PTH and the Regulation of Mesenchymal Cells within the Bone Marrow Niche
Abstract
:1. Introduction
2. Role and Function of Mesenchymal Cells in the BM Niche
2.1. Skeletal Stem Cells
2.2. Osteoblasts and Osteocytes
2.3. Adipocytes
2.4. Endothelial Cells (ECs)
3. The Direct Interactions of PTH with BM Cells
3.1. Skeletal Stem Cells (SSCs) as Targets of PTH
3.1.1. Proliferation and Osteogenesis of SSCs Induced by PTH
3.1.2. Adipogenesis and Its Regulation by PTH
3.1.3. Chondrogenesis and Its Regulation by PTH
3.1.4. PTH and Its Regulation of Angiogenesis
3.1.5. Indirect Effect of PTH on Hematopoiesis via Regulating SSCs
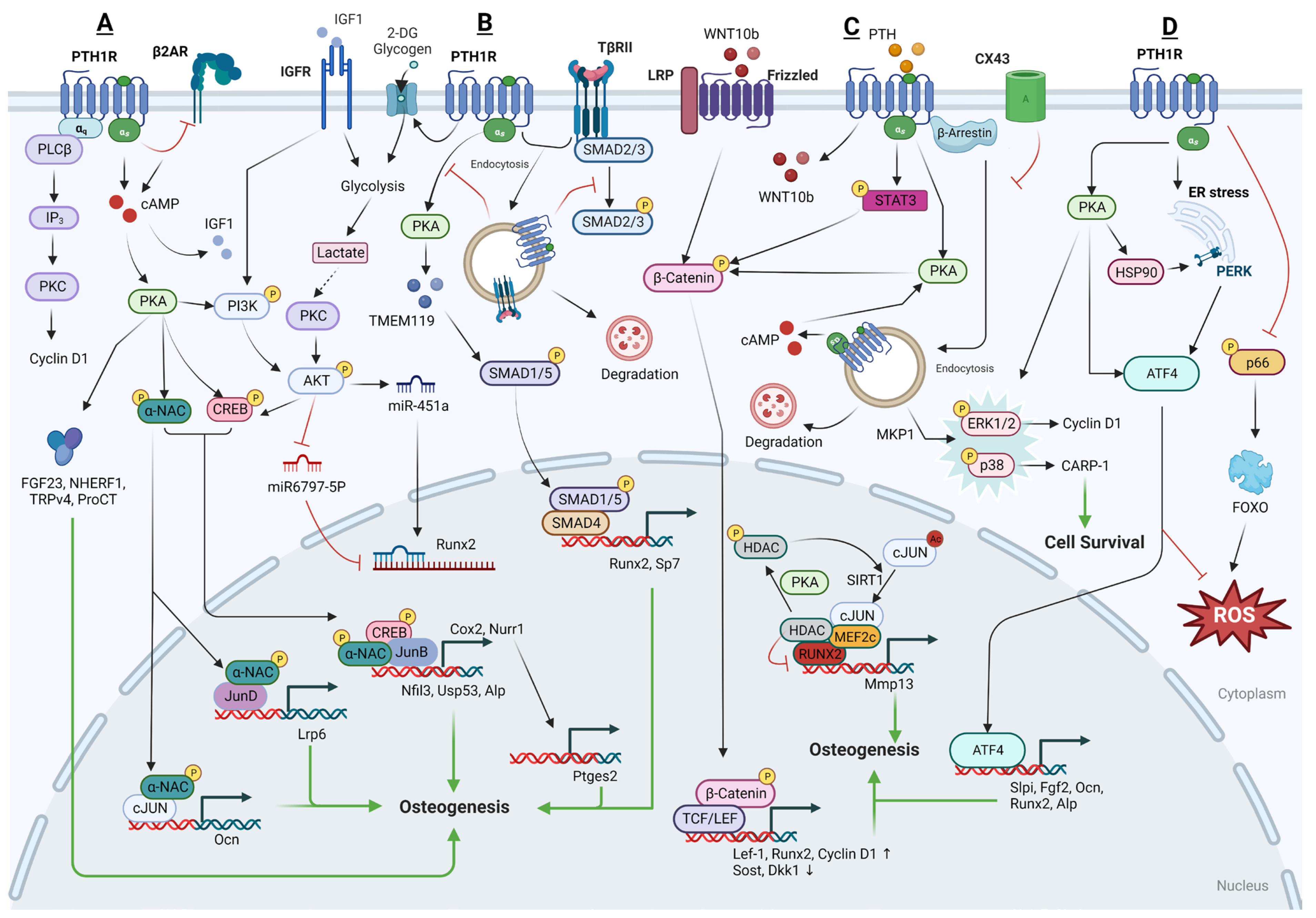
3.1.6. The Downstream Regulatory Networks of PTH in SSCs
- a.
- cAMP/PKA pathway and Ca2+/PKC pathway
- b.
- Wnt signaling pathway
- c.
- TGF-β signaling pathway
- d.
- BMP signaling pathway
- e.
- MAPK pathway
- f.
- Key effectors of PTH action
- g.
- Other modulators of PTH
3.2. Osteoblasts and Osteocytes as Targets
- (1)
- Cellular actions of PTH contribute to increased bone formation: anabolism of bone
3.2.1. Osteoblasts
- a.
- The downstream regulatory network of PTH action in osteoblasts
- PKA and PKC pathways
- PKA pathway
- PKC pathway in osteoblasts
- Wnt pathway in osteoblasts
- TGF-β pathways in osteoblasts
- IGF1-IGF1R signaling in osteoblasts
- MAPK pathway in osteoblasts
- Extracellular Signal-Regulated Kinases (ERK) pathway
- p38 pathway
- MMP13 pathway in osteoblasts
- Endoplasmic reticulum (ER) stress and oxidative stress
- Other pathways induced by PTH
- b.
- Cellular processes and other regulatory mechanisms mediated by PTH in osteoblasts
- PTH1R endocytosis
- Bioenergetic pathways activated by PTH
- Micro-RNA
3.2.2. Osteocytes and PTH
- The downstream regulatory network of PTH function in osteocytes
- PKA-SIK pathway
- Wnt pathway
- Notch pathway
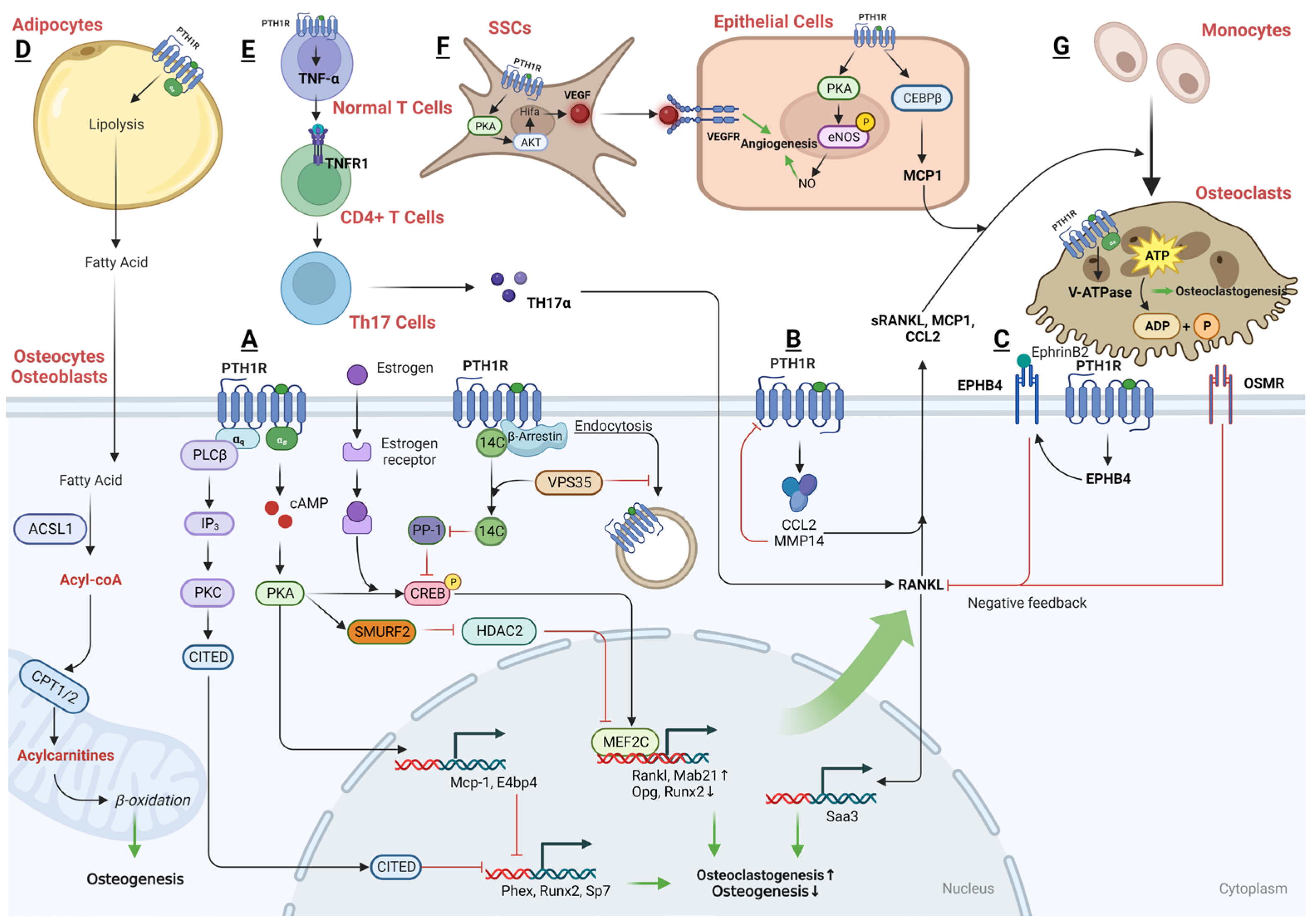
3.2.3. The Downstream Regulatory Networks of PTH That Are Anabolic in T Cells
- (2)
- Cellular actions of PTH as a catabolic modulator
3.2.4. The Downstream Regulatory Networks of PTH That Are Catabolic in Both Osteoblasts and Osteocytes
- PKA and PKC pathways
- PKA pathway
- PKC pathway
- OPG-RANKL
- PTH1R endocytosis
3.2.5. The Downstream Regulatory Networks of PTH That Are Catabolic in T Cells
3.3. Adipocytes and PTH
3.4. ECs and PTH
3.5. PTHrP as the Therapeutic Target for Skeletal Metastasis of Unknown Primary (SMUP)
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Hao, Z.; Hu, Y.; Li, J. Parathyroid hormone and its related peptides in bone metabolism. Biochem. Pharmacol. 2021, 192, 114669. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Fogelman, I.; Hampson, G. Treatment of post-menopausal osteoporosis: Beyond bisphosphonates. J. Endocrinol. Investig. 2015, 38, 13–29. [Google Scholar] [CrossRef]
- Rendina-Ruedy, E.; Rosen, C.J. Parathyroid hormone (PTH) regulation of metabolic homeostasis: An old dog teaches us new tricks. Mol. Metab. 2022, 60, 101480. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.-Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Schipani, E. PTH and stem cells. J. Endocrinol. Investig. 2011, 34, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Morita, Y.; Ooehara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef]
- Agarwal, P.; Isringhausen, S.; Li, H.; Paterson, A.J.; He, J.; Gomariz, A.; Nagasawa, T.; Nombela-Arrieta, C.; Bhatia, R. Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell 2019, 24, 769–784 e766. [Google Scholar] [CrossRef]
- Omatsu, Y.; Sugiyama, T.; Kohara, H.; Kondoh, G.; Fujii, N.; Kohno, K.; Nagasawa, T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010, 33, 387–399. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Baryawno, N.; Przybylski, D.; Kowalczyk, M.S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K.D.; Mercier, F.; Tabaka, M.; Hofree, M.; et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 2019, 177, 1915–1932 e1916. [Google Scholar] [CrossRef]
- Zaidi, M.; Yuen, T.; Sun, L.; Rosen, C.J. Regulation of Skeletal Homeostasis. Endocr. Rev. 2018, 39, 701–718. [Google Scholar] [CrossRef]
- Ding, P.; Gao, C.; Gao, Y.; Liu, D.; Li, H.; Xu, J.; Chen, X.; Huang, Y.; Zhang, C.; Zheng, M.; et al. Osteocytes regulate senescence of bone and bone marrow. Elife 2022, 11, 81480. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; McGuinness, C.S.; Doherty-Boyd, W.S.; Salmeron-Sanchez, M.; Donnelly, H.; Dalby, M.J. Current insights into the bone marrow niche: From biology in vivo to bioengineering ex vivo. Biomaterials 2022, 286, 121568. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.J.; de Araujo, I.M.; Carvalho, A.L.; Elias, J., Jr.; Salmon, C.E.; Nogueira-Barbosa, M.H. The Relationship of Fat Distribution and Insulin Resistance with Lumbar Spine Bone Mass in Women. PLoS ONE 2015, 10, e0129764. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.J.A.; Rosen, C.J. Marrow Adipocytes: Origin, Structure, and Function. Annu. Rev. Physiol. 2020, 82, 461–484. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Wang, T.; Lin, J.M. Role of Marrow Adipocytes in Regulation of Energy Metabolism and Bone Homeostasis. Curr. Osteoporos. Rep. 2018, 16, 116–122. [Google Scholar] [CrossRef]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kwekkeboom, J.C.; Westhoff, S.; Giera, M.; Rombouts, Y.; van Harmelen, V.; Huizinga, T.W.; Deelder, A.; Kloppenburg, M.; Toes, R.E. Adipocyte-derived lipids modulate CD4+ T-cell function. Eur. J. Immunol. 2013, 43, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Chen, J.; De Angelis, J.; Singh, A.; Owen-Woods, C.; Ding, Z.; Pujol, J.M.; Kumar, N.; Zeng, F.; Ramasamy, S.K.; et al. Lymphatic vessels in bone support regeneration after injury. Cell 2023, 186, 382–397. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gensure, R.C.; Gardella, T.J.; Juppner, H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem. Biophys. Res. Commun. 2005, 328, 666–678. [Google Scholar] [CrossRef]
- Nishida, S.; Yamaguchi, A.; Tanizawa, T.; Endo, N.; Mashiba, T.; Uchiyama, Y.; Suda, T.; Yoshiki, S.; Takahashi, H. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone 1994, 15, 717–723. [Google Scholar] [CrossRef]
- van der Horst, G.; Farih-Sips, H.; Löwik, C.W.; Karperien, M. Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 2233–2244. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Jones, E.; Giannoudis, P.V. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J. Orthop. Trauma 2010, 24, 552–556. [Google Scholar] [CrossRef]
- Kulkarni, N.H.; Wei, T.; Kumar, A.; Dow, E.R.; Stewart, T.R.; Shou, J.; N’Cho, M.; Sterchi, D.L.; Gitter, B.D.; Higgs, R.E.; et al. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J. Cell. Biochem. 2007, 102, 1504–1518. [Google Scholar] [CrossRef]
- Yang, M.; Arai, A.; Udagawa, N.; Zhao, L.; Nishida, D.; Murakami, K.; Hiraga, T.; Takao-Kawabata, R.; Matsuo, K.; Komori, T.; et al. Parathyroid Hormone Shifts Cell Fate of a Leptin Receptor-Marked Stromal Population from Adipogenic to Osteoblastic Lineage. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Dorado, G.; Giner, M.; Montoya, M.J.; Navarro-Valverde, C.; Díez-Pérez, A.; Quesada-Gómez, J.M. Proof of Concept on Functionality Improvement of Mesenchymal Stem-Cells, in Postmenopausal Osteoporotic Women Treated with Teriparatide (PTH1-34), After Suffering Atypical Fractures. Calcif. Tissue Int. 2019, 104, 631–640. [Google Scholar] [CrossRef]
- Osagie-Clouard, L.; Sanghani-Kerai, A.; Coathup, M.; Meeson, R.; Briggs, T.; Blunn, G. The influence of parathyroid hormone 1-34 on the osteogenic characteristics of adipose- and bone-marrow-derived mesenchymal stem cells from juvenile and ovarectomized rats. Bone Jt. Res. 2019, 8, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xia, H.; Kang, L.; Sun, Q.; Su, Z.; Hao, C.; Xue, Y. Effects of Intermittent Parathyroid Hormone 1-34 Administration on Circulating Mesenchymal Stem Cells in Postmenopausal Osteoporotic Women. J. Pharmacol. Exp. Ther. 2019, 25, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.J.; Wang, F.L.; Rodriguez-Rojas, A.M.; Wu, Z.; Trice, W.J.; Hoffman, S.J.; Votta, B.; Stroup, G.B.; Kumar, S.; Nuttall, M.E. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone 2006, 39, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, W.; Wei, L.; Zhou, Q.; Yang, G.; Qian, N.; Tang, Y.; Gao, Y.; Jiang, X. Early effects of parathyroid hormone on vascularized bone regeneration and implant osseointegration in aged rats. Biomaterials 2018, 179, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Casado-Diaz, A.; Santiago-Mora, R.; Quesada, J.M. The N- and C-terminal domains of parathyroid hormone-related protein affect differently the osteogenic and adipogenic potential of human mesenchymal stem cells. Exp. Mol. Med. 2010, 42, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lan, S.; Zhu, J.; Lin, T.; Zhang, X.; Siclari, V.A.; Altman, A.R.; Cengel, K.A.; Liu, X.S.; Qin, L. PTH prevents the adverse effects of focal radiation on bone architecture in young rats. Bone 2013, 55, 449–457. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, S.; Du, X.; Bai, B. Parathyroid Hormone-Induced Bone Marrow Mesenchymal Stem Cell Chondrogenic Differentiation and its Repair of Articular Cartilage Injury in Rabbits. Med. Sci. Monit. Basic Res. 2016, 22, 132–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Kumagai, K.; Saito, T. Effect of parathyroid hormone on early chondrogenic differentiation from mesenchymal stem cells. J. Orthop. Surg. Res. 2014, 9, 68. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Holz, J.D.; Rutkowski, T.; Wang, Y.; Zhu, Z.; Dong, Y. Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS ONE 2013, 8, e74255. [Google Scholar] [CrossRef]
- Music, E.; Futrega, K.; Palmer, J.S.; Kinney, M.; Lott, B.; Klein, T.J.; Doran, M.R. Intermittent parathyroid hormone (1-34) supplementation of bone marrow stromal cell cultures may inhibit hypertrophy, but at the expense of chondrogenesis. Stem Cell Res. Ther. 2020, 11, 321. [Google Scholar] [CrossRef]
- Shao, L.T.; Luo, L.; Qiu, J.H.; Deng, D.Y.B. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res. Ther. 2022, 24, 96. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, T.; Cui, H.; Lv, Y.; Gao, D.; Ruan, C.; Zhang, X.; Zhang, Y. Parathyroid hormone (1-34) promotes the effects of 3D printed scaffold-seeded bone marrow mesenchymal stem cells on meniscus regeneration. Stem Cell Res. Ther. 2020, 11, 328. [Google Scholar] [CrossRef]
- Sun, Q.; Zhen, G.; Li, T.P.; Guo, Q.; Li, Y.; Su, W.; Xue, P.; Wang, X.; Wan, M.; Guan, Y.; et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. Elife 2021, 10, 66532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pi, C.; Cui, C.; Zhou, Y.; Liu, B.; Liu, J.; Xu, X.; Zhou, X.; Zheng, L. PTHrP promotes subchondral bone formation in TMJ-OA. Int. J. Oral Sci. 2022, 14, 37. [Google Scholar] [CrossRef]
- Zhao, S.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshida, T.; Haraguchi, M.; de Freitas, P.H.L.; Li, M.; Tei, K.; et al. Intermittent PTH Administration Increases Bone-Specific Blood Vessels and Surrounding Stromal Cells in Murine Long Bones. Calcif. Tissue Int. 2021, 108, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, C.; Shi, H.; Cheng, Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS ONE 2019, 14, e0226163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.; Ren, B.; Feng, Q.; Li, B.; Hao, Z.; Chen, T.; Hu, Y.; Huang, Y.; Zhang, Q.; et al. A Novel PTH-Related Peptide Combined With 3D Printed Macroporous Titanium Alloy Scaffold Enhances Osteoporotic Osseointegration. Adv. Healthc. Mater. 2023, 12, e2301604. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.J.; Kim, M.Y.; Lim, Y.J.; Seol, I.J.; Jin, H.J.; Jang, Y.K.; Choi, S.J.; Oh, W.; Cho, Y.H.; et al. Human parathyroid hormone increases the mRNA expression of the IGF system and hematopoietic growth factors in osteoblasts, but does not influence expression in mesenchymal stem cells. J. Pediatr. Hematol. Oncol. 2012, 34, 491–496. [Google Scholar] [CrossRef]
- Yao, H.; Miura, Y.; Yoshioka, S.; Miura, M.; Hayashi, Y.; Tamura, A.; Iwasa, M.; Sato, A.; Hishita, T.; Higashi, Y.; et al. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells 2014, 32, 2245–2255. [Google Scholar] [CrossRef]
- Bedi, B.; Li, J.Y.; Tawfeek, H.; Baek, K.H.; Adams, J.; Vangara, S.S.; Chang, M.K.; Kneissel, M.; Weitzmann, M.N.; Pacifici, R. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc. Natl. Acad. Sci. USA 2012, 109, E725–E733. [Google Scholar] [CrossRef]
- Cho, S.W.; Pirih, F.Q.; Koh, A.J.; Michalski, M.; Eber, M.R.; Ritchie, K.; Sinder, B.; Oh, S.; Al-Dujaili, S.A.; Lee, J.; et al. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. J. Biol. Chem. 2013, 288, 6814–6825. [Google Scholar] [CrossRef]
- Wang, C.; Ning, H.; Gao, J.; Xue, T.; Zhao, M.; Jiang, X.; Zhu, X.; Guo, X.; Li, H.; Wang, X. Disruption of hematopoiesis attenuates the osteogenic differentiation capacity of bone marrow stromal cells. Stem Cell Res. Ther. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Schepers, K.; Pietras, E.M.; Reynaud, D.; Flach, J.; Binnewies, M.; Garg, T.; Wagers, A.J.; Hsiao, E.C.; Passegue, E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013, 13, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Hammerick, K.E.; Challen, G.A.; Goodell, M.A.; Kasper, F.K.; Mikos, A.G. Investigating the role of hematopoietic stem and progenitor cells in regulating the osteogenic differentiation of mesenchymal stem cells in vitro. J. Orthop. Res. 2011, 29, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Valderrabano, R.J.; Buzkova, P.; Chang, P.Y.; Zakai, N.A.; Fink, H.A.; Robbins, J.A.; Wu, J.Y.; Lee, J.S. Associations of hemoglobin and change in hemoglobin with risk of incident hip fracture in older men and women: The cardiovascular health study. Osteoporos. Int. 2021, 32, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, C.; Zheng, X.; Gao, M. Co-cultivation of progenitor cells enhanced osteogenic gene expression and angiogenesis potential in vitro. J. Int. Med. Res. 2021, 49, 3000605211004024. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Song, J.; Shiozawa, Y.; Wang, J.; Wang, Z.; Williams, B.; Havens, A.; Schneider, A.; Ge, C.; Franceschi, R.T.; et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells 2008, 26, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Childress, P.; Hood, M., Jr.; Alvarez, M.; Kacena, M.A.; Hanlon, M.; McKee, B.; Bidwell, J.P.; Yang, F.C. Nmp4/CIZ suppresses the parathyroid hormone anabolic window by restricting mesenchymal stem cell and osteoprogenitor frequency. Stem Cells Dev. 2013, 22, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, M.; Wu, R.; Meng, H.; He, Y.; Wang, B.; Zhou, X.; Zhu, G. Insensitive to PTH of CD8(+) T cells regulate bone marrow mesenchymal stromal cell in aplastic anemia patients. Int. J. Med. Sci. 2020, 17, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.; Lu, W.; Louie, A.; Nissenson, R. Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes. Endocrine 2012, 42, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Cui, H. Intermittent parathyroid hormone (1-34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp. Ther. Med. 2016, 11, 2399–2406. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wu, X.; Wang, F.; Wang, Y.; Gao, Q.; Liu, H.; Hu, Y.; Su, J.; Jing, Y. PTHG2 Reduces Bone Loss in Ovariectomized Mice by Directing Bone Marrow Mesenchymal Stem Cell Fate. Stem Cells Int. 2021, 2021, 8546739. [Google Scholar] [CrossRef]
- Zhou, S.; Bueno, E.M.; Kim, S.W.; Amato, I.; Shen, L.; Hahne, J.; Bleiberg, I.; Morley, P.; Glowacki, J. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging. Cell 2011, 10, 780–788. [Google Scholar] [CrossRef]
- Choudhary, S.; Huang, H.; Raisz, L.; Pilbeam, C. Anabolic effects of PTH in cyclooxygenase-2 knockout osteoblasts in vitro. Biochem. Biophys. Res. Commun. 2008, 372, 536–541. [Google Scholar] [CrossRef]
- Kulebyakin, K.; Tyurin-Kuzmin, P.; Sozaeva, L.; Voloshin, N.; Nikolaev, M.; Chechekhin, V.; Vigovskiy, M.; Sysoeva, V.; Korchagina, E.; Naida, D.; et al. Dynamic Balance between PTH1R-Dependent Signal Cascades Determines Its Pro- or Anti-Osteogenic Effects on MSC. Cells 2022, 11, 3519. [Google Scholar] [CrossRef]
- Kuo, S.W.; Rimando, M.G.; Liu, Y.S.; Lee, O.K. Intermittent Administration of Parathyroid Hormone 1-34 Enhances Osteogenesis of Human Mesenchymal Stem Cells by Regulating Protein Kinase Cδ. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E. MANAGEMENT OF ENDOCRINE DISEASE: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44. [Google Scholar] [CrossRef] [PubMed]
- Yukata, K.; Xie, C.; Li, T.F.; Takahata, M.; Hoak, D.; Kondabolu, S.; Zhang, X.; Awad, H.A.; Schwarz, E.M.; Beck, C.A.; et al. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone 2014, 62, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Tian, Y.; Lin, Y.; Huang, Q.; Xue, Y. Evaluating Osteogenic Differentiation of Osteoblastic Precursors Upon Intermittent Administration of PTH/IGFBP7. Front. Pharmacol. 2022, 13, 839035. [Google Scholar] [CrossRef]
- Cui, C.; Zheng, L.; Fan, Y.; Zhang, J.; Xu, R.; Xie, J.; Zhou, X. Parathyroid hormone ameliorates temporomandibular joint osteoarthritic-like changes related to age. Cell Prolif. 2020, 53, e12755. [Google Scholar] [CrossRef]
- Ogita, M.; Rached, M.T.; Dworakowski, E.; Bilezikian, J.P.; Kousteni, S. Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology 2008, 149, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, X.; Yang, C.; Crane, J.; Xian, L.; Lu, W.; Wan, M.; Cao, X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2001–2014. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Qin, Z.; Yuan, H.; Li, B.; Pan, Y.; Wang, X.; Du, X.; Hao, S.; Du, Y.; et al. Parathyroid hormone ameliorates osteogenesis of human bone marrow mesenchymal stem cells against glucolipotoxicity through p38 MAPK signaling. IUBMB Life 2021, 73, 213–222. [Google Scholar] [CrossRef]
- Qin, Z.; Hua, S.; Chen, H.; Wang, Z.; Wang, H.; Xu, J.; Wang, Y.; Chen, W.; Zhou, W. Parathyroid hormone promotes the osteogenesis of lipopolysaccharide-induced human bone marrow mesenchymal stem cells through the JNK MAPK pathway. Biosci. Rep. 2021, 41, 20. [Google Scholar] [CrossRef]
- Mwale, F.; Yao, G.; Ouellet, J.A.; Petit, A.; Antoniou, J. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng. Part A 2010, 16, 3449–3455. [Google Scholar] [CrossRef]
- Zhu, J.; Siclari, V.A.; Liu, F.; Spatz, J.M.; Chandra, A.; Divieti Pajevic, P.; Qin, L. Amphiregulin-EGFR signaling mediates the migration of bone marrow mesenchymal progenitors toward PTH-stimulated osteoblasts and osteocytes. PLoS ONE 2012, 7, e50099. [Google Scholar] [CrossRef]
- Jay, F.F.; Vaidya, M.; Porada, S.M.; Andrukhova, O.; Schneider, M.R.; Erben, R.G. Amphiregulin lacks an essential role for the bone anabolic action of parathyroid hormone. Mol. Cell. Endocrinol. 2015, 417, 158–165. [Google Scholar] [CrossRef]
- Quach, J.M.; Walker, E.C.; Allan, E.; Solano, M.; Yokoyama, A.; Kato, S.; Sims, N.A.; Gillespie, M.T.; Martin, T.J. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J. Biol. Chem. 2011, 286, 4186–4198. [Google Scholar] [CrossRef]
- Le, P.T.; Liu, H.; Alabdulaaly, L.; Vegting, Y.; Calle, I.L.; Gori, F.; Lanske, B.; Baron, R.; Rosen, C.J. The role of Zfp467 in mediating the pro-osteogenic and anti-adipogenic effects on bone and bone marrow niche. Bone 2021, 144, 115832. [Google Scholar] [CrossRef]
- Liu, H.; Wada, A.; Le, I.; Le, P.T.; Lee, A.W.F.; Zhou, J.; Gori, F.; Baron, R.; Rosen, C.J. PTH regulates osteogenesis and suppresses adipogenesis through Zfp467 in a feed-forward, PTH1R-cyclic AMP-dependent manner. Elife 2023, 12, 83345. [Google Scholar] [CrossRef]
- Atkinson, E.G.; Adaway, M.; Horan, D.J.; Korff, C.; Klunk, A.; Orr, A.L.; Ratz, K.; Bellido, T.; Plotkin, L.I.; Robling, A.G.; et al. Conditional Loss of Nmp4 in Mesenchymal Stem Progenitor Cells Enhances PTH-Induced Bone Formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2023, 38, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Muheremu, A.; Bai, X.; Zou, X.; Lin, T.; Chen, B. PTH(1-34) activates the migration and adhesion of BMSCs through the rictor/mTORC2 pathway. Int. J. Mol. Med. 2020, 46, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardo, G.; Galderisi, U.; Fiorito, C.; Squillaro, T.; Cito, L.; Cipollaro, M.; Giordano, A.; Napoli, C. Dual role of parathyroid hormone in endothelial progenitor cells and marrow stromal mesenchymal stem cells. J. Cell. Physiol. 2010, 222, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, E.M. Anabolic effects of intermittent PTH on osteoblasts. Curr. Mol. Pharmacol. 2012, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gilchrist, A.; Stern, P.H. Antagonist minigenes identify genes regulated by parathyroid hormone through G protein-selective and G protein co-regulated mechanisms in osteoblastic cells. Cell. Signal. 2011, 23, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Aarnisalo, P.; Chubb, R.; Poulton, I.J.; Guo, J.; Nachtrab, G.; Kimura, T.; Swami, S.; Saeed, H.; Chen, M.; et al. Loss of Gsα in the Postnatal Skeleton Leads to Low Bone Mass and a Blunted Response to Anabolic Parathyroid Hormone Therapy. J. Biol. Chem. 2016, 291, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J. PTH1R Actions on Bone Using the cAMP/Protein Kinase A Pathway. Front. Endocrinol. 2021, 12, 833221. [Google Scholar] [CrossRef]
- Martin, T.J.; Sims, N.A.; Seeman, E. Physiological and Pharmacological Roles of PTH and PTHrP in Bone Using Their Shared Receptor, PTH1R. Endocr. Rev. 2021, 42, 383–406. [Google Scholar] [CrossRef]
- Tong, G.; Meng, Y.; Hao, S.; Hu, S.; He, Y.; Yan, W.; Yang, D. Parathyroid Hormone Activates Phospholipase C (PLC)-Independent Protein Kinase C Signaling Pathway via Protein Kinase A (PKA)-Dependent Mechanism: A New Defined Signaling Route Would Induce Alternative Consideration to Previous Conceptions. J. Pharmacol. Exp. Ther. 2017, 23, 1896–1906. [Google Scholar] [CrossRef]
- Cupp, M.E.; Nayak, S.K.; Adem, A.S.; Thomsen, W.J. Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J. Pharmacol. Exp. Ther. 2013, 345, 404–418. [Google Scholar] [CrossRef]
- Balani, D.H.; Ono, N.; Kronenberg, H.M. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J. Clin. Investig. 2017, 127, 3327–3338. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Yang, L.; Zhou, Y.; Wang, Y. Dose-dependence of PTH-related peptide-1 on the osteogenic induction of MC3T3-E1 cells in vitro. Medicine 2017, 96, e6637. [Google Scholar] [CrossRef] [PubMed]
- Kir, M.C.; Onal, M.O.; Uluer, E.T.; Ulman, C.; Inan, S. Continuous and intermittent parathyroid hormone administration promotes osteogenic differentiation and activity of programmable cells of monocytic origin. Biotech. Histochem. 2022, 97, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Esbrit, P.; Alcaraz, M.J. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem. Pharmacol. 2013, 85, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hasegawa, T.; Sasaki, M.; Hongo, H.; Tsuboi, K.; Shimizu, T.; Ota, M.; Haraguchi, M.; Takahata, M.; Oda, K.; et al. Frequency of Teriparatide Administration Affects the Histological Pattern of Bone Formation in Young Adult Male Mice. Endocrinology 2016, 157, 2604–2620. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Jilka, R.L.; Almeida, M.; Roberson, P.K.; Manolagas, S.C. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology 2010, 151, 2641–2649. [Google Scholar] [CrossRef]
- Motyl, K.J.; McCauley, L.K.; McCabe, L.R. Amelioration of type I diabetes-induced osteoporosis by parathyroid hormone is associated with improved osteoblast survival. J. Cell. Physiol. 2012, 227, 1326–1334. [Google Scholar] [CrossRef]
- Bouleftour, W.; Bouet, G.; Granito, R.N.; Thomas, M.; Linossier, M.T.; Vanden-Bossche, A.; Aubin, J.E.; Lafage-Proust, M.H.; Vico, L.; Malaval, L. Blocking the expression of both bone sialoprotein (BSP) and osteopontin (OPN) impairs the anabolic action of PTH in mouse calvaria bone. J. Cell. Physiol. 2015, 230, 568–577. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, M.; Zhang, Q.; Liu, H.; Xu, Y.; Shu, L.; Zhang, J.; Miao, D.; Ren, Y. Synergistic effects of high dietary calcium and exogenous parathyroid hormone in promoting osteoblastic bone formation in mice. Br. J. Nutr. 2015, 113, 909–922. [Google Scholar] [CrossRef]
- Jang, M.G.; Lee, J.Y.; Yang, J.Y.; Park, H.; Kim, J.H.; Kim, J.E.; Shin, C.S.; Kim, S.Y.; Kim, S.W. Intermittent PTH treatment can delay the transformation of mature osteoblasts into lining cells on the periosteal surfaces. J. Bone Miner. Metab. 2016, 34, 532–539. [Google Scholar] [CrossRef]
- Kim, S.W.; Pajevic, P.D.; Selig, M.; Barry, K.J.; Yang, J.Y.; Shin, C.S.; Baek, W.Y.; Kim, J.E.; Kronenberg, H.M. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.A.; Stephen, L.A.; Jayash, S.N.; Myers, K.; Little, K.; Hopkinson, M.; Pitsillides, A.A.; MacRae, V.E.; Millan, J.L.; Staines, K.A.; et al. Increased PHOSPHO1 and alkaline phosphatase expression during the anabolic bone response to intermittent parathyroid hormone delivery. Cell Biochem. Funct. 2023, 41, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Osagie-Clouard, L.; Sanghani, A.; Coathup, M.; Briggs, T.; Bostrom, M.; Blunn, G. Parathyroid hormone 1-34 and skeletal anabolic action: The use of parathyroid hormone in bone formation. Bone Jt. Res. 2017, 6, 14–21. [Google Scholar] [CrossRef]
- Pellicelli, M.; Miller, J.A.; Arabian, A.; Gauthier, C.; Akhouayri, O.; Wu, J.Y.; Kronenberg, H.M.; St-Arnaud, R. The PTH-Gαs-protein kinase A cascade controls αNAC localization to regulate bone mass. Mol. Cell. Biol. 2014, 34, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Pellicelli, M.; Hariri, H.; Miller, J.A.; St-Arnaud, R. Lrp6 is a target of the PTH-activated αNAC transcriptional coregulator. Biochim. Et Biophys. Acta. Gene Regul. Mech. 2018, 1861, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hariri, H.; Pellicelli, M.; St-Arnaud, R. Nfil3, a target of the NACA transcriptional coregulator, affects osteoblast and osteocyte gene expression differentially. Bone 2020, 141, 115624. [Google Scholar] [CrossRef]
- Hariri, H.; Addison, W.N.; St-Arnaud, R. Ubiquitin specific peptidase Usp53 regulates osteoblast versus adipocyte lineage commitment. Sci. Rep. 2021, 11, 8418. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Liu, L.; Blair, H.C.; Friedman, P.A. NHERF1 regulation of PTH-dependent bimodal Pi transport in osteoblasts. Bone 2013, 52, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Pozo, A.; Regnier, M.; Lizotte, J.; Martineau, C.; Scorza, T.; Moreau, R. Cyclic adenosine monophosphate-dependent activation of transient receptor potential vanilloid 4 (TRPV4) channels in osteoblast-like MG-63 cells. Cell. Signal. 2020, 66, 109486. [Google Scholar] [CrossRef] [PubMed]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Ren. Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- Knab, V.M.; Corbin, B.; Andrukhova, O.; Hum, J.M.; Ni, P.; Rabadi, S.; Maeda, A.; White, K.E.; Erben, R.G.; Jüppner, H.; et al. Acute Parathyroid Hormone Injection Increases C-Terminal but Not Intact Fibroblast Growth Factor 23 Levels. Endocrinology 2017, 158, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Bivi, N.; Farrow, E.; Lezcano, V.; Plotkin, L.I.; White, K.E.; Bellido, T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011, 49, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Baranowsky, A.; Jahn, D.; Jiang, S.; Yorgan, T.; Ludewig, P.; Appelt, J.; Albrecht, K.K.; Otto, E.; Knapstein, P.; Donat, A.; et al. Procalcitonin is expressed in osteoblasts and limits bone resorption through inhibition of macrophage migration during intermittent PTH treatment. Bone Res. 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, R.; Wehbi, V.L.; Hayata, T.; Moriya, S.; Feinstein, T.N.; Ezura, Y.; Nagao, M.; Saita, Y.; Hemmi, H.; Notomi, T.; et al. Anabolic action of parathyroid hormone regulated by the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 7433–7438. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Hayata, T.; Notomi, T.; Aryal, S.; Nakamaoto, T.; Izu, Y.; Kawasaki, M.; Yamada, T.; Shirakawa, J.; Kaneko, K.; et al. PTH regulates β2-adrenergic receptor expression in osteoblast-like MC3T3-E1 cells. J. Cell. Biochem. 2015, 116, 142–148. [Google Scholar] [CrossRef]
- Huang, H.; Chikazu, D.; Voznesensky, O.S.; Herschman, H.R.; Kream, B.E.; Drissi, H.; Pilbeam, C.C. Parathyroid hormone induction of cyclooxygenase-2 in murine osteoblasts: Role of the calcium-calcineurin-NFAT pathway. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 819–829. [Google Scholar] [CrossRef]
- Choi, H.; Magyar, C.E.; Nervina, J.M.; Tetradis, S. Different duration of parathyroid hormone exposure distinctively regulates primary response genes Nurr1 and RANKL in osteoblasts. PLoS ONE 2018, 13, e0208514. [Google Scholar] [CrossRef]
- Estus, T.L.; Choudhary, S.; Pilbeam, C.C. Prostaglandin-mediated inhibition of PTH-stimulated β-catenin signaling in osteoblasts by bone marrow macrophages. Bone 2016, 85, 123–130. [Google Scholar] [CrossRef]
- Aslan, D.; Andersen, M.D.; Gede, L.B.; de Franca, T.K.; Jørgensen, S.R.; Schwarz, P.; Jørgensen, N.R. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scand. J. Clin. Lab. Investig. 2012, 72, 14–22. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Yang, D.; Bouxsein, M.L.; Thomas, C.C.; Schipani, E.; Bringhurst, F.R.; Kronenberg, H.M. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology 2010, 151, 3502–3513. [Google Scholar] [CrossRef]
- Laxman, N.; Rubin, C.J.; Mallmin, H.; Nilsson, O.; Tellgren-Roth, C.; Kindmark, A. Second generation sequencing of microRNA in Human Bone Cells treated with Parathyroid Hormone or Dexamethasone. Bone 2016, 84, 181–188. [Google Scholar] [CrossRef]
- Yu, C.; Xuan, M.; Zhang, M.; Yao, Q.; Zhang, K.; Zhang, X.; Guo, J.; Song, L. Postnatal deletion of β-catenin in osterix-expressing cells is necessary for bone growth and intermittent PTH-induced bone gain. J. Bone Miner. Metab. 2018, 36, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Y.; Fu, Q.; He, M. Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol. Cell. Biochem. 2011, 355, 211–216. [Google Scholar] [CrossRef]
- Saidak, Z.; Le Henaff, C.; Azzi, S.; Marty, C.; Marie, P.J. Low-dose PTH increases osteoblast activity via decreased Mef2c/Sost in senescent osteopenic mice. J. Endocrinol. 2014, 223, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; O’Brien, C.A.; Bartell, S.M.; Weinstein, R.S.; Manolagas, S.C. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, J.; Harada, H.; Noda, M.; Ezura, Y. PTH-Induced Osteoblast Proliferation Requires Upregulation of the Ubiquitin-Specific Peptidase 2 (Usp2) Expression. Calcif. Tissue Int. 2016, 98, 306–315. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Yang, D.; Bouxsein, M.L.; Saito, H.; Galvin, R.J.; Kuhstoss, S.A.; Thomas, C.C.; Schipani, E.; Baron, R.; et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.Q.; Wu, J.J.; Troiano, N.; Insogna, K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J. Bone Miner. Metab. 2011, 29, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Uyama, M.; Kawanami, M.; Tamura, M. Wasf2: A novel target of intermittent parathyroid hormone administration. Int. J. Mol. Med. 2013, 31, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Nakashima, K.; Schipani, E.; Hayata, T.; Ezura, Y.; Soma, K.; Kronenberg, H.M.; Noda, M. Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. J. Cell. Physiol. 2012, 227, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, T.; Zhou, C.; Huang, L.; Li, Y.; Wang, H.; Duan, P.; Zou, S.; Mei, L. Parathyroid hormone increases alveolar bone homoeostasis during orthodontic tooth movement in rats with periodontitis via crosstalk between STAT3 and β-catenin. Int. J. Oral Sci. 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lin, T.; Zhu, J.; Tong, W.; Huo, Y.; Jia, H.; Zhang, Y.; Liu, X.S.; Cengel, K.; Xia, B.; et al. PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J. Biol. Chem. 2015, 290, 157–167. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; He, S.; Yang, J.; Liu, D.; Bao, Q.; Qin, H.; Du, W.; Zhong, X.; Chen, C.; et al. Preactivation of β-catenin in osteoblasts improves the osteoanabolic effect of PTH in type 1 diabetic mice. J. Cell. Physiol. 2020, 235, 1480–1493. [Google Scholar] [CrossRef]
- Hisa, I.; Inoue, Y.; Hendy, G.N.; Canaff, L.; Kitazawa, R.; Kitazawa, S.; Komori, T.; Sugimoto, T.; Seino, S.; Kaji, H. Parathyroid hormone-responsive Smad3-related factor, Tmem119, promotes osteoblast differentiation and interacts with the bone morphogenetic protein-Runx2 pathway. J. Biol. Chem. 2011, 286, 9787–9796. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Le Henaff, C.; Johnson, J.; He, Z.; Rifkin, D.B.; Partridge, N.C. Osteoblastic monocyte chemoattractant protein-1 (MCP-1) mediation of parathyroid hormone’s anabolic actions in bone implicates TGF-β signaling. Bone 2021, 143, 115762. [Google Scholar] [CrossRef]
- Atfi, A.; Baron, R. PTH battles TGF-beta in bone. Nat. Cell Biol. 2010, 12, 205–207. [Google Scholar] [CrossRef]
- Qiu, T.; Wu, X.; Zhang, F.; Clemens, T.L.; Wan, M.; Cao, X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat. Cell Biol. 2010, 12, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.W.M.; Chan, A.S.; Pavlos, N.J.; Sims, N.A.; Martin, T.J. Brief exposure to full length parathyroid hormone-related protein (PTHrP) causes persistent generation of cyclic AMP through an endocytosis-dependent mechanism. Biochem. Pharmacol. 2019, 169, 113627. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Kawaguchi, H.; Higashikawa, A.; Hirata, M.; Miura, T.; Saito, T.; Nakamura, K.; Chung, U.I.; Ogata, N. Mechanisms underlying catabolic and anabolic functions of parathyroid hormone on bone by combination of culture systems of mouse cells. J. Cell. Biochem. 2010, 109, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Di Somma, C.; Vuolo, L.; Guerra, E.; Scarano, E.; Colao, A. Role of IGF-I on PTH effects on bone. J. Endocrinol. Investig. 2010, 33 (Suppl. S7), 22–26. [Google Scholar] [PubMed]
- Qiu, T.; Crane, J.L.; Xie, L.; Xian, L.; Xie, H.; Cao, X. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 2018, 6, 5. [Google Scholar] [CrossRef]
- Ardura, J.A.; Portal-Núñez, S.; Castelbón-Calvo, I.; Martínez de Toda, I.; De la Fuente, M.; Esbrit, P. Parathyroid Hormone-Related Protein Protects Osteoblastic Cells From Oxidative Stress by Activation of MKP1 Phosphatase. J. Cell. Physiol. 2017, 232, 785–796. [Google Scholar] [CrossRef]
- Schneider, M.R.; Dahlhoff, M.; Andrukhova, O.; Grill, J.; Glösmann, M.; Schüler, C.; Weber, K.; Wolf, E.; Erben, R.G. Normal epidermal growth factor receptor signaling is dispensable for bone anabolic effects of parathyroid hormone. Bone 2012, 50, 237–244. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, C.; Zhang, J.; Li, Y.; Li, T.; Zhang, C.; Chen, J.; Bai, D.; Yin, X.; Zou, S. Intermittent parathyroid hormone promotes cementogenesis in a PKA- and ERK1/2-dependent manner. J. Periodontol. 2019, 90, 1002–1013. [Google Scholar] [CrossRef]
- Mahalingam, C.D.; Sampathi, B.R.; Sharma, S.; Datta, T.; Das, V.; Abou-Samra, A.B.; Datta, N.S. MKP1-dependent PTH modulation of bone matrix mineralization in female mice is osteoblast maturation stage specific and involves P-ERK and P-p38 MAPKs. J. Endocrinol. 2012, 216, 315–329. [Google Scholar] [CrossRef]
- Datta, N.S.; Kolailat, R.; Fite, A.; Pettway, G.; Abou-Samra, A.B. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell. Signal. 2010, 22, 457–466. [Google Scholar] [CrossRef]
- Datta, N.S.; Samra, T.A.; Mahalingam, C.D.; Datta, T.; Abou-Samra, A.B. Role of PTH1R internalization in osteoblasts and bone mass using a phosphorylation-deficient knock-in mouse model. J. Endocrinol. 2010, 207, 355–365. [Google Scholar] [CrossRef]
- Thouverey, C.; Caverzasio, J. Suppression of p38α MAPK Signaling in Osteoblast Lineage Cells Impairs Bone Anabolic Action of Parathyroid Hormone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 985–993. [Google Scholar] [CrossRef]
- Sharma, S.; Mahalingam, C.D.; Das, V.; Jamal, S.; Levi, E.; Rishi, A.K.; Datta, N.S. Cell cycle and apoptosis regulatory protein (CARP)-1 is expressed in osteoblasts and regulated by PTH. Biochem. Biophys. Res. Commun. 2013, 436, 607–612. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Onal, M.; Pike, J.W. Selective regulation of Mmp13 by 1,25(OH)(2)D(3), PTH, and Osterix through distal enhancers. J. Steroid Biochem. Mol. Biol. 2016, 164, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishnan, V.; Balasubramanian, A.; Mahalingam, G.; Partridge, N.C.; Ramachandran, I.; Selvamurugan, N. Parathyroid hormone-induced down-regulation of miR-532-5p for matrix metalloproteinase-13 expression in rat osteoblasts. J. Cell. Biochem. 2018, 119, 6181–6193. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Selvamurugan, N.; Westendorf, J.J.; Olson, E.N.; Partridge, N.C. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J. Biol. Chem. 2010, 285, 9616–9626. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Nakatani, T.; He, Z.; Partridge, N.C. Parathyroid hormone regulates histone deacetylase (HDAC) 4 through protein kinase A-mediated phosphorylation and dephosphorylation in osteoblastic cells. J. Biol. Chem. 2014, 289, 21340–21350. [Google Scholar] [CrossRef]
- Malavika, D.; Shreya, S.; Raj Priya, V.; Rohini, M.; He, Z.; Partridge, N.C.; Selvamurugan, N. miR-873-3p targets HDAC4 to stimulate matrix metalloproteinase-13 expression upon parathyroid hormone exposure in rat osteoblasts. J. Cell. Physiol. 2020, 235, 7996–8009. [Google Scholar] [CrossRef]
- Lee, M.; Partridge, N.C. Parathyroid hormone activation of matrix metalloproteinase-13 transcription requires the histone acetyltransferase activity of p300 and PCAF and p300-dependent acetylation of PCAF. J. Biol. Chem. 2010, 285, 38014–38022. [Google Scholar] [CrossRef]
- Nakatani, T.; Partridge, N.C. MEF2C Interacts With c-FOS in PTH-Stimulated Mmp13 Gene Expression in Osteoblastic Cells. Endocrinology 2017, 158, 3778–3791. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Shimizu, E.; McBurney, M.W.; Partridge, N.C. Sirtuin 1 is a negative regulator of parathyroid hormone stimulation of matrix metalloproteinase 13 expression in osteoblastic cells: Role of sirtuin 1 in the action of PTH on osteoblasts. J. Biol. Chem. 2015, 290, 8373–8382. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, M.; Li, Y.; Li, C.; Tang, S.; Qu, X.; Feng, N.; Wu, Y. The PERK-EIF2α-ATF4 signaling branch regulates osteoblast differentiation and proliferation by PTH. Am. J. Physiol. Endocrinol. Metab. 2019, 316, e590–e604. [Google Scholar] [CrossRef]
- Morimoto, A.; Kikuta, J.; Nishikawa, K.; Sudo, T.; Uenaka, M.; Furuya, M.; Hasegawa, T.; Hashimoto, K.; Tsukazaki, H.; Seno, S.; et al. SLPI is a critical mediator that controls PTH-induced bone formation. Nat. Commun. 2021, 12, 2136. [Google Scholar] [CrossRef]
- Fei, Y.; Xiao, L.; Hurley, M.M. The impaired bone anabolic effect of PTH in the absence of endogenous FGF2 is partially due to reduced ATF4 expression. Biochem. Biophys. Res. Commun. 2011, 412, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yi, L.; Weng, T.; Huang, J.; Luo, F.; Jiang, W.; Xian, C.J.; Du, X.; Chen, L. Fibroblast Growth Factor Receptor 3 Deficiency Does Not Impair the Osteoanabolic Action of Parathyroid Hormone on Mice. Int. J. Biol. Sci. 2016, 12, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Loots, G.G.; Yellowley, C.E.; Dosé, A.C.; Genetos, D.C. Parathyroid hormone regulation of hypoxia-inducible factor signaling in osteoblastic cells. Bone 2015, 81, 97–103. [Google Scholar] [CrossRef]
- Jilka, R.L.; Almeida, M.; Ambrogini, E.; Han, L.; Roberson, P.K.; Weinstein, R.S.; Manolagas, S.C. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 2010, 9, 851–867. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Ardura, J.A.; Lozano, D.; Martínez de Toda, I.; De la Fuente, M.; Herrero-Beaumont, G.; Largo, R.; Esbrit, P. Parathyroid hormone-related protein exhibits antioxidant features in osteoblastic cells through its N-terminal and osteostatin domains. Bone Jt. Res. 2018, 7, 58–68. [Google Scholar] [CrossRef]
- Uda, Y.; Saini, V.; Petty, C.A.; Alshehri, M.; Shi, C.; Spatz, J.M.; Santos, R.; Newell, C.M.; Huang, T.Y.; Kochen, A.; et al. Parathyroid hormone signaling in mature osteoblasts/osteocytes protects mice from age-related bone loss. Aging 2021, 13, 25607–25642. [Google Scholar] [CrossRef]
- Prideaux, M.; Dallas, S.L.; Zhao, N.; Johnsrud, E.D.; Veno, P.A.; Guo, D.; Mishina, Y.; Harris, S.E.; Bonewald, L.F. Parathyroid Hormone Induces Bone Cell Motility and Loss of Mature Osteocyte Phenotype through L-Calcium Channel Dependent and Independent Mechanisms. PLoS ONE 2015, 10, e0125731. [Google Scholar] [CrossRef]
- Al-Dujaili, S.A.; Koh, A.J.; Dang, M.; Mi, X.; Chang, W.; Ma, P.X.; McCauley, L.K. Calcium Sensing Receptor Function Supports Osteoblast Survival and Acts as a Co-Factor in PTH Anabolic Actions in Bone. J. Cell. Biochem. 2016, 117, 1556–1567. [Google Scholar] [CrossRef]
- Lu, X.; Ding, Y.; Niu, Q.; Xuan, S.; Yang, Y.; Jin, Y.; Wang, H. ClC-3 chloride channel mediates the role of parathyroid hormone [1-34] on osteogenic differentiation of osteoblasts. PLoS ONE 2017, 12, e0176196. [Google Scholar] [CrossRef]
- Agas, D.; Amaroli, A.; Lacava, G.; Yanagawa, T.; Sabbieti, M.G. Loss of p62 impairs bone turnover and inhibits PTH-induced osteogenesis. J. Cell. Physiol. 2020, 235, 7516–7529. [Google Scholar] [CrossRef]
- Martín-Guerrero, E.; Tirado-Cabrera, I.; Buendía, I.; Alonso, V.; Gortázar, A.R.; Ardura, J.A. Primary cilia mediate parathyroid hormone receptor type 1 osteogenic actions in osteocytes and osteoblasts via Gli activation. J. Cell. Physiol. 2020, 235, 7356–7369. [Google Scholar] [CrossRef]
- Vanessa, L.; Wehbi, H.P.S.; Feinstein, T.N.; Calero, G.; Romero, G.; Vilardaga, J.-P. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc. Natl. Acad. Sci. USA 2013, 110, 1530–1535. [Google Scholar]
- Peterson, Y.K.; Luttrell, L.M. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol. Rev. 2017, 69, 256–297. [Google Scholar] [CrossRef] [PubMed]
- White, A.D.; Jean-Alphonse, F.G.; Fang, F.; Pena, K.A.; Liu, S.; Konig, G.M.; Inoue, A.; Aslanoglou, D.; Gellman, S.H.; Kostenis, E.; et al. G(q/11)-dependent regulation of endosomal cAMP generation by parathyroid hormone class B GPCR. Proc. Natl. Acad. Sci. USA 2020, 117, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Pei, G. Beta-arrestin signaling and regulation of transcription. J. Cell Sci. 2007, 120 Pt 2, 213–218. [Google Scholar] [CrossRef]
- Pacheco-Costa, R.; Davis, H.; Atkinson, E.; Katchburian, E.; Plotkin, L.; Reginato, R. Osteocytic connexin 43 is not required for the increase in bone mass induced by intermittent PTH administration in male mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 16, 45–57. [Google Scholar]
- Bivi, N.; Lezcano, V.; Romanello, M.; Bellido, T.; Plotkin, L.I. Connexin43 interacts with βarrestin: A pre-requisite for osteoblast survival induced by parathyroid hormone. J. Cell. Biochem. 2011, 112, 2920–2930. [Google Scholar] [CrossRef]
- Stegen, S.; Devignes, C.S.; Torrekens, S.; Van Looveren, R.; Carmeliet, P.; Carmeliet, G. Glutamine Metabolism in Osteoprogenitors Is Required for Bone Mass Accrual and PTH-Induced Bone Anabolism in Male Mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2021, 36, 604–616. [Google Scholar] [CrossRef]
- Alekos, N.S.; Kushwaha, P.; Kim, S.P.; Li, Z.; Abood, A.; Dirckx, N.; Aja, S.; Kodama, J.; Garcia-Diaz, J.G.; Otsuru, S.; et al. Mitochondrial β-oxidation of adipose-derived fatty acids by osteoblasts fuels parathyroid hormone-induced bone formation. J. Clin. Investig. 2023, 8, 165604. [Google Scholar] [CrossRef]
- Zoidis, E.; Ghirlanda-Keller, C.; Schmid, C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol. Cell. Biochem. 2011, 348, 33–42. [Google Scholar] [CrossRef]
- Esen, E.; Lee, S.Y.; Wice, B.M.; Long, F. PTH Promotes Bone Anabolism by Stimulating Aerobic Glycolysis via IGF Signaling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 1959–1968. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Zhang, K.; Li, Y.; Xu, M.; Tang, S.; Qu, X.; Li, C. Lactate enhanced the effect of parathyroid hormone on osteoblast differentiation via GPR81-PKC-Akt signaling. Biochem. Biophys. Res. Commun. 2018, 503, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Karvande, A.; Kushwaha, P.; Ahmad, N.; Adhikary, S.; Kothari, P.; Tripathi, A.K.; Khedgikar, V.; Trivedi, R. Glucose dependent miR-451a expression contributes to parathyroid hormone mediated osteoblast differentiation. Bone 2018, 117, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Vishal, M.; Shreya, S.; Malavika, D.; Rajpriya, V.; He, Z.; Partridge, N.C.; Selvamurugan, N. Parathyroid hormone-stimulation of Runx2 during osteoblast differentiation via the regulation of lnc-SUPT3H-1:16 (RUNX2-AS1:32) and miR-6797-5p. Biochimie 2019, 158, 43–52. [Google Scholar] [CrossRef]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on osteocyte function. Bone 2013, 54, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Tu, X.; Pacheco-Costa, R.; McAndrews, K.; Edwards, R.; Pellegrini, G.G.; Kuhlenschmidt, K.; Olivos, N.; Robling, A.; Peacock, M.; et al. Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Michopoulos, A.; Yovos, J.G. PTH and PTHR1 in osteocytes. New insights into old partners. Hormones 2017, 16, 150–160. [Google Scholar] [CrossRef]
- Mosca, M.J.; He, Z.; Ricarte, F.R.; Le Henaff, C.; Partridge, N.C. Differential effects of PTH (1-34), PTHrP (1-36) and abaloparatide on the murine osteoblast transcriptome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ricarte, F.R.; Le Henaff, C.; Kolupaeva, V.G.; Gardella, T.J.; Partridge, N.C. Parathyroid hormone(1-34) and its analogs differentially modulate osteoblastic Rankl expression via PKA/SIK2/SIK3 and PP1/PP2A-CRTC3 signaling. J. Biol. Chem. 2018, 293, 20200–20213. [Google Scholar] [CrossRef]
- Smargiassi, A.; Bertacchini, J.; Checchi, M.; Potì, F.; Tenedini, E.; Montosi, G.; Magarò, M.S.; Amore, E.; Cavani, F.; Ferretti, M.; et al. WISP-2 expression induced by Teriparatide treatment affects in vitro osteoblast differentiation and improves in vivo osteogenesis. Mol. Cell. Endocrinol. 2020, 513, 110817. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, Q.; Yu, B.; Xie, H.; Wang, W.; Shi, C.; Crane, J.L.; Cao, X.; Wan, M. Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Revollo, L.; Kading, J.; Jeong, S.Y.; Li, J.; Salazar, V.; Mbalaviele, G.; Civitelli, R. N-cadherin restrains PTH activation of Lrp6/β-catenin signaling and osteoanabolic action. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.G.; Cremers, S.; Rubin, M.R.; McMahon, D.J.; Sliney, J., Jr.; Lazaretti-Castro, M.; Silverberg, S.J.; Bilezikian, J.P. Circulating sclerostin in disorders of parathyroid gland function. J. Clin. Endocrinol. Metab. 2011, 96, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.F., Jr.; Barry, K.J.; Tulum, I.; Kobayashi, T.; Harris, S.E.; Bringhurst, F.R.; Pajevic, P.D. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J. Endocrinol. 2011, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Allen, M.R.; Condon, K.; Lezcano, V.; Ronda, A.C.; Galli, C.; Olivos, N.; Passeri, G.; O’Brien, C.A.; Bivi, N.; et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W.; Xie, L.; Luo, X.; Cao, X.; Wan, M. Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression. Ann. N. Y. Acad. Sci. 2016, 1364, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Uda, Y.; Azab, E.; Kochen, A.; Santos, R.; Shi, C.; Kobayashi, T.; Wein, M.N.; Divieti Pajevic, P. Effects of histone deacetylase inhibitor Scriptaid and parathyroid hormone on osteocyte functions and metabolism. J. Biol. Chem. 2019, 294, 9722–9733. [Google Scholar] [CrossRef]
- Wein, M.N.; Liang, Y.; Goransson, O.; Sundberg, T.B.; Wang, J.; Williams, E.A.; O’Meara, M.J.; Govea, N.; Beqo, B.; Nishimori, S.; et al. SIKs control osteocyte responses to parathyroid hormone. Nat. Commun. 2016, 7, 13176. [Google Scholar] [CrossRef]
- Yang, H.; Dong, J.; Xiong, W.; Fang, Z.; Guan, H.; Li, F. N-cadherin restrains PTH repressive effects on sclerostin/SOST by regulating LRP6-PTH1R interaction. Ann. N. Y. Acad. Sci. 2016, 1385, 41–52. [Google Scholar] [CrossRef]
- Fortunati, D.; Reppe, S.; Fjeldheim, A.K.; Nielsen, M.; Gautvik, V.T.; Gautvik, K.M. Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. Matrix Biol. 2010, 29, 594–601. [Google Scholar] [CrossRef]
- Bonnet, N.; Conway, S.J.; Ferrari, S.L. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc. Natl. Acad. Sci. USA 2012, 109, 15048–15053. [Google Scholar] [CrossRef]
- Saito, H.; Gasser, A.; Bolamperti, S.; Maeda, M.; Matthies, L.; Jähn, K.; Long, C.L.; Schlüter, H.; Kwiatkowski, M.; Saini, V.; et al. TG-interacting factor 1 (Tgif1)-deficiency attenuates bone remodeling and blunts the anabolic response to parathyroid hormone. Nat. Commun. 2019, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. Parathyroid hormone inhibits Notch signaling in osteoblasts and osteocytes. Bone 2017, 103, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Bridgewater, D.; Schilling, L.; Zanotti, S. Canonical Notch activation in osteocytes causes osteopetrosis. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E171–E182. [Google Scholar] [CrossRef] [PubMed]
- Yorgan, T.; Vollersen, N.; Riedel, C.; Jeschke, A.; Peters, S.; Busse, B.; Amling, M.; Schinke, T. Osteoblast-specific Notch2 inactivation causes increased trabecular bone mass at specific sites of the appendicular skeleton. Bone 2016, 87, 136–146. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; McAndrews, K.; Wu, G.; Orr, A.L.; Ferrari, A.; Tu, X.; Srinivasan, V.; Roodman, G.D.; Ebetino, F.H.; Boeckman, R.K., Jr.; et al. The Notch pathway regulates the bone gain induced by PTH anabolic signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22196. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Liao, L.-M.; Wu, H.; Lin, Y.-P.; Yu, S. PTH promotes rabbit tibial fracture healing via the Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1616–1623. [Google Scholar]
- Li, J.Y.; Walker, L.D.; Tyagi, A.M.; Adams, J.; Weitzmann, M.N.; Pacifici, R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 43–54. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F.; Buondonno, I.; Fornelli, G.; Spertino, E.; D’Amico, L.; Marchetti, M.; Lucchiari, M.; Roato, I.; Isaia, G.C. Treatment with intermittent PTH increases Wnt10b production by T cells in osteoporotic patients. Osteoporos. Int. 2015, 26, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Li, J.Y.; Walker, L.D.; Tyagi, A.M.; Reott, M.A.; Yu, M.; Adams, J.; Weitzmann, M.N.; Pacifici, R. T cell-expressed CD40L potentiates the bone anabolic activity of intermittent PTH treatment. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. T cells, osteoblasts, and osteocytes: Interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 2016, 1364, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, W.; Li, S.; Ma, J.; Zhang, H.; Li, Z.; Zhang, L.; Zhang, B.; Li, Z.; Liang, X.; et al. Bovine parathyroid hormone enhances osteoclast bone resorption by modulating V-ATPase through PTH1R. Int. J. Mol. Med. 2016, 37, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, W.; Li, S.; Cui, T.; Li, Z.; Zhang, B.; Li, Z.; Wu, J.; Liang, X.; Lin, Z.; et al. The effect of bovine parathyroid hormone withdrawal on MC3T3-E1 cell proliferation and phosphorus metabolism. PLoS ONE 2015, 10, e0120402. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Marengi, D.A.; Barry, K.J.; Fulzele, K.S.; Heiden, E.; Liu, X.; Dedic, C.; Maeda, A.; Lotinun, S.; Baron, R.; et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 2013, 288, 20122–20134. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. CCL2/Monocyte Chemoattractant Protein 1 and Parathyroid Hormone Action on Bone. Front. Endocrinol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Johnson, J.; Le Henaff, C.; Bitel, C.L.; Tamasi, J.A.; Partridge, N.C. Catabolic Effects of Human PTH (1-34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism. Sci. Rep. 2017, 7, 15300. [Google Scholar] [CrossRef]
- Pellicelli, M.; Taheri, M.; St-Louis, M.; Bériault, V.; Desgroseillers, L.; Boileau, G.; Moreau, A. PTHrP(1-34)-mediated repression of the PHEX gene in osteoblastic cells involves the transcriptional repressor E4BP4. J. Cell. Physiol. 2012, 227, 2378–2387. [Google Scholar] [CrossRef]
- Dela Cruz, A.; Grynpas, M.D.; Mitchell, J. Overexpression of Gα11 in Osteoblast Lineage Cells Suppresses the Osteoanabolic Response to Intermittent PTH and Exercise. Calcif. Tissue Int. 2016, 99, 423–434. [Google Scholar] [CrossRef]
- Ogata, N.; Shinoda, Y.; Wettschureck, N.; Offermanns, S.; Takeda, S.; Nakamura, K.; Segre, G.V.; Chung, U.I.; Kawaguchi, H. G alpha(q) signal in osteoblasts is inhibitory to the osteoanabolic action of parathyroid hormone. J. Biol. Chem. 2011, 286, 13733–13740. [Google Scholar] [CrossRef]
- Lin, Z.; Feng, R.; Li, J.; Meng, Y.; Yuan, L.; Fu, Z.; Guo, J.; Bringhurst, F.R.; Yang, D. Nuclear translocation of CBP/p300-interacting protein CITED1 induced by parathyroid hormone requires serine phosphorylation at position 79 in its 63-84 domain. Cell. Signal. 2014, 26, 2436–2445. [Google Scholar] [CrossRef]
- Romero, G.; Sneddon, W.B.; Yang, Y.; Wheeler, D.; Blair, H.C.; Friedman, P.A. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J. Biol. Chem. 2010, 285, 14756–14763. [Google Scholar] [CrossRef]
- Babey, M.; Wang, Y.; Kubota, T.; Fong, C.; Menendez, A.; ElAlieh, H.Z.; Bikle, D.D. Gender-Specific Differences in the Skeletal Response to Continuous PTH in Mice Lacking the IGF1 Receptor in Mature Osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Ben-awadh, A.N.; Delgado-Calle, J.; Tu, X.; Kuhlenschmidt, K.; Allen, M.R.; Plotkin, L.I.; Bellido, T. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology 2014, 155, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.; Fenne, I.S.; Flågeng, M.H.; Almås, B.; Lien, E.A.; Mellgren, G. Estradiol determines the effects of PTH on ERα-dependent transcription in MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2014, 450, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.P.; Khan, K.; Yadav, P.S.; Singh, A.K.; Nag, A.; Prasahar, P.; Mittal, M.; China, S.P.; Tewari, M.C.; Nagar, G.K.; et al. BMP signaling is required for adult skeletal homeostasis and mediates bone anabolic action of parathyroid hormone. Bone 2016, 92, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Park, Y.J.; Libermann, T.A.; Cho, J.Y. PTH regulates myleoid ELF-1-like factor (MEF)-induced MAB-21-like-1 (MAB21L1) expression through the JNK1 pathway. J. Cell. Biochem. 2011, 112, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Obri, A.; Makinistoglu, M.P.; Zhang, H.; Karsenty, G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J. Cell Biol. 2014, 205, 771–780. [Google Scholar] [CrossRef]
- Yang, Y.; Blair, H.C.; Shapiro, I.M.; Wang, B. The Proteasome Inhibitor Carfilzomib Suppresses Parathyroid Hormone-induced Osteoclastogenesis through a RANKL-mediated Signaling Pathway. J. Biol. Chem. 2015, 290, 16918–16928. [Google Scholar] [CrossRef] [PubMed]
- Heckt, T.; Keller, J.; Peters, S.; Streichert, T.; Chalaris, A.; Rose-John, S.; Mell, B.; Joe, B.; Amling, M.; Schinke, T. Parathyroid hormone induces expression and proteolytic processing of Rankl in primary murine osteoblasts. Bone 2016, 92, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Goetjen, A.; Estus, T.; Jacome-Galarza, C.E.; Aguila, H.L.; Lorenzo, J.; Pilbeam, C. Serum Amyloid A3 Secreted by Preosteoclasts Inhibits Parathyroid Hormone-stimulated cAMP Signaling in Murine Osteoblasts. J. Biol. Chem. 2016, 291, 3882–3894. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Hancock, B.; Likine, E.F.; Sato, A.Y.; McAndrews, K.; Sanudo, C.; Bruzzaniti, A.; Riancho, J.A.; Tonra, J.R.; Bellido, T. MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 2878–2890. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.L.; Chen, P.; Yu, A.X.; Kong, M.; Tan, Z.; Tsang, K.Y.; Zhou, Z.; Cheah, K.S.E. MMP14 cleaves PTH1R in the chondrocyte-derived osteoblast lineage, curbing signaling intensity for proper bone anabolism. Elife 2023, 12, 82142. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.C.; Poulton, I.J.; McGregor, N.E.; Ho, P.W.; Allan, E.H.; Quach, J.M.; Martin, T.J.; Sims, N.A. Sustained RANKL response to parathyroid hormone in oncostatin M receptor-deficient osteoblasts converts anabolic treatment to a catabolic effect in vivo. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Takyar, F.M.; Tonna, S.; Ho, P.W.; Crimeen-Irwin, B.; Baker, E.K.; Martin, T.J.; Sims, N.A. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 912–925. [Google Scholar] [CrossRef]
- Xiong, L.; Xia, W.F.; Tang, F.L.; Pan, J.X.; Mei, L.; Xiong, W.C. Retromer in Osteoblasts Interacts With Protein Phosphatase 1 Regulator Subunit 14C, Terminates Parathyroid Hormone’s Signaling, and Promotes Its Catabolic Response. EBioMedicine 2016, 9, 45–60. [Google Scholar] [CrossRef]
- Pacifici, R. The Role of IL-17 and TH17 Cells in the Bone Catabolic Activity of PTH. Front. Immunol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Shukla, P.; Singh, D. Combination of PTH (1-34) with anti-IL17 prevents bone loss by inhibiting IL-17/N-cadherin mediated disruption of PTHR1/LRP-6 interaction. Bone 2017, 105, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; D’Amelio, P.; Robinson, J.; Walker, L.D.; Vaccaro, C.; Luo, T.; Tyagi, A.M.; Yu, M.; Reott, M.; Sassi, F.; et al. IL-17A Is Increased in Humans with Primary Hyperparathyroidism and Mediates PTH-Induced Bone Loss in Mice. Cell Metab. 2015, 22, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Yu, M.; Tyagi, A.M.; Vaccaro, C.; Hsu, E.; Adams, J.; Bellido, T.; Weitzmann, M.N.; Pacifici, R. IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wang, Q.; Han, Y.; Li, J.; Yang, X.J.; Miao, D. Parathyroid hormone administration improves bone marrow microenvironment and partially rescues haematopoietic defects in Bmi1-null mice. PLoS ONE 2014, 9, e93864. [Google Scholar] [CrossRef] [PubMed]
- Maridas, D.E.; Rendina-Ruedy, E.; Helderman, R.C.; DeMambro, V.E.; Brooks, D.; Guntur, A.R.; Lanske, B.; Bouxsein, M.L.; Rosen, C.J. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, H.; Yamamoto, T.; Zhao, S.; Hongo, H.; Abe, M.; Ishizu, H.; Yoshino, H.; Luiz de Freitas, P.H.; Li, M.; Hasegawa, T. Histological functions of parathyroid hormone on bone formation and bone blood vessels. J. Oral Biosci. 2022, 64, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Chen, D.; Lee, J.; Gu, X.; Alaaeddine, G.; Li, J.; Wei, L.; Yu, S.P. Mobilization of endogenous bone marrow derived endothelial progenitor cells and therapeutic potential of parathyroid hormone after ischemic stroke in mice. PLoS ONE 2014, 9, e87284. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, P.; Zhou, H.; Wan, B.; Yin, J.; Huang, Y.; Li, Q.; Yin, G.; Fan, J. Lack of endogenous parathyroid hormone delays fracture healing by inhibiting vascular endothelial growth factor—mediated angiogenesis. Int. J. Mol. Med. 2018, 42, 171–181. [Google Scholar] [CrossRef]
- Prisby, R.; Menezes, T.; Campbell, J. Vasodilation to PTH (1-84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone 2013, 54, 68–75. [Google Scholar] [CrossRef]
- Gohin, S.; Carriero, A.; Chenu, C.; Pitsillides, A.A.; Arnett, T.R.; Marenzana, M. The anabolic action of intermittent parathyroid hormone on cortical bone depends partly on its ability to induce nitric oxide-mediated vasorelaxation in BALB/c mice. Cell Biochem. Funct. 2016, 34, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Vanden-Bossche, A.; Malaval, L.; Normand, M.; Jannot, M.; Chaux, R.; Vico, L.; Lafage-Proust, M.H. Parathyroid hormone 1-84 targets bone vascular structure and perfusion in mice: Impacts of its administration regimen and of ovariectomy. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.; Menezes, T.; Campbell, J.; Bice, A.; Hood, B.; Prisby, R. Mechanisms of vasodilation to PTH 1-84, PTH 1-34, and PTHrP 1-34 in rat bone resistance arteries. Osteoporos. Int. 2016, 27, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Lagumdzija, A.; Ou, G.; Petersson, M.; Bucht, E.; Gonon, A.; Pernow, Y. Inhibited anabolic effect of insulin-like growth factor-I on stromal bone marrow cells in endothelial nitric oxide synthase-knockout mice. Acta Physiol. Scand. 2004, 182, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Caire, R.; Roche, B.; Picot, T.; Aanei, C.M.; He, Z.; Campos, L.; Thomas, M.; Malaval, L.; Vico, L.; Lafage-Proust, M.H. Parathyroid Hormone Remodels Bone Transitional Vessels and the Leptin Receptor-Positive Pericyte Network in Mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiao, G.; Galson, D.L.; Nishio, Y.; Mizokami, A.; Keller, E.T.; Yao, Z.; Zhang, J. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int. J. Cancer 2007, 121, 724–733. [Google Scholar] [CrossRef]
- Byrne, N.M.; Summers, M.A.; McDonald, M.M. Tumor Cell Dormancy and Reactivation in Bone: Skeletal Biology and Therapeutic Opportunities. JBMR Plus 2019, 3, e10125. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Riese, D.J., II; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 574667. [Google Scholar] [CrossRef]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.H.; et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef]
- Solimando, A.G.; Brandl, A.; Mattenheimer, K.; Graf, C.; Ritz, M.; Ruckdeschel, A.; Stuhmer, T.; Mokhtari, Z.; Rudelius, M.; Dotterweich, J.; et al. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia 2018, 32, 736–743. [Google Scholar] [CrossRef]
- Caino, M.C.; Chae, Y.C.; Vaira, V.; Ferrero, S.; Nosotti, M.; Martin, N.M.; Weeraratna, A.; O’Connell, M.; Jernigan, D.; Fatatis, A.; et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J. Clin. Investig. 2013, 123, 2907–2920. [Google Scholar] [CrossRef]
- Rudelius, M.; Rosenfeldt, M.T.; Leich, E.; Rauert-Wunderlich, H.; Solimando, A.G.; Beilhack, A.; Ott, G.; Rosenwald, A. Inhibition of focal adhesion kinase overcomes resistance of mantle cell lymphoma to ibrutinib in the bone marrow microenvironment. Haematologica 2018, 103, 116–125. [Google Scholar] [CrossRef]
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat. Rev. Clin Oncol. 2017, 14, 155–167. [Google Scholar] [CrossRef]
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers 2019, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Fizazi, K.; Stopeck, A.T.; Henry, D.H.; Smith, M.R.; Shore, N.; Martin, M.; Vadhan-Raj, S.; Brown, J.E.; Richardson, G.E.; et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer 2016, 53, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Via, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; Bondarenko, I.; Brincat, S.; Hotko, Y.; Munster, P.N.; Chmielowska, E.; Fumoleau, P.; Ward, R.; Bardy-Bouxin, N.; Leip, E.; et al. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann. Oncol. 2012, 23, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Southby, J.; Kissin, M.W.; Danks, J.A.; Hayman, J.A.; Moseley, J.M.; Henderson, M.A.; Bennett, R.C.; Martin, T.J. Immunohistochemical Localization of Parathyroid Hormone-related Protein in Human Breast Cancer. Cancer Res. 1991, 50, 7710–7716. [Google Scholar]
- Shen, X.; Rychahou, P.G.; Evers, B.M.; Falzon, M. PTHrP increases xenograft growth and promotes integrin α6β4 expression and Akt activation in colon cancer. Cancer Lett. 2007, 258, 241–252. [Google Scholar] [CrossRef]
- Henderson, M.A.; Danks, J.A.; Slavin, J.L.; Byrnes, G.B.; Choong, P.F.; Spillane, J.B.; Hopper, J.L.; Martin, T.J. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006, 66, 2250–2256. [Google Scholar] [CrossRef]
- Soki, F.N.; Park, S.I.; McCauley, L.K. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol. 2012, 8, 803–817. [Google Scholar] [CrossRef]
- Manilay, J.O.; Zouali, M. Tight relationships between B lymphocytes and the skeletal system. Trends Mol. Med. 2014, 20, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.S.; Lynch, C.C. Proteolytic Regulation of Parathyroid Hormone-Related Protein: Functional Implications for Skeletal Malignancy. Int. J. Mol. Sci. 2019, 20, 2814. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; McCauley, L.K. Skeletal metastasis: Established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev. 2006, 25, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Antwi, S.O.; Sartorius, K.; Zheng, X.; Li, X. Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer. Cancers 2022, 14, 4888. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, L.; Rosen, C.J. PTH and the Regulation of Mesenchymal Cells within the Bone Marrow Niche. Cells 2024, 13, 406. https://doi.org/10.3390/cells13050406
Liu H, Liu L, Rosen CJ. PTH and the Regulation of Mesenchymal Cells within the Bone Marrow Niche. Cells. 2024; 13(5):406. https://doi.org/10.3390/cells13050406
Chicago/Turabian StyleLiu, Hanghang, Linyi Liu, and Clifford J. Rosen. 2024. "PTH and the Regulation of Mesenchymal Cells within the Bone Marrow Niche" Cells 13, no. 5: 406. https://doi.org/10.3390/cells13050406





