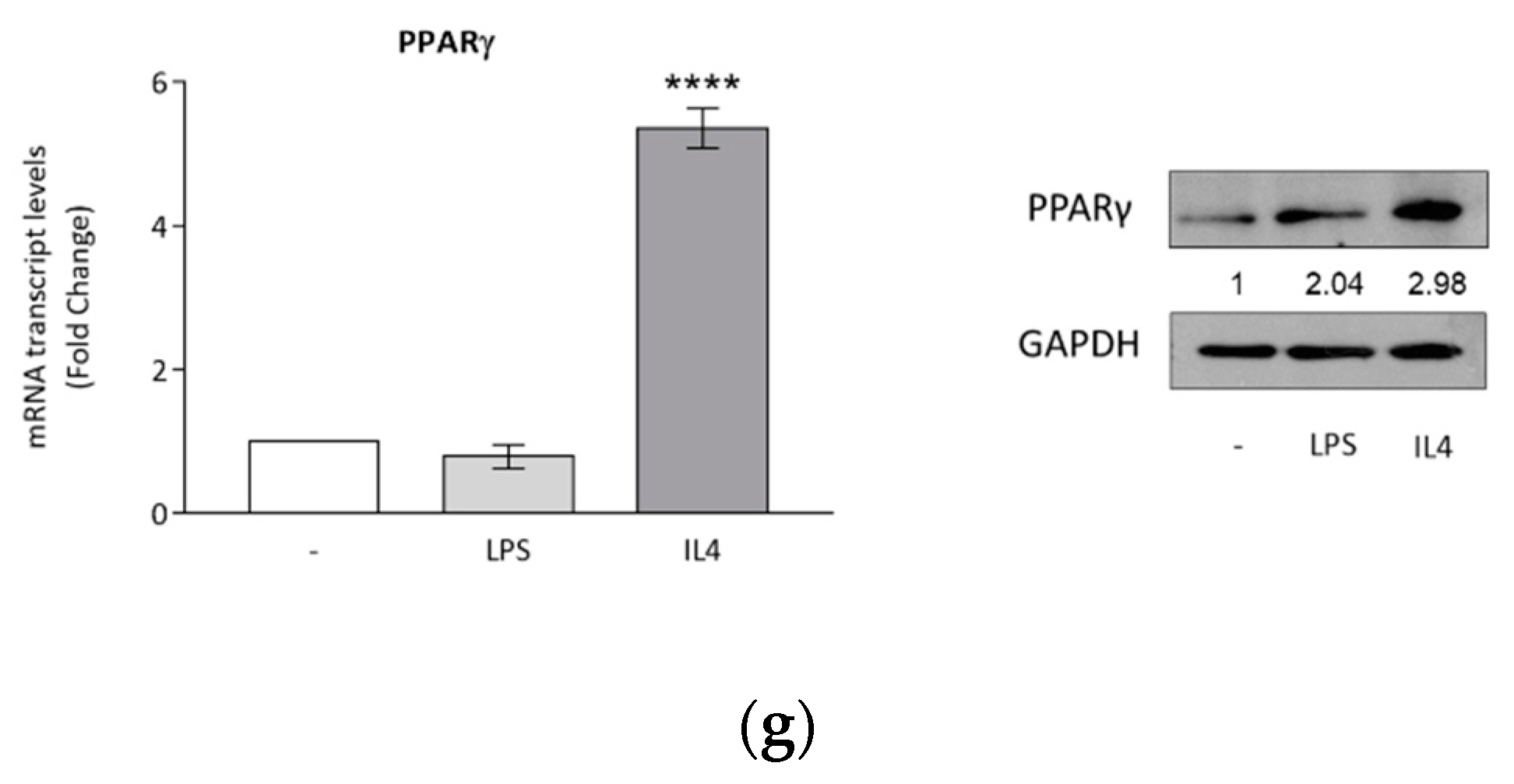
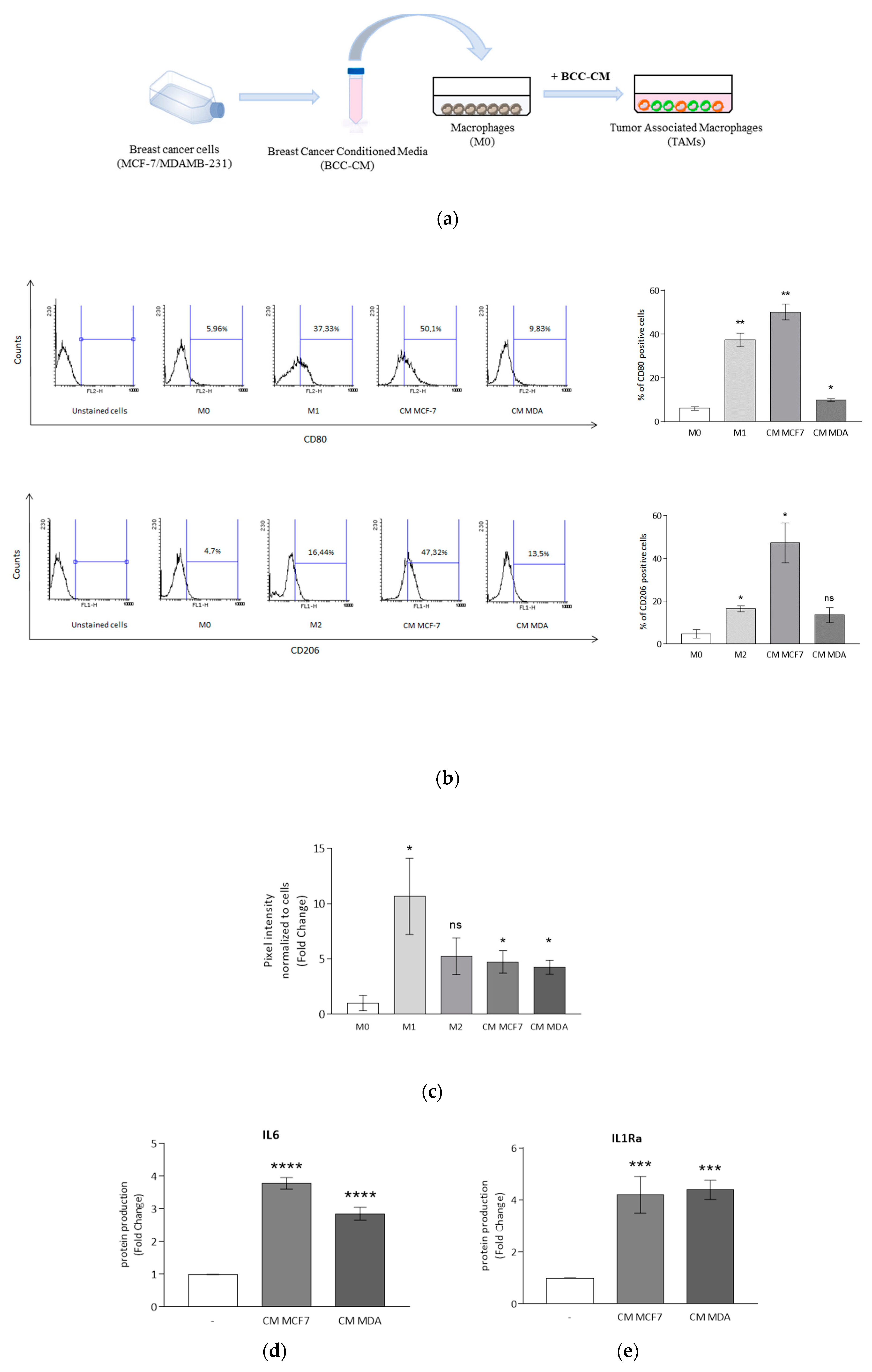
3.2. Breast Cancer Cells Drive Macrophage Polarization of THP-1 Cells
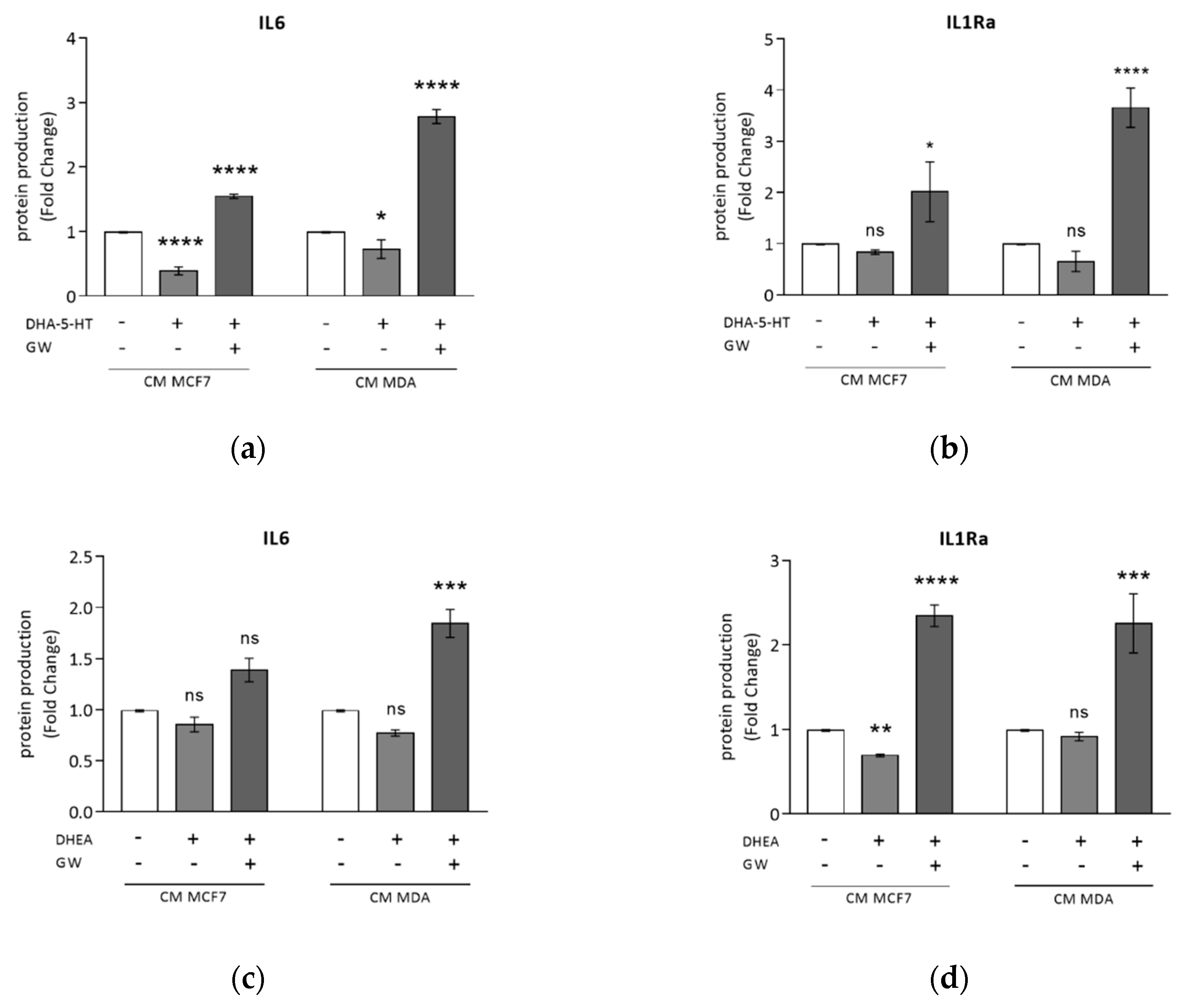
3.3. Rosiglitazone Negatively Affects Cytokine Secretion Induced by Breast Cancer Cell Conditioned Media
3.4. DHA Conjugates DHEA and DHA-5-HT Counteract TAM Cytokine Secretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nature Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.H.; McGarvey, K.M.; Baylin, S.B. The cancer epigenome—Components and functional correlates. Genes Dev. 2006, 20, 3215–3231. [Google Scholar] [CrossRef]
- Woutersen, R.A.; Appel, M.J.; van Garderen-Hoetmer, A.; Wijnands, M.V. Dietary fat and carcinogenesis. Mutat. Res. 1999, 443, 111–127. [Google Scholar] [CrossRef]
- Willett, W.C. Diet and cancer: One view at the start of the millennium. Cancer Epidemiol. Biomark. Prev. 2001, 10, 3–8. [Google Scholar]
- Gerber, M. N-3 fatty acids and cancers: A systematic update review of epidemiological studies. Br. J. Nutr. 2012, 107, S228–S239. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and n-6 and n-3 polyunsaturated fatty acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef]
- Thiebaut, A.C.; Chajes, V.; Gerber, M.; Boutron-Ruault, M.C.; Joulin, V.; Lenoir, G.; Berrino, F.; Riboli, E.; Benichou, J.; Clavel-Chapelon, F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer 2009, 124, 924–931. [Google Scholar] [CrossRef]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic Acid Induces Apoptosis in MCF7 Cells In Vitro and In Vivo via Reactive Oxygen Species Formation and Caspase 8 Activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef]
- Rose, D.P.; Connolly, J.M.; Rayburn, J.; Coleman, M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995, 87, 587–592. [Google Scholar] [CrossRef]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long chain omega-3 (n-3) fatty acids and human health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- Gani, O.A. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 7, 6. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Ting, A.T.; Seed, B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Reddy, R.C. Immunomodulatory role of PPAR-gamma in alveolar macrophages. J. Investig. Med. 2008, 56, 522–527. [Google Scholar] [CrossRef]
- Catalano, S.; Mauro, L.; Bonofiglio, D.; Pellegrino, M.; Qi, H.; Rizza, P.; Vizza, D.; Bossi, G.; Andò, S. In vivo and in vitro evidence that PPARγ Ligands are antagonists of leptin signaling in breast cancer. Am. J. Pathol. 2011, 179, 1030–1040. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Qi, H.; Gabriele, S.; Catalano, S.; Aquila, S.; Belmonte, M.; Andò, S. Peroxisome proliterator-activated receptor γ inhibits follicular and anaplastic thyroid carcinoma cells growth by upregulating p21 Cip1/WAF1 gene in a Sp1-dependent manner. Endocr. Relat. Cancer 2008, 15, 545–557. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Gabriele, S.; Aquila, S.; Qi, H.; Belmonte, M.; Catalano, S.; Andò, S. Peroxisome proliferator-activated receptor gamma activates fas ligand gene promoter inducing apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 2009, 113, 423–434. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Cione, E.; Vizza, D.; Perri, M.; Pingitore, A.; Qi, H.; Catalano, S.; Rovito, D.; Genchi, G.; Andò, S. Bid as a potential target of apoptotic effects exerted by low doses of PPARγ and RXR ligands in breast cancer cells. Cell Cycle 2011, 10, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, J.; Balvers, M.; Witkamp, R. N-acyl amines of docosahexaenoic acid and other n–3 polyunsatured fatty acids—from fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013, 169, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging Class of Omega-3 Fatty Acid Endocannabinoids and Their Derivatives. Prostaglandins Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- De Bus, I.; Witkamp, R.; Zuilhof, H.; Albada, B.; Balvers, M. The role of n-3 PUFA-derived fatty acid derivatives and their oxygenated metabolites in the modulation of inflammation. Prostaglandins Lipid Mediat. 2019, 144, 106351. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Berger, A.; Crozier, G.; Bisogno, T. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef]
- Brown, I.; Wahle, K.W.; Cascio, M.G.; Smoum-Jaouni, R.; Mechoulam, R.; Pertwee, R.G.; Heys, S.D. Omega-3 N-acylethanolamines are endogenously synthesised from omega-3 fatty acids in different human prostate and breast cancer cell lines. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 305–310. [Google Scholar] [CrossRef]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Plastina, P.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim. Biophys. Acta 2010, 1801, 1107–1114. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; De Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA Ethanolamides DHEA and EPEA Induce Autophagy Through PPARγ Activation in MCF7 Breast Cancer Cells. J. Cell. Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef]
- Meijerink, J.; Plastina, P.; Vincken, J.-P.; Poland, M.; Attya, M.; Balvers, M.; Gruppen, H.; Gabriele, B.; Witkamp, R.F. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: Evidence for a new link between fish oil and inflammation. Br. J. Nutr. 2011, 105, 1798–1807. [Google Scholar] [CrossRef]
- Meijerink, J.; Poland, M.; Balvers, M.G.J.; Plastina, P.; Lute, C.; Dwarkasing, J.; van Norren, K.; Witkamp, R.F. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. Br. J. Pharmacol. 2015, 172, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.M.; Voortman, T.; Balvers, M.G.J.; Hendriks, H.F.J.; Wortelboer, H.M.; Witkamp, R.F. Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract. Biochim. Biophys. Acta 2011, 1811, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Poland, M.; ten Klooster, J.P.; Wang, Z.; Pieters, R.; Boekschoten, M.; Witkamp, R.; Meijerink, J. Docosahexaenoyl serotonin, an endogenously formed n-3 fatty acid-serotonin conjugate has anti-inflammatory properties by attenuating IL-23-IL-17 signaling in macrophages. Biochim. Biophys. Acta 2016, 1861, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Balvers, M.G.J.; Hendriks, H.F.J.; Wilpshaar, T.; van Heek, T.; Witkamp, R.F.; Meijerink, J. Docosahexaenoyl serotonin emerges as most potent inhibitor of IL-17 and CCL-20 released by blood mononuclear cells from a series of N-acyl serotonins identified in human intestinal tissue. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Laviron, M.; Boissonnas, A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019, 10, 1799. [Google Scholar] [CrossRef]
- Evans, R.; Alexander, P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature 1970, 228, 620–622. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Caux, C.; Ramos, R.N.; Prendergast, G.C.; Bendriss-Vermare, N.; Ménétrier-Caux, C. A milestone review on how macrophages affect tumor growth. Cancer Res. 2016, 76, 6439–6442. [Google Scholar] [CrossRef]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2017, 36, 229–239. [Google Scholar] [CrossRef]
- Cione, E.; Plastina, P.; Pingitore, A.; Perri, M.; Caroleo, M.C.; Fazio, A.; Witkamp, R.; Meijerink, J. Capsaicin Analogues Derived from n-3 Polyunsaturated Fatty Acids (PUFAs) Reduce Inflammatory Activity of Macrophages and Stimulate Insulin Secretion by β-Cells In Vitro. Nutrients 2019, 11, 915. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Plastina, P.; Barone, I.; De Amicis, F.; Mauro, L.; Rizza, P.; Lanzino, M.; Catalano, S.; Bonofiglio, D.; et al. Omega-3 DHA- and EPA-Dopamine Conjugates Induce PPARgamma-Dependent Breast Cancer Cell Death through Autophagy and Apoptosis. Biochim. Biophys. Acta Gen. Sub. 2015, 1850, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Plastina, P.; Vincken, J.-P.; Jansen, R.; Balvers, M.; ten Klooster, J.P.; Gruppen, H.; Witkamp, R.; Meijerink, J. N-Docosahexaenoyl dopamine, an endocannabinoid-like conjugate of dopamine and the n-3 fatty acid docosahexaenoic acid, attenuates lipopolysaccharide-induced activation of microglia and macrophages via COX-2. ACS Chem. Neurosci. 2017, 8, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Leggio, A.; Barone, I.; De Marco, R.; Gelsomino, L.; Campana, A.; Malivindi, R.; Panza, S.; Giordano, C.; Liguori, A.; et al. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J. Cell. Mol. Med. 2015, 19, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L.; et al. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Luo, R.-Z.; Peng, R.-J.; Wang, S.-S.; Xue, C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco. Targets Ther. 2014, 7, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Jakopin, Ž.; Corsini, E. THP-1 Cells and Pro-inflammatory Cytokine Production: An in Vitro Tool for Functional Characterization of NOD1/NOD2 Antagonists. Int. J. Mol. Sci. 2019, 20, 4265. [Google Scholar] [CrossRef] [PubMed]
- Madhvi, A.; Mishra, H.; Leisching, G.R.; Mahlobo, P.Z.; Baker, B. Comparison of human monocyte derived macrophages and THP1-like macrophages as in vitro models for M. tuberculosis infection. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101355. [Google Scholar] [CrossRef]
- Stewart, D.A.; Yang, Y.; Makowski, L.; Troester, M.A. Basal-like Breast Cancer Cells Induce Phenotypic and Genomic Changes in Macrophages. Mol. Cancer Res. 2012, 10, 727–738. [Google Scholar] [CrossRef]
- Benner, B.; Scarberry, L.; Suarez-Kelly, L.P.; Duggan, M.C.; Campbell, A.R.; Smith, E.; Lapurga, G.; Jiang, K.; Butchar, J.P.; Tridandapani, S.; et al. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J. Immunother. Cancer 2019, 7, 140. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Buess, M.; Rajski, M.; Vogel-Durrer, B.M.; Herrmann, R.; Rochlitz, C. Tumor-endothelial interaction links the CD44(þ)/CD24(-) phenotype with poor prognosis in early-stage breast cancer. Neoplasia 2009, 11, 987–1002. [Google Scholar] [CrossRef]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011, 71, 614–624. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Mazurak, V.C. n-3 polyunsaturated fatty acids: The potential role for supplementation in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 246–251. [Google Scholar] [CrossRef]
- Fearon, K.C.; Barber, M.D.; Moses, A.G.; Ahmedzai, S.H.; Taylor, G.S.; Tisdale, M.J.; Murray, G.D. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J. Clin. Oncol. 2006, 24, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- van Norren, K.; Kegler, D.; Argiles, J.M.; Luiking, Y.; Gorselink, M.; Laviano, A.; Arts, K.; Faber, J.; Jansen, H.; van der Beek, E.M.; et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br. J. Cancer 2009, 100, 713–722. [Google Scholar] [CrossRef]
- Faber, J.; Vos, P.; Kegler, D.; van Norren, K.; Argiles, J.M.; Laviano, A.; Garssen, J.; van Helvoort, A. Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br. J. Cancer 2008, 99, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Turk, H.F.; Liddle, D.M.; De Boer, A.A.; Power, K.A.; Ma, D.W.; Robinson, L.E. n-3 polyunsaturated fatty acids and mechanisms to mitigate inflammatory paracrine signaling in obesity-associated breast cancer. Nutrients 2014, 6, 4760. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Lee, Y.S.; Mayoral, R.; Oh, D.Y.; Siu, J.T.; Webster, N.J.; Sears, D.D.; Olefsky, J.M.; Ellies, L.G. Omega-3 fatty acids reduce obesity-induced tumor progression independent of gpr120 in a mouse model of postmenopausal breast cancer. Oncogene 2014, 34, 3504–3513. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Muller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Wahle, K.W.; Smoum, R.; Mechoulam, R.; Ross, R.A.; Pertwee, R.G.; Heys, S.D. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 2010, 31, 1584–1591. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wong, J.; Conklin, D.S. PPARγ maintains ERBB2-positive breast cancer stem cells. Oncogene 2013, 32, 5512–5521. [Google Scholar] [CrossRef]
- Clark, R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002, 71, 388–400. [Google Scholar] [CrossRef]
- Wang, L.H.; Yang, X.Y.; Zhang, X.; Huang, J.; Hou, J.; Li, J.; Xiong, H.; Mihalic, K.; Zhu, H.; Xiao, W.; et al. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.D.; Conrad, D.; Glass, C.K. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by12/15-lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Huynh, H.; Chen, P.; Peña-Llopis, S.; Wan, Y. Macrophage PPARγ inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone. eLife 2016, 5, e18501. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. https://doi.org/10.3390/cells9010174
Gionfriddo G, Plastina P, Augimeri G, Catalano S, Giordano C, Barone I, Morelli C, Giordano F, Gelsomino L, Sisci D, et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells. 2020; 9(1):174. https://doi.org/10.3390/cells9010174
Chicago/Turabian StyleGionfriddo, Giulia, Pierluigi Plastina, Giuseppina Augimeri, Stefania Catalano, Cinzia Giordano, Ines Barone, Catia Morelli, Francesca Giordano, Luca Gelsomino, Diego Sisci, and et al. 2020. "Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer" Cells 9, no. 1: 174. https://doi.org/10.3390/cells9010174
APA StyleGionfriddo, G., Plastina, P., Augimeri, G., Catalano, S., Giordano, C., Barone, I., Morelli, C., Giordano, F., Gelsomino, L., Sisci, D., Witkamp, R., Andò, S., van Norren, K., & Bonofiglio, D. (2020). Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells, 9(1), 174. https://doi.org/10.3390/cells9010174









