The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer
Abstract
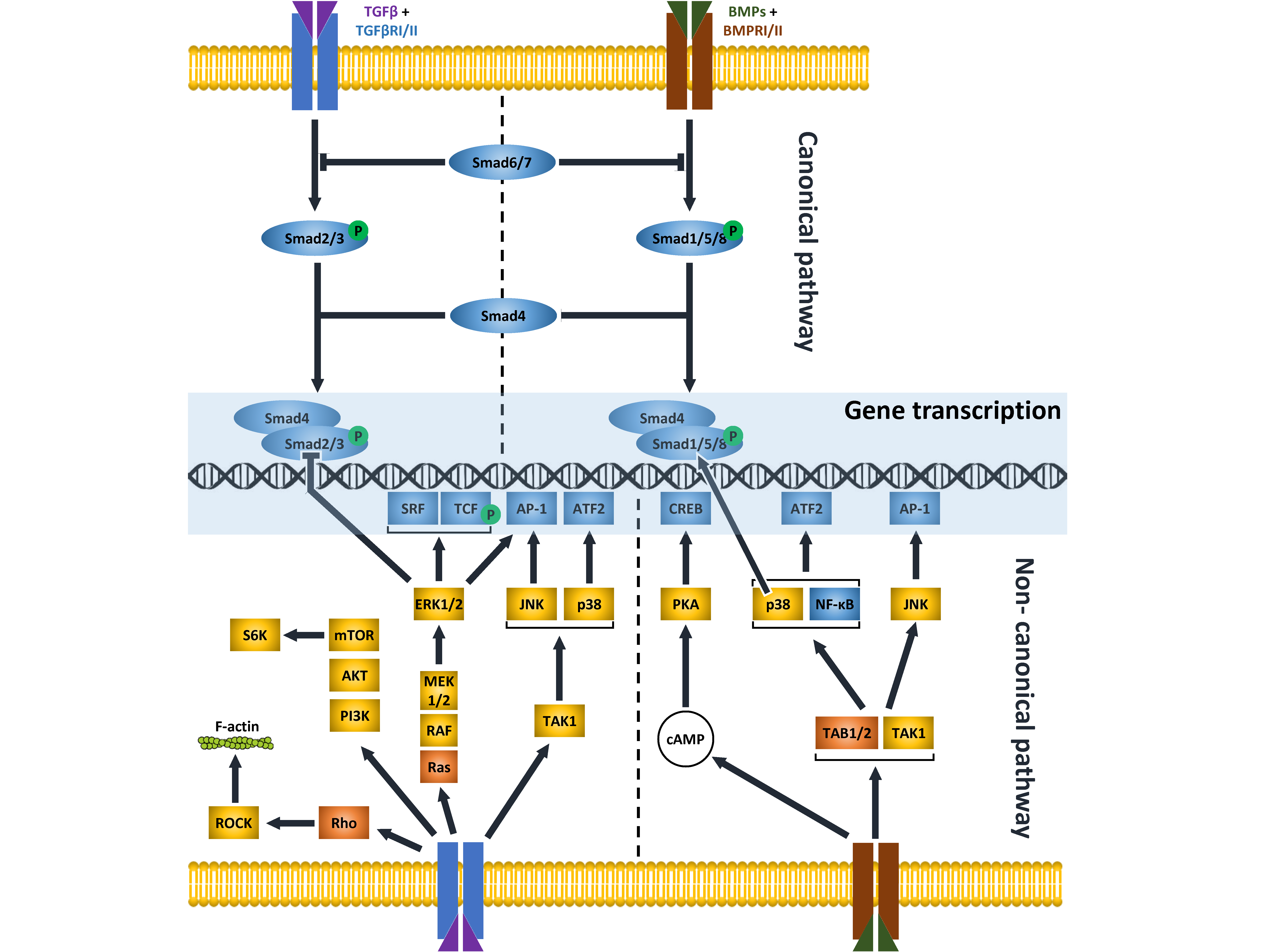
1. TGFβ and BMP Pathways
2. TGFβ and BMPs in Development
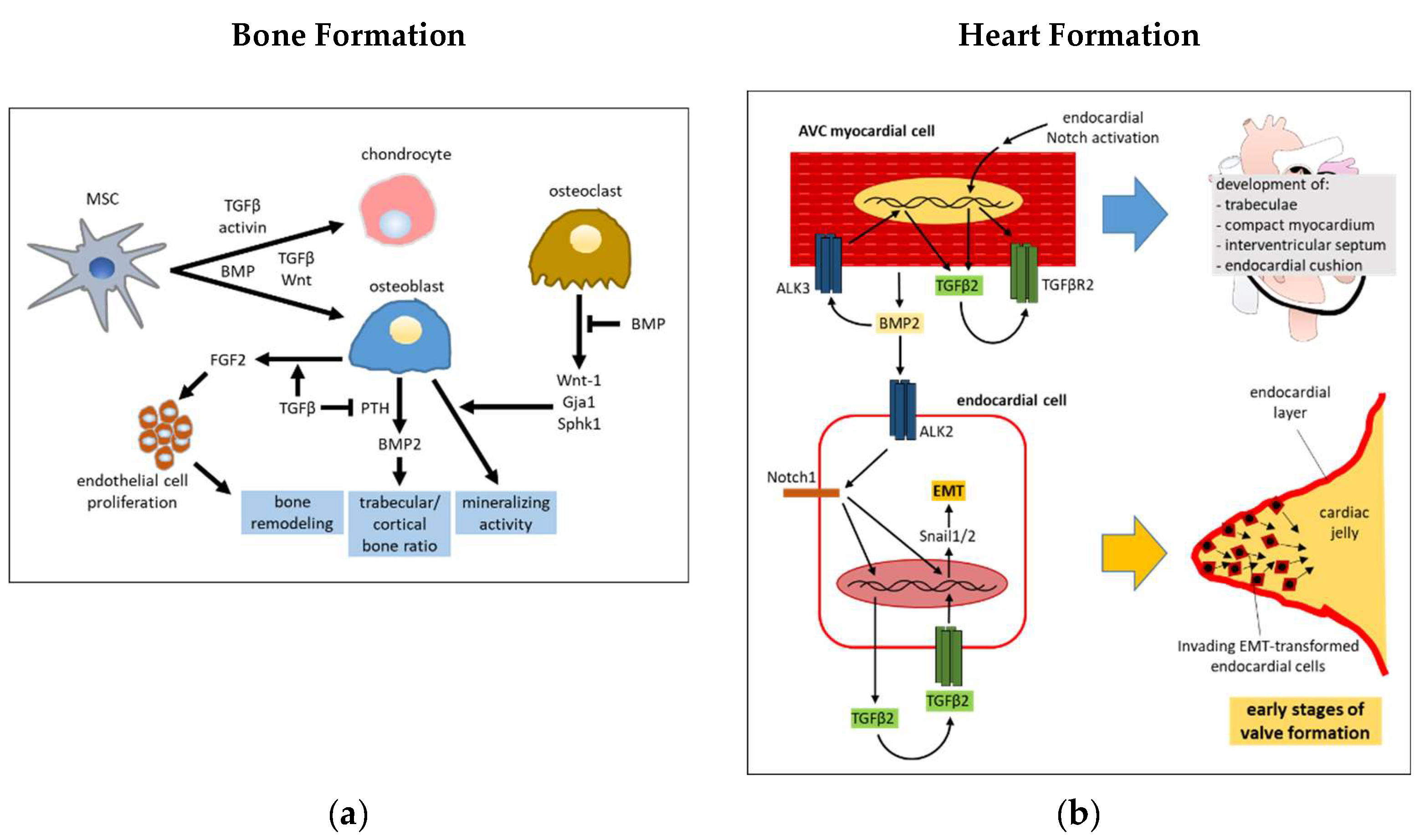
2.1. Bone Morphogenesis
2.2. Heart Development
3. Role of TGFβ-BMP Pathway Crosstalk in Fibrosis
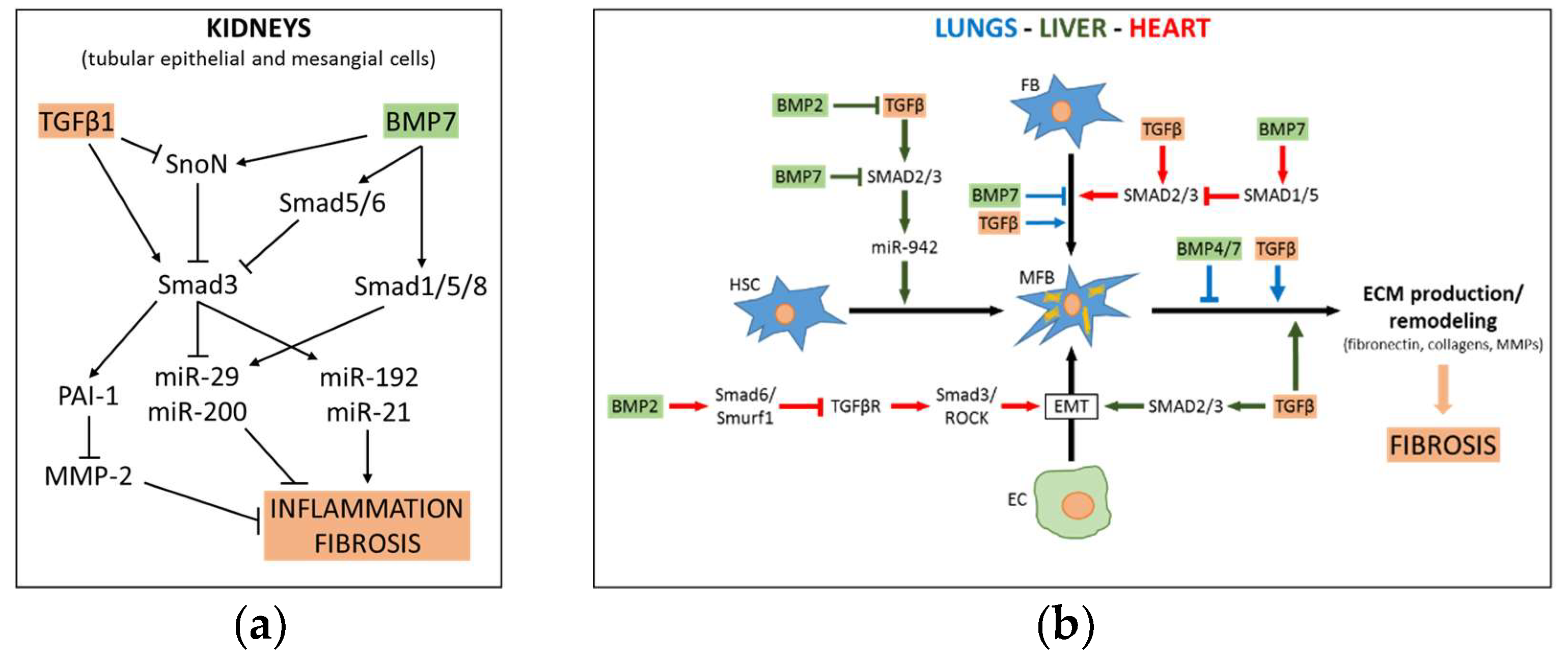
3.1. TGFβ-BMP pathway in Liver Fibrosis
3.2. TGFβ-BMP Pathway in Renal Fibrosis
3.3. TGFβ-BMP Pathway in Pulmonary Fibrosis
3.4. TGFβ-BMP Pathway in Myocardial Fibrosis
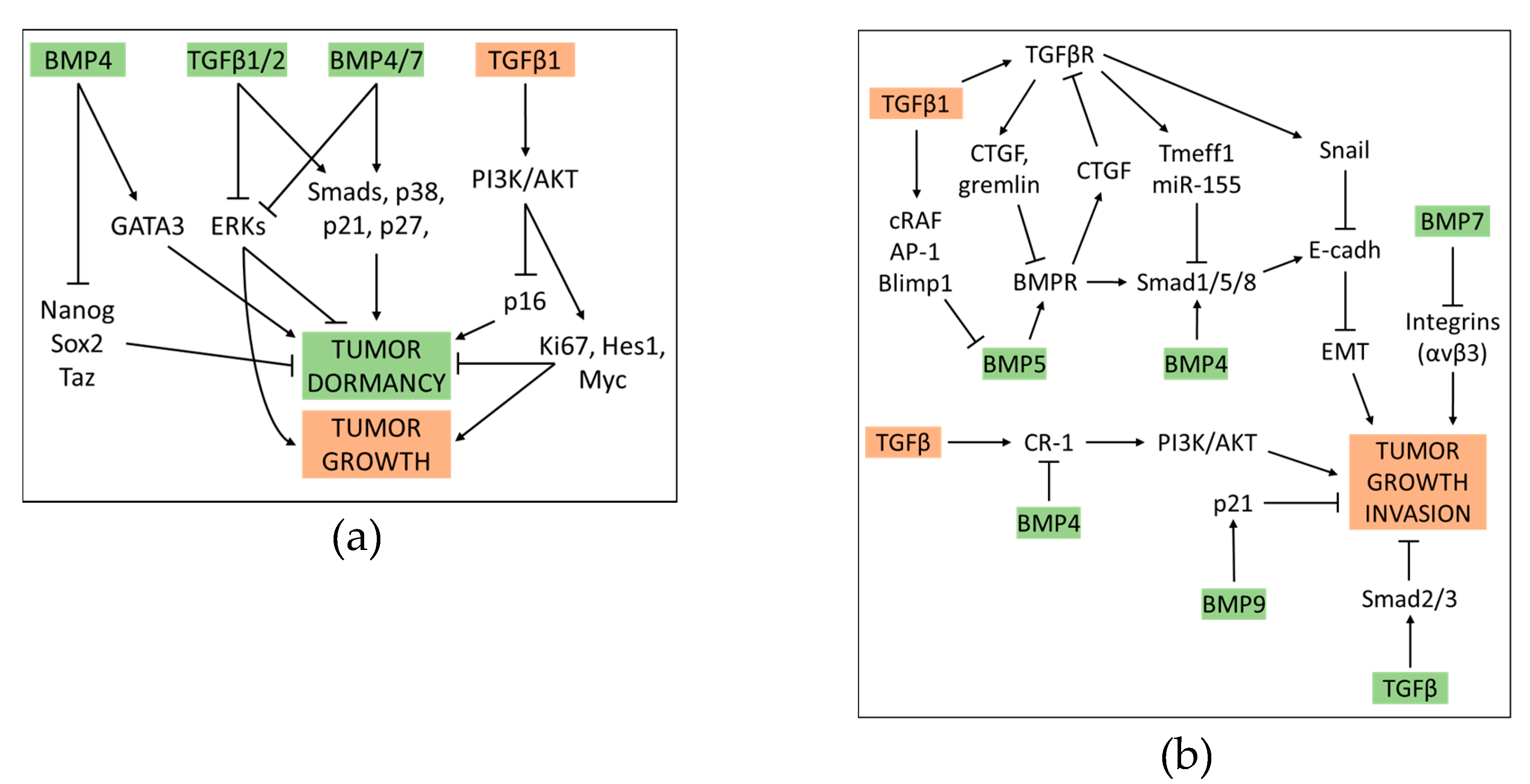
4. Crosstalk between BMP and TGFβ Pathway in Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dituri, F.; Mancarella, S.; Cigliano, A.; Chieti, A.; Giannelli, G. TGFβ as Multifaceted Orchestrator in HCC Progression: Signaling, EMT, Immune Microenvironment, and Novel Therapeutic Perspectives. Semin. Liver Dis. 2019, 39, 53–69. [Google Scholar] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGFβ and the TGFβ Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Boil. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-SMAD pathways in TGFβ signaling. Cell Res. 2009, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGFβ/BMP and other pathways. Cell Res. 2009, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Miyazono, K. Regulation of TGFβ family signaling by inhibitory SMADs. Cold Spring Harb. Perspect. Biol. 2017, 9, a022095. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419. [Google Scholar] [CrossRef]
- Dijke, P.T.; Goumans, M.-J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef]
- Pardali, E.; Goumans, M.-J.; Dijke, P.T. Signaling by members of the TGFβ family in vascular morphogenesis and disease. Trends Cell Boil. 2010, 20, 556–567. [Google Scholar] [CrossRef]
- Nickel, J.; Dreyer, M.K.; Kirsch, T.; Sebald, W. The Crystal Structure of the BMP-2:BMPR-IA Complex and the Generation of BMP-2 Antagonists. J. Bone Jt. Surgery-American Vol. 2001, 83. [Google Scholar] [CrossRef]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Lavery, K.; Swain, P.; Falb, D.; Alaoui-Ismaili, M.H. BMP-2/4 and BMP-6/7 Differentially Utilize Cell Surface Receptors to Induce Osteoblastic Differentiation of Human Bone Marrow-derived Mesenchymal Stem Cells. J. Boil. Chem. 2008, 283, 20948–20958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.C.; Bang, E.; Rejon, C.A.; Libasci, V.; Pertchenko, P.; Hébert, T.E.; Bernard, D.J. Bone Morphogenetic Protein 2 Stimulates Noncanonical SMAD2/3 Signaling via the BMP Type 1A Receptor in Gonadotrope-Like Cells: Implications for FSH Synthesis. Endocrinology 2014, 155, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chang, H.-M.; Cheng, J.-C.; Chu, G.; Leung, P.C.; Yang, G. ALK2/ALK3-BMPR2/ACVR2A Mediate BMP2-Induced Downregulation of Pentraxin 3 Expression in Human Granulosa-Lutein Cells. Endocrinology 2017, 158, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, T.-G.; Hao, J.; Hu, J.; Wang, J.; Simmons, H.; Miura, S.; Mishina, Y.; Zhao, G.-Q. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA 2004, 101, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.-J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef]
- Tillet, E.; Bailly, S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 5, 456. [Google Scholar] [CrossRef]
- Perron, J.C.; Dodd, J. Inductive specification and axonal orientation of spinal neurons mediated by divergent bone morphogenetic protein signaling pathways. Neural Dev. 2011, 6, 36. [Google Scholar] [CrossRef]
- Perron, J.C.; Dodd, J. ActRIIA and BMPRII Type II BMP Receptor Subunits Selectively Required for SMAD4-Independent BMP7-Evoked Chemotaxis. PLoS ONE 2009, 4, e8198. [Google Scholar] [CrossRef]
- Olsen, O.E.; Sankar, M.; Elsaadi, S.; Hella, H.; Buene, G.; Darvekar, S.R.; Misund, K.; Katagiri, T.; Knaus, P.; Holien, T. BMPR2 inhibits activin and BMP signaling via wild-type ALK2. J. Cell Sci. 2018, 131, jcs213512. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Fleming, R.E. Releasing the FKBP12 brake on hepcidin. Blood 2017, 130, 2049–2050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mullen, A.C.; Wrana, J.L. TGFβ Family Signaling in Embryonic and Somatic Stem Cell Renewal and Differentiation. Cold Spring Harb. Perspect. Boil. 2017, 9, a022186. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Xi, Q. TGFβ control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Levine, A.J.; Besser, D.; Hemmati-Brivanlou, A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005, 132, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Vallier, L.; Touboul, T.; Chng, Z.; Brimpari, M.; Hannan, N.; Millan, E.; Smithers, L.E.; Trotter, M.; Rugg-Gunn, P.; Weber, A.; et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE 2009, 4, e6082. [Google Scholar] [CrossRef] [PubMed]
- Sakaki-Yumoto, M.; Liu, J.; Ramalho-Santos, M.; Yoshida, N.; Derynck, R. SMAD2 Is Essential for Maintenance of the Human and Mouse Primed Pluripotent Stem Cell State. J. Boil. Chem. 2013, 288, 18546–18560. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Derynck, R. Transforming growth factor-β inhibits adipocyte differentiation by SMAD3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 2003, 278, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B. In VitroChondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic Differentiation of Cultured Human Mesenchymal Stem Cells from Marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef]
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Mokhtari, R.B.; Kumar, S.; Maalouf, J.; Arab, S.; Yeger, H.; Farhat, W.A. Spatio-Temporal Distribution of SMADs and Role of SMADs/TGFβ/BMP-4 in the Regulation of Mouse Bladder Organogenesis. PLoS ONE 2013, 8, e61340. [Google Scholar] [CrossRef] [PubMed]
- Holtzhausen, A.; Golzio, C.; How, T.; Lee, Y.-H.; Schiemann, W.P.; Katsanis, N.; Blobe, G.C. Novel bone morphogenetic protein signaling through SMAD2 and SMAD3 to regulate cancer progression and development. FASEB J. 2014, 28, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGFβ/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.-P. TGFβ and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Boil. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGFΒ1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Sobue, T.; Gravely, T.; Hand, A.; Min, Y.K.; Pilbeam, C.; Raisz, L.G.; Zhang, X.; LaRocca, D.; Florkiewicz, R.; Hurley, M.M. Regulation of Fibroblast Growth Factor 2 and Fibroblast Growth Factor Receptors by Transforming Growth Factor β in Human Osteoblastic MG-63 Cells. J. Bone Miner. Res. 2002, 17, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Boil. 1998, 141, 1659–1673. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGFβ signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef]
- Hudnall, A.M.; Arthur, J.W.; Lowery, J.W. Clinical Relevance and Mechanisms of Antagonism Between the BMP and Activin/TGFβ Signaling Pathways. J. Am. Osteopat. Assoc. 2016, 116, 452. [Google Scholar] [CrossRef]
- Re’Em, T.; Witte, F.; Willbold, E.; Ruvinov, E.; Cohen, S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 2012, 8, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.; Astleford, K.; Blixt, N.C.; Jensen, E.D.; Gopalakrishnan, R.; Mansky, K.C. SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors. PLoS ONE 2018, 13, e0203404. [Google Scholar] [CrossRef] [PubMed]
- Singhatanadgit, W.; Salih, V.; Olsen, I. Up-regulation of bone morphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. J. Cell. Physiol. 2006, 209, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Zhang, K.; Liu, K.; Shao, X.; Ding, Z.; Wang, F.; Hong, Y.; Zhu, M.; Li, H. Transferrin receptor facilitates TGFβ and BMP signaling activation to control craniofacial morphogenesis. Cell Death Dis. 2016, 7, e2282. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, J.; Ito, Y.; Bringas, P.; Deng, C.; Chai, Y. Ectodermal SMAD4 and p38 MAPK are functionally redundant in mediating TGFβ/BMP signaling during tooth and palate development. Dev. Cell 2008, 15, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhan, Y.; Gou, X.; Chen, Y.; Yang, G. TGFβ signaling inhibits canonical BMP signaling pathway during palate development. Cell Tissue Res. 2018, 371, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Eid, K.; Glowacki, J. Cooperation between TGFβ and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J. Bone Miner. Res. 2004, 19, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.Y.; Yang, X.; Chen, D.; Zuscik, M.; O’Keefe, R.J. Axin1 and Axin2 Are Regulated by TGFβ and Mediate Cross-talk between TGFβ and Wnt Signaling Pathways. Ann. New York Acad. Sci. 2007, 1116, 82–99. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, T.L.; Centrella, M. Novel links among Wnt and TGFβsignaling and Runx2. Mol. Endocrinol. 2010, 24, 587–597. [Google Scholar] [CrossRef]
- Bosch, M.H.V.D.; Blom, A.B.; Van Lent, P.L.; Van Beuningen, H.M.; Davidson, E.N.B.; Van Der Kraan, P.M.; Berg, W.B.V.D. Canonical Wnt signaling skews TGFβ signaling in chondrocytes towards signaling via ALK1 and SMAD 1/5/8. Cell. Signal. 2014, 26, 951–958. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, X.; Zhang, F.; Clemens, T.L.; Wan, M.; Cao, X. TGFβ type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat. Cell Biol. 2010, 12, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edwards, J.R.; Ko, S.-Y.; Dong, S.; Liu, H.; Oyajobi, B.O.; Papasian, C.; Deng, H.-W.; Zhao, M. Transcriptional Regulation of BMP2 Expression by the PTH-CREB Signaling Pathway in Osteoblasts. PLoS ONE 2011, 6, e20780. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Joergensen, B.; Bornhaeuser, M.; Ehninger, G.; Werner, C. Mesenchymal Stem Cells (MSC) can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kurpinski, K.; Lam, H.; Chu, J.; Wang, A.; Kim, A.; Tsay, E.; Agrawal, S.; Schaffer, D.V.; Li, S. Transforming Growth Factor-β and Notch Signaling Mediate Stem Cell Differentiation into Smooth Muscle Cells. Stem Cells 2010, 28, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Kang, H.-A.; Park, M.; Lee, H.-Y.; Choi, H.-R.; Yun, C.-H.; Oh, J.-W.; Kang, H.-S. Interleukin-24 attenuates β-glycerophosphate-induced calcification of vascular smooth muscle cells by inhibiting apoptosis, the expression of calcification and osteoblastic markers, and the Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2012, 428, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, J.M.; Muñoz-Castañeda, J.R.; Herencia, C.; De Oca, A.M.; Estepa, J.C.; Canalejo, R.; Rodriguez-Ortiz, M.E.; Martínez, P.P.; Aguilera-Tejero, E.; Canalejo, A.; et al. In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/β-catenin activation. Am. J. Physiol. Physiol. 2012, 303, F1136–F1144. [Google Scholar] [CrossRef]
- Guerrero, F.; Herencia, C.; Almadén, Y.; Martinez-Moreno, J.M.; De Oca, A.M.; Rodríguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Canalejo, A.; Florio, M.; López, I.; et al. TGFβ Prevents Phosphate-Induced Osteogenesis through Inhibition of BMP and Wnt/β-Catenin Pathways. PLoS ONE 2014, 9, e89179. [Google Scholar] [CrossRef]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGFβ signaling during heart valve development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef]
- Wang, J.; Sridurongrit, S.; Dudas, M.; Thomas, P.; Nagy, A.; Schneider, M.D.; Epstein, J.A.; Kaartinen, V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev. Boil. 2005, 286, 299–310. [Google Scholar] [CrossRef]
- Yamagishi, T.; Nakajima, Y.; Miyazono, K.; Nakamura, H. Bone morphogenetic protein-2 acts synergistically with transforming growth factor-β3 during endothelial-mesenchymal transformation in the developing chick heart. J. Cell. Physiol. 1999, 180, 35–45. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yamagishi, T.; Hokari, S.; Nakamura, H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: Roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP). Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2000, 258, 119–127. [Google Scholar] [CrossRef]
- Sugi, Y.; Yamamura, H.; Okagawa, H.; Markwald, R.R. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev. Boil. 2004, 269, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, M.-F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Luna-Zurita, L.; Prados, B.; Grego-Bessa, J.; Luxán, G.; Del Monte, G.; Benguría, A.; Adams, R.H.; Pérez-Pomares, J.M.; De La Pompa, J.L. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Investig. 2010, 120, 3493–3507. [Google Scholar] [CrossRef] [PubMed]
- Gaussin, V.; Van De Putte, T.; Mishina, Y.; Hanks, M.C.; Zwijsen, A.; Huylebroeck, D.; Behringer, R.R.; Schneider, M.D. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc. Natl. Acad. Sci. USA 2002, 99, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, L.A.; Grego-Bessa, J.; Raya, A.; Bertran, E.; Pérez-Pomares, J.M.; Díez, J.; Aranda, S.; Palomo, S.; McCormick, F.; Izpisúa-Belmonte, J.C.; et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genome Res. 2004, 18, 99–115. [Google Scholar] [CrossRef]
- Niessen, K.; Fu, Y.; Chang, L.; Hoodless, P.A.; McFadden, D.; Karsan, A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J. Cell Boil. 2008, 182, 315–325. [Google Scholar] [CrossRef]
- Moskowitz, I.P.; Wang, J.; Peterson, M.A.; Pu, W.T.; Mackinnon, A.C.; Oxburgh, L.; Chu, G.C.; Sarkar, M.; Berul, C.; Smoot, L.; et al. Cardiac-specific transcription factor genes SMAD4 and Gata4 cooperatively regulate cardiac valve development. Proc. Natl. Acad. Sci. USA 2011, 108, 4006–4011. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGFβ: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012, 586, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; He, W.; Lin, X.; Kiss, L.P.; Liu, Y. SMAD ubiquitination regulatory factor-2 in the fibrotic kidney: Regulation, target specificity, and functional implication. Am. J. Physiol. Physiol. 2008, 294, F1076–F1083. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, M.; Feng, X.-H. Smurf2 Is a Ubiquitin E3 Ligase Mediating Proteasome-dependent Degradation of SMAD2 in Transforming Growth Factor-β Signaling. J. Boil. Chem. 2000, 275, 36818–36822. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chung, A.C.K.; Lan, H.Y. Role of the TGFβ/BMP-7/SMAD pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGFβ/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Non-SMAD TGFβ signals. J. Cell Sci. 2005, 118, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Addante, A.; Sánchez, A. BMP signalling at the crossroad of liver fibrosis and regeneration. Int. J. Mol. Sci. 2018, 19, 39. [Google Scholar] [CrossRef]
- Katsuno, Y.; Qin, J.; Oses-Prieto, J.A.; Wang, H.; Jackson-Weaver, O.; Zhang, T.; Lamouille, S.; Wu, J.; Burlingame, A.; Xu, J.; et al. Arginine methylation of SMAD7 by PRMT1 in TGFβ–induced epithelial–mesenchymal transition and epithelial stem-cell generation. J. Boil. Chem. 2018, 293, 13059–13072. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepato-Biliary-Pancreatic Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Perepelyuk, M.; Cosgrove, B.D.; Tsai, S.J.; Lee, G.Y.; Mauck, R.L.; Wells, R.G.; Burdick, J.A. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci. Rep. 2016, 6, 21387. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Hui, J.-J.; Gombos, Z.; McCormick, M.E.; Wang, A.Y.; Uemura, M.; Mick, R.; Janmey, P.A.; Furth, E.E.; Wells, R.G. Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am. J. Physiol. Liver Physiol. 2007, 293, G1147–G1154. [Google Scholar] [CrossRef] [PubMed]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic Stellate Cells and Liver Fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar] [PubMed]
- Park, S.-A.; Kim, M.-J.; Park, S.-Y.; Kim, J.-S.; Lim, W.; Nam, J.-S.; Sheen, Y.Y. TIMP-1 mediates TGFβ-dependent crosstalk between hepatic stellate and cancer cells via FAK signaling. Sci. Rep. 2015, 5, 16492. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGFβ signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xue, D.; Shen, D.; Ma, W.; Zhang, J.; Wang, X.; Zhang, W.; Wu, L.; Pan, K.; Yang, Y.; et al. MicroRNA-942 mediates hepatic stellate cell activation by regulating BAMBI expression in human liver fibrosis. Arch. Toxicol. 2018, 92, 2935–2946. [Google Scholar] [CrossRef]
- Chen, B.-L.; Peng, J.; Li, Q.-F.; Yang, M.; Wang, Y.; Chen, W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-β/SMAD signaling. World J. Gastroenterol. 2013, 19, 1405–1415. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, X.; Wang, S.; Yang, L.; Gao, H.; Yang, C. The Anti-Fibrotic Effect of Bone Morphogenic Protein-7(BMP-7) on Liver Fibrosis. Int. J. Med. Sci. 2013, 10, 441–450. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Huang, Y.-H.; Chu, T.-H.; Chen, C.-L.; Lin, P.-R.; Huang, S.-C.; Wu, D.-C.; Huang, C.-C.; Hu, T.-H.; Kao, Y.-H.; et al. BMP-2 restoration aids in recovery from liver fibrosis by attenuating TGFβ1 signaling. Lab. Investig. 2018, 98, 999–1013. [Google Scholar] [CrossRef]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Soofi, A.; Wolf, K.I.; Emont, M.P.; Qi, N.; Martinez-Santibanez, G.; Grimley, E.; Ostwani, W.; Dressler, G.R. The kielin/chordin-like protein (KCP) attenuates high-fat diet-induced obesity and metabolic syndrome in mice. J. Boil. Chem. 2017, 292, 9051–9062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Gagliardini, E.; Benigni, A. Role of anti-TGFβ antibodies in the treatment of renal injury. Cytokine Growth Factor Rev. 2006, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, R.; Dobberfuhl, A.D.; Cooley, C.; Overstreet, J.M.; Patel, S.; Goldschmeding, R.; Meldrum, K.K.; Higgins, P.J. Induction of renal fibrotic genes by TGFβ1 requires EGFR activation, p53 and reactive oxygen species. Cell. Signal. 2013, 25, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Hanai, J.-I.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGFβ1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Luo, D.D.; Phillips, A.; Fraser, D. Bone Morphogenetic Protein-7 Inhibits Proximal Tubular Epithelial Cell SMAD3 Signaling via Increased SnoN Expression. Am. J. Pathol. 2010, 176, 1139–1147. [Google Scholar] [CrossRef]
- Wang, S.; Hirschberg, R. Bone morphogenetic protein-7 signals opposing transforming growth factor β in mesangial cells. J. Biol. Chem. 2004, 279, 23200–23206. [Google Scholar] [CrossRef]
- Wang, S.; Hirschberg, R. BMP7 antagonizes TGFβ-dependent fibrogenesis in mesangial cells. Am. J. Physiol. Physiol. 2003, 284, F1006–F1013. [Google Scholar] [CrossRef]
- Sugimoto, H.; LeBleu, V.S.; Basukonda, D.; Keck, P.; Taduri, G.; Bechtel, W.; Okada, H.; Carlson, W.; Bey, P.; Rusckowski, M.; et al. Activin–like kinase–3 activity is important for kidney regeneration and reversal of fibrosis. Nat. Med. 2012, 18, 396–404. [Google Scholar] [CrossRef]
- Loureiro, J.; Schilte, M.; Aguilera, A.; Albar-Vizcaíno, P.; Ramírez-Huesca, M.; Perez-Lozano, M.L.; González-Mateo, G.T.; Aroeira, L.S.; Selgas, R.; Mendoza, L.; et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol. Dial. Transplant. 2010, 25, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.T.J.; Ranghini, E.J.; Grimley, E.; Lee, P.H.; Dressler, G.R. High-throughput screens for agonists of bone morphogenetic protein (BMP) signaling identify potent benzoxazole compounds. J. Boil. Chem. 2019, 294, 3125–3136. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Aschner, Y.; Downey, G.P. Transforming growth factor-B: Master regulator of the respiratory system in health and disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 647–655. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, E.; Cailotto, F.; De Vooght, V.; Aznar-Lopez, C.; Vanoirbeek, J.A.; Luyten, F.P.; Lories, R.J.U. Enhanced endogenous bone morphogenetic protein signaling protects against bleomycin induced pulmonary fibrosis. Respir. Res. 2015, 16, 816. [Google Scholar] [CrossRef] [PubMed]
- Pegorier, S.; Campbell, G.A.; Kay, A.B.; Lloyd, C.M. Bone Morphogenetic Protein (BMP)-4 and BMP-7 regulate differentially Transforming Growth Factor (TGF)-β1 in normal human lung fibroblasts (NHLF). Respir. Res. 2010, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Myllärniemi, M.; Lindholm, P.; Ryynänen, M.J.; Kliment, C.R.; Salmenkivi, K.; Keski-Oja, J.; Kinnula, V.L.; Oury, T.D.; Koli, K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rezov, V.; Joensuu, E.; Vartiainen, V.; Rönty, M.; Yin, M.; Myllärniemi, M.; Koli, K. Pirfenidone decreases mesothelioma cell proliferation and migration via inhibition of ERK and AKT and regulates mesothelioma tumor microenvironment in vivo. Sci. Rep. 2018, 8, 10070. [Google Scholar] [CrossRef]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; Van Laar, J.M. Interleukin-6 (IL-6) Trans Signaling Drives a STAT3-dependent Pathway That Leads to Hyperactive Transforming Growth Factor-β (TGFβ) Signaling Promoting SMAD3 Activation and Fibrosis via Gremlin Protein. J. Boil. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Zhu, Z.; Yang, G.; An, G.; Li, X.; Niu, P.; Chen, L.; Tian, L. BMP-7 attenuated silica-induced pulmonary fibrosis through modulation of the balance between TGFβ/SMAD and BMP-7/SMAD signaling pathway. Chem. Interact. 2016, 243, 72–81. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Hear. Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, A.; Li, L.; Zhao, G.; Jia, J.; Wang, K.; Ge, J.; Zou, Y. Up-regulation of BMP-2 antagonizes TGFβ1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/SMAD6 complex. J. Cell. Mol. Med. 2012, 16, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Jiang, B.; Liu, D. Bone Morphogenetic Protein-7 Antagonizes Myocardial Fibrosis Induced by Atrial Fibrillation by Restraining Transforming Growth Factor-β (TGFβ)/SMADs Signaling. Med. Sci. Monit. 2016, 22, 3457–3468. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cheng, X.; Lu, J.; Li, X. Exogenous BMP-7 facilitates the recovery of cardiac function after acute myocardial infarction through counteracting TGFβ1 signaling pathway. Tohoku J. Exp. Med. 2017, 244, 1–6. [Google Scholar] [CrossRef]
- Massague, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGFβ and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef]
- Mazzocca, A.; Fransvea, E.; Dituri, F.; Lupo, L.; Antonaci, S.; Giannelli, G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor β blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology 2010, 51, 523–534. [Google Scholar] [CrossRef]
- Rani, B.; Malfettone, A.; Dituri, F.; Soukupova, J.; Lupo, L.; Mancarella, S.; Fabregat, I.; Giannelli, G. Galunisertib suppresses the staminal phenotype in hepatocellular carcinoma by modulating CD44 expression. Cell Death Dis. 2018, 9, 373. [Google Scholar] [CrossRef]
- Cascione, M.; Leporatti, S.; Dituri, F.; Giannelli, G. Transforming growth factor-β promotes morphomechanical effects involved in epithelial to mesenchymal transition in living hepatocellular carcinoma. Int. J. Mol. Sci. 2019, 20, 108. [Google Scholar] [CrossRef]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, Q.; Li, Y.; Yang, Z.; Yang, L.; Zong, Z.; Wang, B.; Meng, X.; Li, Q.; Liu, J.; et al. Transforming growth factor-beta1 suppresses hepatocellular carcinoma proliferation via activation of Hippo signaling. Oncotarget 2017, 8, 29785–29794. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zaidi, S.; Rao, S.; Chen, J.S.; Phan, L.; Farci, P.; Su, X.; Shetty, K.; White, J.; Zamboni, F.; et al. Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway. Gastroenterology 2018, 154, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Rao, S.; Gough, N.R.; Schultz, A.; Li, X.; Lorenzi, P.L.; Robertson, G.; Kwong, L.N.; Datto, M.; Roszik, J.; et al. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGFβ Superfamily. Cell Syst. 2018, 7, 422–437.e7. [Google Scholar]
- Liu, Y.; Chen, J.; Yang, Y.; Zhang, L.; Jiang, W.G. Molecular impact of bone morphogenetic protein 7, on lung cancer cells and its clinical significance. Int. J. Mol. Med. 2012, 9, 1016–1024. [Google Scholar]
- Maegdefrau, U.; Bosserhoff, A.-K. BMP activated SMAD signaling strongly promotes migration and invasion of hepatocellular carcinoma cells. Exp. Mol. Pathol. 2012, 92, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wang, J.; Lu, P.; Si, X.; Han, K.; Ruan, T.; Lu, J. BMP10 inhibited the growth and migration of gastric cancer cells. Tumor Biol. 2016, 37, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Kodach, L.L.; Wiercinska, E.; De Miranda, N.F.; Bleuming, S.A.; Musler, A.R.; Peppelenbosch, M.P.; Dekker, E.; Brink, G.R.V.D.; Van Noesel, C.J.; Morreau, H.; et al. The Bone Morphogenetic Protein Pathway Is Inactivated in the Majority of Sporadic Colorectal Cancers. Gastroenterology 2008, 134, 1332–1341.e3. [Google Scholar] [CrossRef]
- Herrera, B.; Van Dinther, M.; Ten Dijke, P.; Inman, G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/SMAD1/SMAD4 to promote ovarian cancer cell proliferation. Cancer Res. 2009, 69, 9254–9262. [Google Scholar] [CrossRef]
- Ye, L.; Kynaston, H.; Jiang, W.G. Bone Morphogenetic Protein-9 Induces Apoptosis in Prostate Cancer Cells, the Role of Prostate Apoptosis Response-4. Mol. Cancer Res. 2008, 6, 1594–1606. [Google Scholar] [CrossRef]
- Lim, M.; Chuong, C.-M.; Roy-Burman, P. PI3K, Erk Signaling in BMP7-Induced Epithelial-Mesenchymal Transition (EMT) of PC-3 Prostate Cancer Cells in 2- and 3-Dimensional Cultures. Horm. Cancer 2011, 2, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Oh, S.C.; Lee, D.-H.; Kim, J.L.; Lee, S.Y.; Kang, M.H.; Lee, S.I.; Kang, S.; Joung, S.Y.; Min, B.W. BMP-2 induces motility and invasiveness by promoting colon cancer stemness through STAT3 activation. Tumor Boil. 2015, 36, 9475–9486. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, T.; Poser, I.; Soncin, F.; Bataille, F.; Moser, M.; Bosserhoff, A.-K. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005, 65. [Google Scholar]
- Cao, Y.; Slaney, C.Y.; Bidwell, B.N.; Parker, B.; Johnstone, C.N.; Rautela, J.; Eckhardt, B.L.; Anderson, R.L. BMP4 Inhibits Breast Cancer Metastasis by Blocking Myeloid-Derived Suppressor Cell Activity. Cancer Res. 2014, 74, 5091–5102. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Sun, X.; Wang, K.; Feng, H.; Liu, Y.; Fei, C.; Wan, S.; Wang, W.; Luo, J.; Shi, Q.; et al. BMP9 inhibits the bone metastasis of breast cancer cells by downregulating CCN2 (connective tissue growth factor, CTGF) expression. Mol. Boil. Rep. 2014, 41, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Ouarné, M.; Bouvard, C.; Boneva, G.; Mallet, C.; Ribeiro, J.; Desroches-Castan, A.; Soleilhac, E.; Tillet, E.; Peyruchaud, O.; Bailly, S. BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 209. [Google Scholar] [CrossRef] [PubMed]
- Townson, J.L.; Wilson, S.M.; Bramwell, V.H.; Groom, A.C.; Naumov, G.N.; Macdonald, I.C.; Chambers, A.F. Ineffectiveness of Doxorubicin Treatment on Solitary Dormant Mammary Carcinoma Cells or Late-developing Metastases. Breast Cancer Res. Treat. 2003, 82, 199–206. [Google Scholar]
- Gao, H.; Chakraborty, G.; Lee-Lim, A.P.; Mo, Q.; Decker, M.; Vonica, A.; Shen, R.; Brogi, E.; Brivanlou, A.H.; Giancotti, F.G. The BMP Inhibitor Coco Reactivates Breast Cancer Cells at Lung Metastatic sites. Cell 2012, 150, 764–779. [Google Scholar] [CrossRef]
- Kobayashi, A.; Okuda, H.; Xing, F.; Pandey, P.R.; Watabe, M.; Hirota, S.; Pai, S.K.; Liu, W.; Fukuda, K.; Chambers, C.; et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 2011, 208, 2641–2655. [Google Scholar] [CrossRef]
- Prunier, C.; Baker, D.; ten Dijke, P.; Ritsma, L. TGFβ Family Signaling Pathways in Cellular Dormancy. Trends Cancer 2019, 5, 66–78. [Google Scholar] [CrossRef]
- Naber, H.P.H.; Wiercinska, E.; Pardali, E.; Van Laar, T.; Nirmala, E.; Sundqvist, A.; Van Dam, H.; Van Der Horst, G.; Van Der Pluijm, G.; Heckmann, B.; et al. BMP-7 inhibits TGFβ-induced invasion of breast cancer cells through inhibition of integrin β 3 expression. Cell. Oncol. 2012, 35, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Koeneman, K.S. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 112–113. [Google Scholar] [CrossRef]
- Buijs, J.T.; Henriquez, N.V.; Van Overveld, P.G.; Que, I.; Schwaninger, R.; Rentsch, C.; Dijke, P.T.; Driouch, K.; Lidereau, R.; Vukicevic, S.; et al. Bone Morphogenetic Protein 7 in the Development and Treatment of Bone Metastases from Breast Cancer. Cancer Res. 2007, 67, 8742–8751. [Google Scholar] [CrossRef] [PubMed]
- Gatza, C.E.; Elderbroom, J.L.; Oh, S.Y.; Starr, M.D.; Nixon, A.B.; Blobe, G.C. The Balance of Cell Surface and Soluble Type III TGFβ Receptor Regulates BMP Signaling in Normal and Cancerous Mammary Epithelial Cells1. Neoplasia 2014, 16, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Tazat, K.; Hector-Greene, M.; Blobe, G.C.; Henis, Y.I. TβRIII independently binds type I and type II TGFβ receptors to inhibit TGFβ signaling. Mol. Boil. Cell 2015, 26, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Hempel, N.; How, T.; Cooper, S.J.; Green, T.R.; Dong, M.; Copland, J.A.; Wood, C.G.; Blobe, G.C. Expression of the type III TGFβ receptor is negatively regulated by TGFβ. Carcinogenesis 2008, 29, 905–912. [Google Scholar] [CrossRef]
- Gordon, K.J.; Kirkbride, K.C.; How, T.; Blobe, G.C. Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a SMAD1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis 2009, 30, 238–248. [Google Scholar] [CrossRef]
- Ning, J.; Zhao, Y.; Ye, Y.; Yu, J. Opposing roles and potential antagonistic mechanism between TGFβ and BMP pathways: Implications for cancer progression. EBioMedicine 2019, 41, 702–710. [Google Scholar] [CrossRef]
- Pardali, E.; Van Der Schaft, D.W.J.; Wiercinska, E.; Gorter, A.; Hogendoorn, P.C.W.; Griffioen, A.W.; Ten Dijke, P. Critical role of endoglin in tumor cell plasticity of Ewing sarcoma and melanoma. Oncogene 2011, 30, 334. [Google Scholar] [CrossRef]
- Romagnoli, M.; Belguise, K.; Yu, Z.; Wang, X.; Landesman-Bollag, E.; Seldin, D.C.; Chalbos, D.; Barillé-Nion, S.; Jézéquel, P.; Seldin, M.L.; et al. Epithelial-to-Mesenchymal Transition Induced by TGFβ1 is Mediated by Blimp-1-Dependent Repression of BMP-5. Cancer Res. 2012, 72, 6268–6278. [Google Scholar] [CrossRef]
- Duangkumpha, K.; Techasen, A.; Loilome, W.; Namwat, N.; Thanan, R.; Khuntikeo, N.; Yongvanit, P. BMP-7 blocks the effects of TGFβ-induced EMT in cholangiocarcinoma. Tumor Boil. 2014, 35, 9667–9676. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Postovit, L.M.; Margaryan, N.V.; Seftor, E.A.; Abbott, D.E.; Seftor, R.E.B.; Salomon, D.S.; Hendrix, M.J.C. Emerging roles of nodal and cripto-1: From embryogenesis to breast cancer progression. Breast Dis. 2007, 29, 91–103. [Google Scholar] [CrossRef]
- Mancino, M.; Strizzi, L.; Wechselberger, C.; Watanabe, K.; Gonzales, M.; Hamada, S.; Normanno, N.; Salomon, D.S.; Bianco, C. Regulation of human cripto-1 gene expression by TGFβ1 and BMP-4 in embryonal and colon cancer cells. J. Cell. Physiol. 2008, 215, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. BMP4 inhibits glioblastoma invasion by promoting E-cadherin and claudin expression. Front. Biosci. 2019, 24, 1060–1070. [Google Scholar] [CrossRef]
- Taguchi, L.; Miyakuni, K.; Morishita, Y.; Morikawa, T.; Fukayama, M.; Miyazono, K.; Ehata, S. c-Ski accelerates renal cancer progression by attenuating transforming growth factor β signaling. Cancer Sci. 2019, 110, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Yoon, S.-M.; Kim, S.; Jeon, Y.-H.; Yoon, B.-H.; Yang, S.-G.; Kim, M.K.; Choe, S.; Kuo, M.M.-C. Bone morphogenetic protein-9 is a potent growth inhibitor of hepatocellular carcinoma and reduces the liver cancer stem cells population. Oncotarget 2016, 7, 73754–73768. [Google Scholar] [CrossRef] [PubMed]
| Ligand | Type 1 Receptor | Type 2 Receptor | Coreceptor | Intracellular Mediator | Ref. |
|---|---|---|---|---|---|
| TGFβ1/2/3 | ALK5 | TβRII | endoglin | SMAD2/3 | [9] |
| TGFβ1/2/3 | ALK5 | TβRII | betaglycan | SMAD2/3 | [10] |
| TGFβ1/2/3 | ALK1 | TβRII | endoglin | SMAD1/5/8 | [10] |
| TGFβ1/2/3 | ALK1/2 | ACVR2A, BMPR2 | SMAD2/3, SMAD1/5/8 | [10] | |
| BMP1 | BMPR1A | [11] | |||
| BMP2 | ALK2/3 | BMPR2, ACVR2A, ACVR2B | SMAD1/5/8, p38 | [12,13] | |
| BMP2 | ALK3 | BMPR2, ACVR2A, ACVR2B | SMAD2/3 | [14] | |
| BMP2 | ALK2/3 | BMPR2, ACVR2A | SMAD1/5/8 | [15] | |
| BMP4 | ALK1/2 | BMPR2 | SMAD1/5 | [16] | |
| BMP4 | ALK3 | BMPR2 | SMAD1/5, p38/ERK | [16] | |
| BMP9/10 | ALK1 | BMPR2, ACVR2A, ACVR2B | endoglin | SMAD1/5/8 | [17,18] |
| BMP6/7 | BMPRI | ACVR2A, BMPR2 | SMAD1/5/8, PI3K | [19] | |
| BMP6/7 | BMPRI | BMPRII | SMAD1/5/8 | [20] | |
| BMP6/7/9 | ALK2 | ACVR2A, ACVR2B | SMAD1/5 | [21] | |
| BMP2/6 | Alk2 | ACVR2A | SMAD1/5/8 | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dituri, F.; Cossu, C.; Mancarella, S.; Giannelli, G. The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells 2019, 8, 1130. https://doi.org/10.3390/cells8101130
Dituri F, Cossu C, Mancarella S, Giannelli G. The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells. 2019; 8(10):1130. https://doi.org/10.3390/cells8101130
Chicago/Turabian StyleDituri, Francesco, Carla Cossu, Serena Mancarella, and Gianluigi Giannelli. 2019. "The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer" Cells 8, no. 10: 1130. https://doi.org/10.3390/cells8101130
APA StyleDituri, F., Cossu, C., Mancarella, S., & Giannelli, G. (2019). The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells, 8(10), 1130. https://doi.org/10.3390/cells8101130






