Patient-Derived Non-Muscular Invasive Bladder Cancer Xenografts of Main Molecular Subtypes of the Tumor for Anti-Pd-l1 Treatment Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Clinical Data, Patient-Derived Xenografts Establishment
2.4. Specific Intervention and Animals’ Surveillance, Pain Control
2.5. Immunohistochemistry (IHC)
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Statistical Data Analysis
3. Results
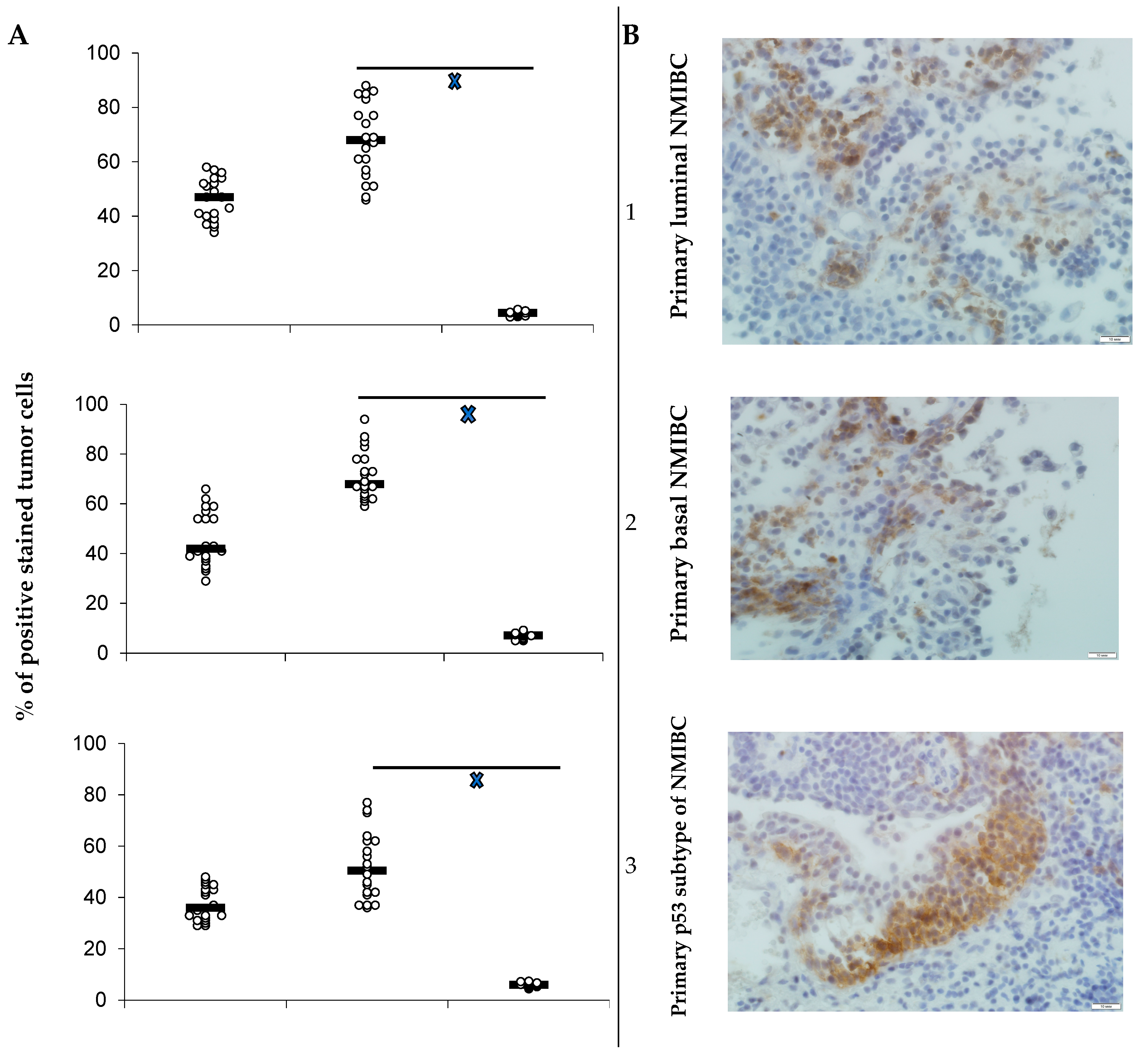
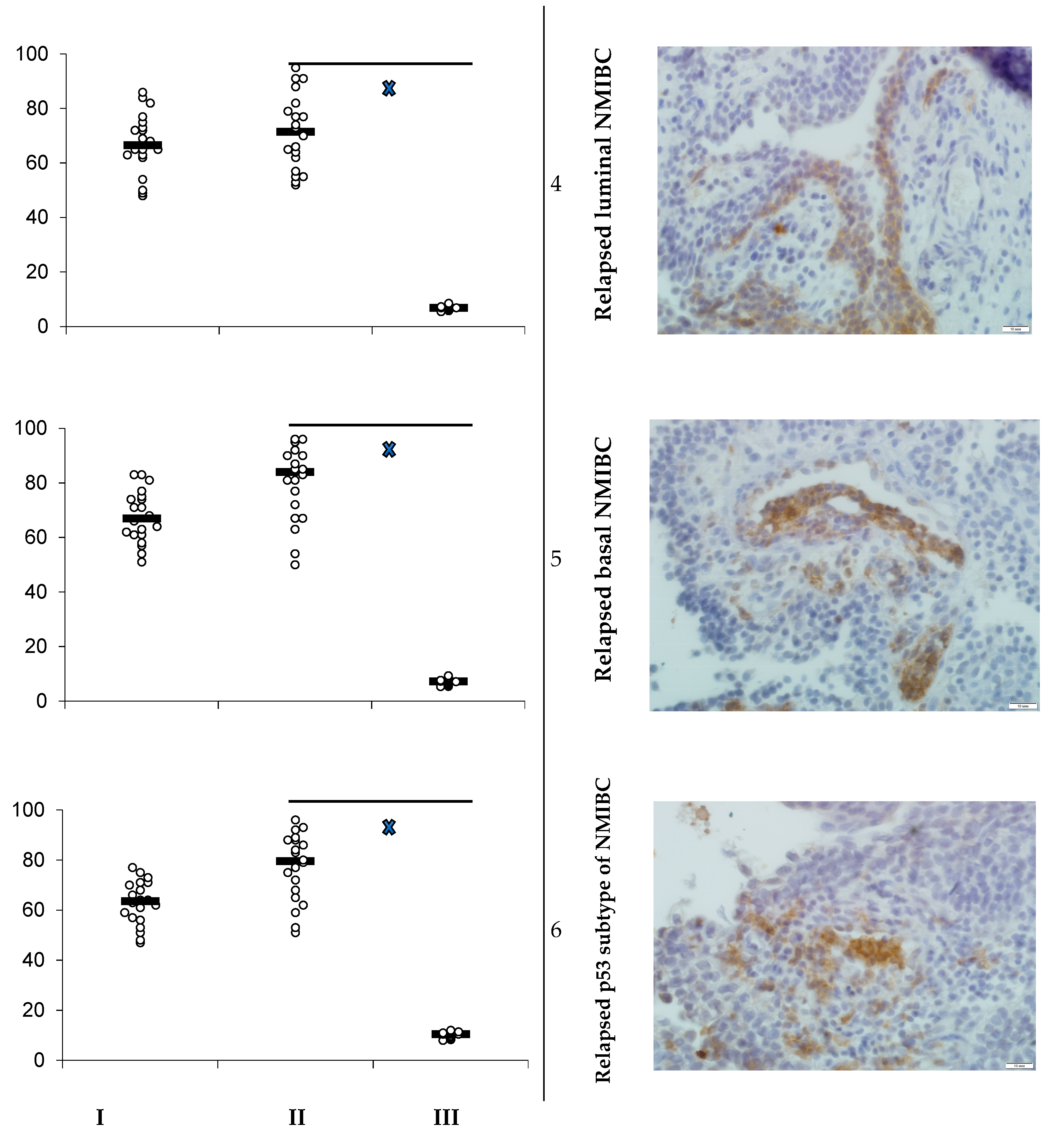
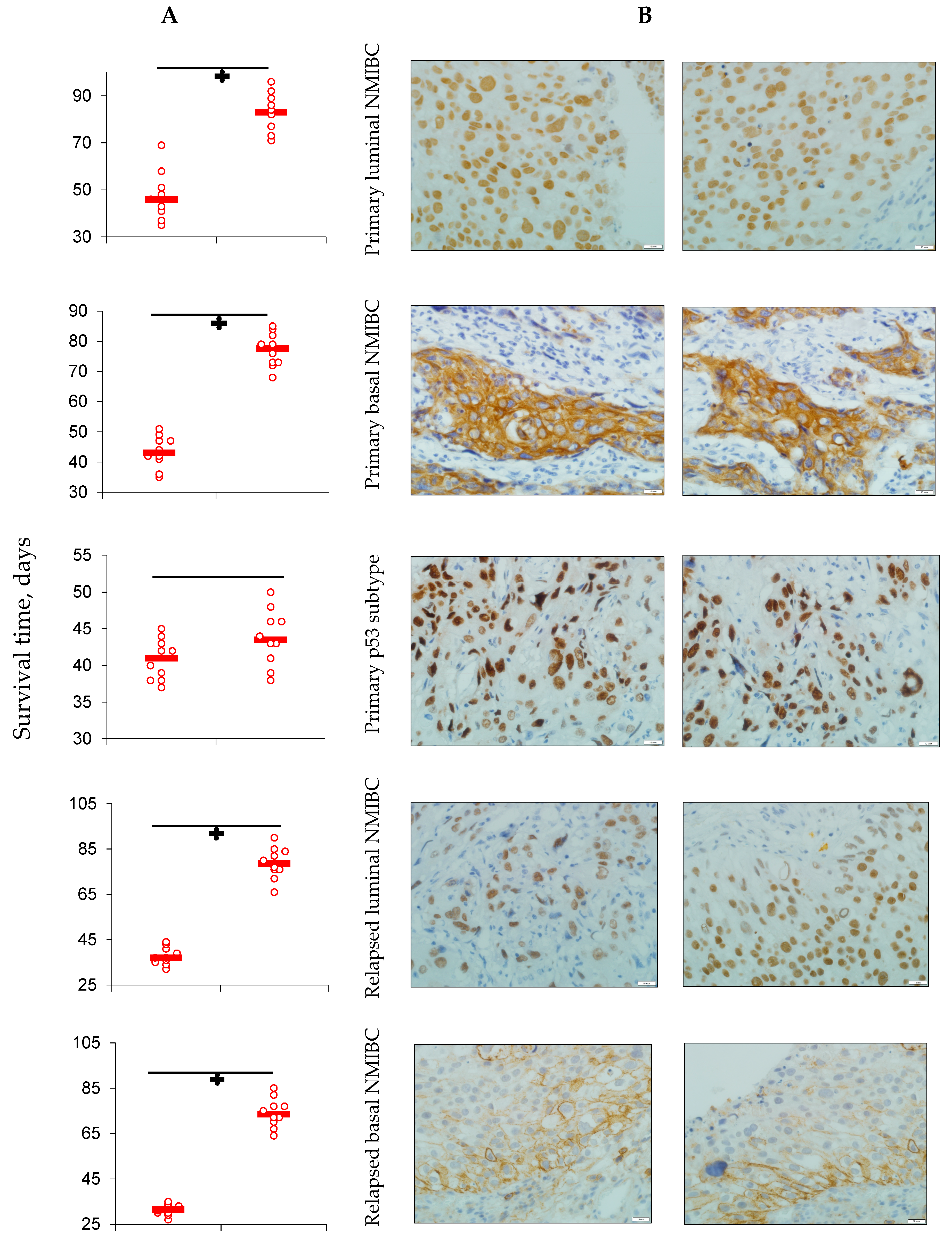
3.1. PD-L1 Expression and Animals’ Survival
3.2. Tumor Growth
3.3. Anti-Metastatic Property
3.4. PDXs’ CD8+ Cells Population and Serum PD-L1 Level Assessment
4. Discussion
5. Conclusions
- Heterotopic primary and relapsed luminal, basal, and p53 subtypes of NMIBC PDXs were established. More than 25% of counted tumor cells of all PDX specimens expressed PD-L1, so the tumors were ranged as PD-L1 positive.
- Specific anti-PD-L1 treatment sufficiently decreased the number of PD-L1-positive cells in PDXs of all Durvalumab-treated mice. Survival of the animals that were PDX carriers was different in subgroups and depended on both the tumor molecular type and intervention implemented. Survival of animals with relapsed lines of bladder cancer was the shortest among non-invasive tumors.
- Anti-PD-L1 intervention prolonged animals’ life expectancy and depressed tumor growth in the majority of subgroups assigned to treatment, except ones with the primary and relapsed p53 subtype of NMIBC.
- Bad response of p53 mutant subtypes of primary and relapsed NMIBC on specific anti-PD-L1 treatment with high probability was associated with low CD8+ subpopulation of T cells representation into the tumors tissue, which led to the loss of the intervention implementation site.
- Durvalumab inhibited metastatic activity in all subgroups of animals that were PD-L1-expressed bladder carcinoma PDX carriers.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. Oncotarget 2016, 12, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Eifler, J.B.; Scarpato, K.R.; Clark, P.E. Management of noninvasive bladder cancers. Curr. Opin. Oncol. 2015, 27, 185–190. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.; Yang, L.; Li, Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 2013, 6, 74. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zheng, F.F.; Mao, Y.L.; Ye, L.F.; Bian, J.; Lai, D.H.; Ye, Y.-L.; Dai, Y.-P. Effects of programmed death-ligand 1 expression on OK-432 immunotherapy following transurethral resection in non-muscle invasive bladder cancer. Oncol. Lett. 2017, 13, 4818–4824. [Google Scholar] [CrossRef] [Green Version]
- Brower, V. Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol. 2016, 17, 275. [Google Scholar] [CrossRef]
- Chijiwa, T.; Kawai, K.; Noguchi, A.; Sato, H.; Hayashi, A.; Cho, H.; Shiozawa, M.; Kishida, T.; Morinaga, S.; Yokose, T.; et al. Establishment of patient-derived cancer xenografts in immunodeficient NOG mice. Int. J. Oncol. 2015, 47, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Xu, H.; Su, Y.; Wu, W.; Hao, L.; Han, C. Establishment of a novel bladder cancer xenograft model in humanized immunodeficient mice. Cell Physiol. Biochem. 2015, 37, 1355–1368. [Google Scholar] [CrossRef]
- Blinova, E.V.; Dudina, M.O.; Suslova, I.R.; Samishina, E.A.; Blinov, D.S.; Roshchin, D.A. Novel aminochromone derivative inhibits tumor growth on xenograft model of lung cancer in mice. J. Adv. Pharm. Technol. Res. 2018, 9, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Sheinin, Y.; Crispen, P.L.; Farmer, S.A.; Lohse, C.M.; Kuntz, S.M.; Leibovich, B.C.; Kwon, E.D.; Frank, I. T-cell coregulatory molecule expression in urothelial cell carcinoma: Clinicopathologic correlations and association with survival. Clin. Cancer Res. 2008, 14, 4800–4808. [Google Scholar] [CrossRef] [PubMed]
- Huebner, D.; Rieger, C.; Bergmann, R.; Ullrich, M.; Meister, S.; Toma, M.; Wiedemuth, R.; Temme, A.; Novotny, V.; Wirth, M.P.; et al. An orthotopic xenograft model for high-risk non-muscle invasive bladder cancer in mice: Influence of mouse strain, tumor cell count, dwell time and bladder pretreatment. BMC Cancer 2017, 17, 790. [Google Scholar] [CrossRef] [PubMed]
- Imfinzi™ (durvalumab). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/durvalumab-imfinzi (accessed on 4 December 2018).
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.J.; et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef]
- Gad, S.C. The mouse. In Animal Models in Toxicology, 2nd ed.; Gad, S.C., Ed.; Taylor and Francis: New York, NY, USA, 2007; pp. 19–147. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against tumor and other biological systems. Cancer Chemother. Rep. 1972, 3, 100–103. [Google Scholar]
- Carbone, L. Pain in laboratory animals: The ethical and regulatory imperatives. PLoS ONE 2011, 6, 21578. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; La Croix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Girard, P.; Verniers, D.; Coppé, M.C.; Pansart, Y.; Gillardin, J.M. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur. J. Pharm. 2008, 584, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, 1002128. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Motamed, K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am. J. Pathol. 2005, 167, 1739–1752. [Google Scholar] [CrossRef]
- Pendleton, C.; Li, Q.; Chesler, D.A.; Yuan, K.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. Mesenchymal stem cells derived from adipose tissue vs. bone marrow: In vitro comparison of their tropism towards gliomas. PLoS ONE 2013, 8, 58198. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Vandeveer, A.J.; Fallon, J.K.; Tighe, R.; Sabzevari, H.; Schlom, J.; Greiner, J.W. Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti-PD-L1 immune checkpoint inhibitor. Cancer Immunol. Res. 2015, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Worst, T.S.; Stoehr, R.; Eckstein, M.; Denzinger, S.; Burger, M.; Hartmann, A. High PDL1 mRNA expression predicts better survival of stage pT1 non-muscle-invasive bladder cancer (NMIBC) patients. Cancer Immunol. Immunother. 2018, 67, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Ploussard, G.; LeMaoult, J.; Desgrandchamps, F. A systematic review of immunotherapy in urologic cancer: Evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur. Urol. 2015, 68, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Robinson, B.D.; Kluth, L.A.; Volkmer, B.G.; Hautmann, R.; Küfer, R.; Zerbib, M.; Kwon, E.; Thompson, R.H.; Boorjian, S.A.; et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. 2014, 40, 121–127. [Google Scholar] [CrossRef]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015, 7, 65–79. [Google Scholar]
- Jovanovic, D.; Roksandic Milenkovic, M.; Kotur Stevuljevic, J.; Markovic, J.; Ceriman, V.; Kontic, M.; Skodric Trifunovic, V. Membrane PD-L1 expression and soluble PD- L1 plasma levels in idiopathic pulmonary fibrosis—A pilot study. J. Thorac. Dis. 2018, 12, 6660–6669. [Google Scholar] [CrossRef]
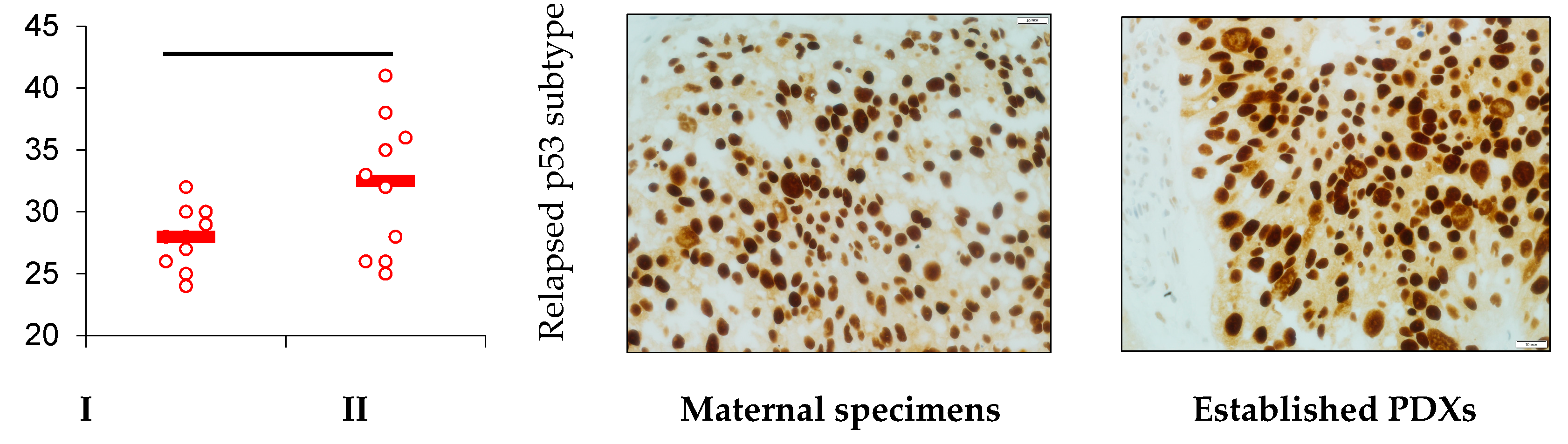
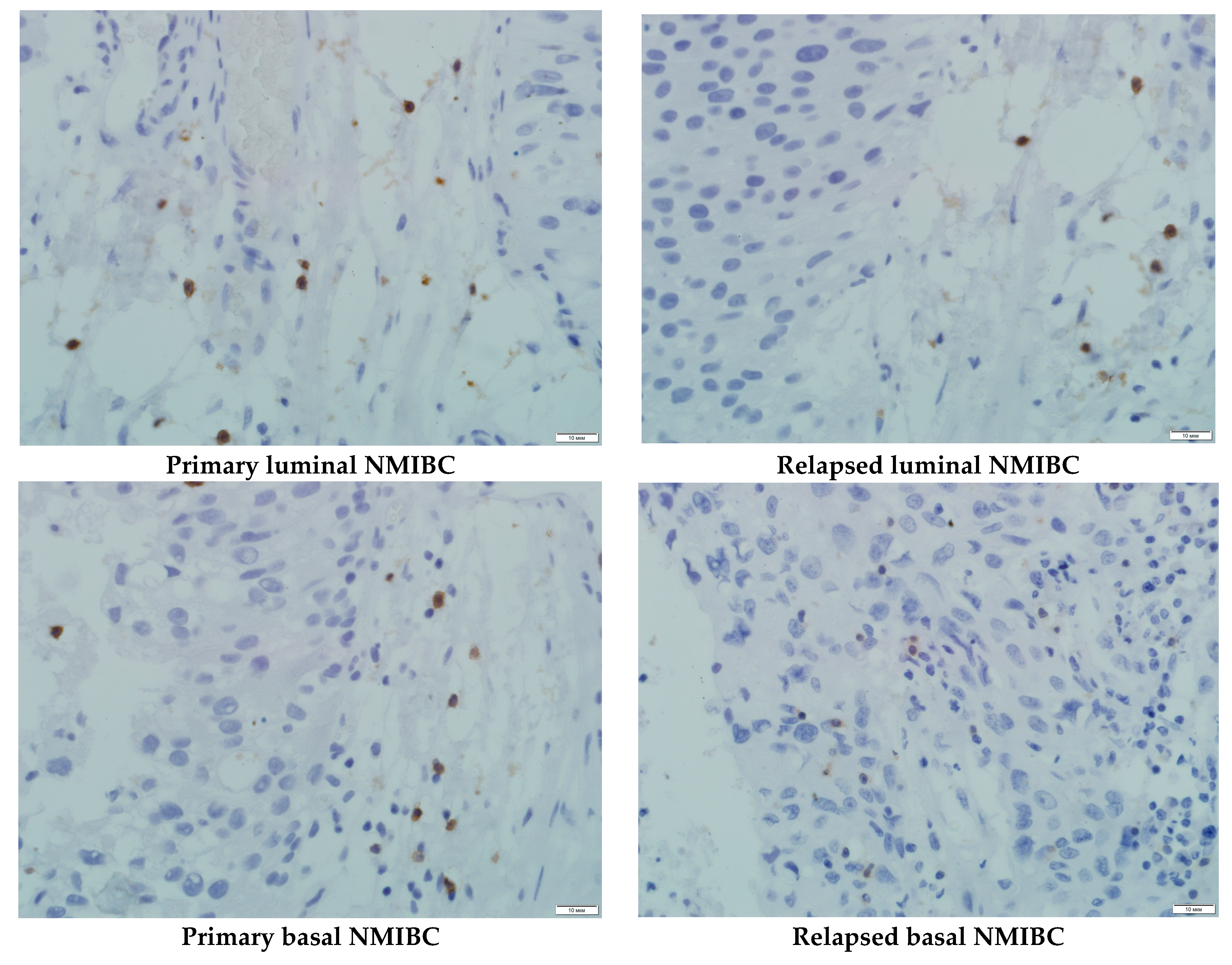
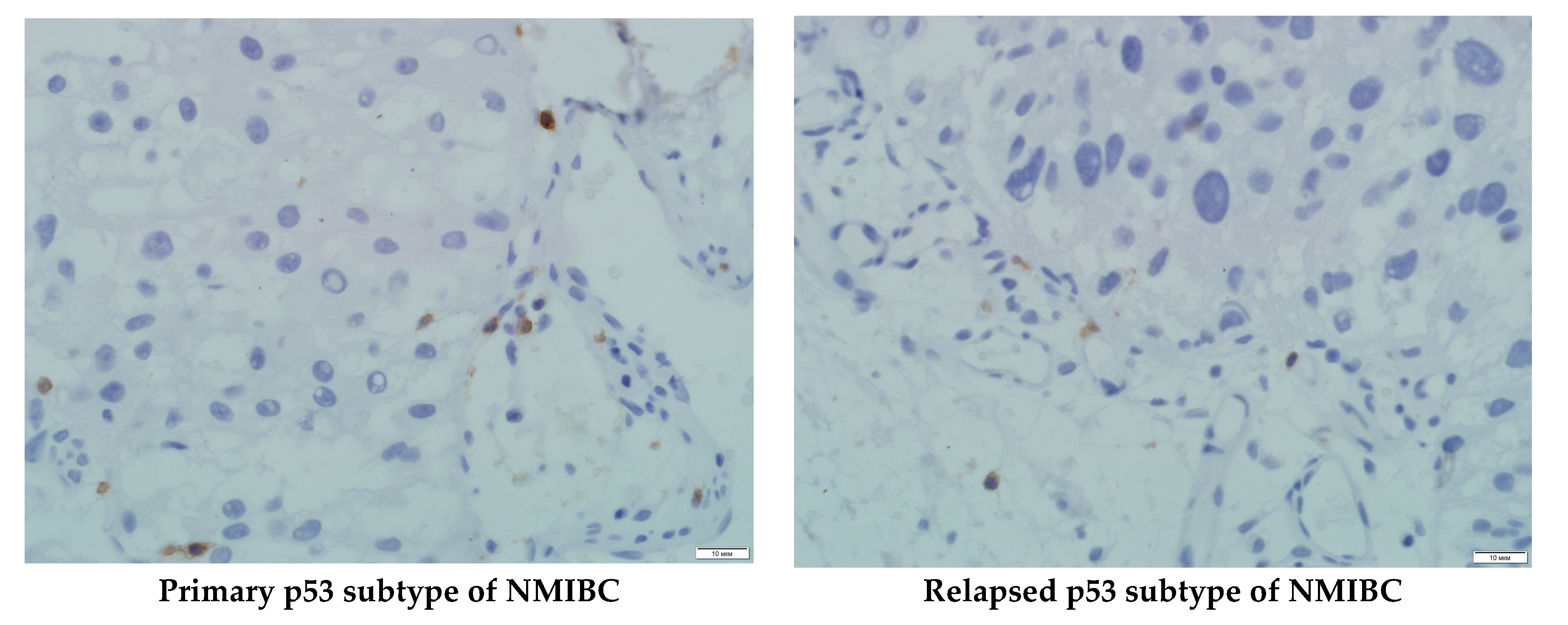
| No | Sex | Age | The Tumor Source | Tumor Histology | Grade, Stage | PDX | PDX’s Metastasis |
|---|---|---|---|---|---|---|---|
| 1 | Male | 47 | Primarynode | Urothelial papillary carcinoma | Grade 1 T1 | Established | Lung |
| 2 | Female | 67 | Primarynode | Glandular carcinoma | Garde 2 T1 | Established | Lung |
| 3 | Male | 61 | Primarynode | Micropapillary carcinoma | Grade 2 T1 | Established | Lung |
| 4 | Male | 53 | Relapsed node | Urothelial papillary carcinoma | Grade 3 T1 | Established | Lung |
| 5 | Female | 72 | Relapsed node | Squamous carcinoma | Grade 2 T1 | Established | Lung |
| 6 | Male | 59 | Relapsed node | Urothelial papillary carcinoma | Grade 3 T1 | Established | Lung |
| Tumor Subtype | T/C Index | Tumor-Doubling Time, Days M ± SEM | Number of Metastasis, M ± SEM | |||
|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | ||||
| Primary luminal NMIBC | 89 | 80 | 76 | V | 9.4 ± 0.3 | 24.6 ± 3.9 |
| D | 21.2 ± 2.8 † | 0 ± 0 † | ||||
| Primary basal NMIBC | 71 | 65 | 63 | V | 10.8 ± 1.6 | 16.5 ± 2.4 |
| D | 17.3 ± 1.7 † | 4.3 ± 1.7 † | ||||
| p53 NMIBC | 32 | 17 | 4 | V | 8.3 ± 0.9 | 44.7 ± 4.5 ‡ |
| D | 12.7 ± 2.6 | 16.2 ± 5.8 † | ||||
| Relapsed luminal NMIBC | 76 | 62 | 50 | V | 9.6 ± 0.7 | 56.1 ± 6.4 ‡ |
| D | 15.2 ± 1.4 † | 13.5 ± 4.3 † | ||||
| Relapsed basal NMIBC | 77 | 58 | 53 | V | 10.0 ± 0.5 | 47.8 ± 6.1 ‡ |
| D | 18.3 ± 1.9 † | 7.2 ± 3.5 † | ||||
| Relapsed p53 NMIBC | 47 | 29 | 17 | V | 10.1 ± 0.7 | 63.4 ± 7.5 ‡ |
| D | 13.4 ± 2.1 | 22.8 ± 5.4 † | ||||
| No | Tumor Subtype | Subgroup | CD8+ Expression, % | sPD-L1, ng/mL | Correlation |
|---|---|---|---|---|---|
| 1 | Primary luminal NMIBC | V | 27.6 ± 2.7 | 17.6 ± 1.4 | r = 0.17 p = 0. 4 |
| D | 33.4 ± 4.1 | 2.7 ± 0.5 † | r = −0.99 p = 0.001 | ||
| 2 | Primary basal NMIBC | V | 18.5 ± 3.2 | 21.8 ± 4.3 | r = 0.15 p = 0.3 |
| D | 25.7 ± 2.9 | 4.1 ± 1.3 † | r = −0.93 p = 0.001 | ||
| 3 | Primary p53 NMIBC | V | 3.6 ± 1.1 ‡ | 31.5 ± 3.6 ‡ | r = −0.99 p = 0.001 |
| D | 5.4 ± 2.3 ‡ | 8.4 ± 2.8 ‡† | r = 0.15 p = 0.3 | ||
| 4 | Relapsed luminal NMIBC | V | 19.2 ± 2.1 | 25.4 ± 2.0 | r = 0.19 p = 0.3 |
| D | 26.1 ± 3.5 | 6.2 ± 0.8 † | r = −0.99 p = 0.001 | ||
| 5 | Relapsed basal NMIBC | V | 22.5 ± 2.1 | 18.4 ± 1.3 | r = 0.16 p = 0.4 |
| D | 27.7 ± 3.8 | 5.2 ± 1.2 † | r = −0.97 p = 0.001 | ||
| 6 | Relapsed p53 NMIBC | V | 2.1 ± 0.9 ‡ | 37.5 ± 3.8 ‡ | r = −0.99 p = 0.001 |
| D | 4.2 ± 1.5 ‡ | 10.3 ± 2.1 ‡† | r = −0.38 p = 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, E.; Roshchin, D.; Kogan, E.; Samishina, E.; Demura, T.; Deryabina, O.; Suslova, I.; Blinov, D.; Zhdanov, P.; Osmanov, U.; et al. Patient-Derived Non-Muscular Invasive Bladder Cancer Xenografts of Main Molecular Subtypes of the Tumor for Anti-Pd-l1 Treatment Assessment. Cells 2019, 8, 526. https://doi.org/10.3390/cells8060526
Blinova E, Roshchin D, Kogan E, Samishina E, Demura T, Deryabina O, Suslova I, Blinov D, Zhdanov P, Osmanov U, et al. Patient-Derived Non-Muscular Invasive Bladder Cancer Xenografts of Main Molecular Subtypes of the Tumor for Anti-Pd-l1 Treatment Assessment. Cells. 2019; 8(6):526. https://doi.org/10.3390/cells8060526
Chicago/Turabian StyleBlinova, Ekaterina, Dmitry Roshchin, Evgenya Kogan, Elena Samishina, Tatiana Demura, Olga Deryabina, Irina Suslova, Dmitry Blinov, Pavel Zhdanov, Usif Osmanov, and et al. 2019. "Patient-Derived Non-Muscular Invasive Bladder Cancer Xenografts of Main Molecular Subtypes of the Tumor for Anti-Pd-l1 Treatment Assessment" Cells 8, no. 6: 526. https://doi.org/10.3390/cells8060526