Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Experimental Design
2.3. Kidney Function Evaluation
2.4. ELISA Techniques
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Western Blotting Analysis
2.7. Histological Examination
2.8. Statistical Analysis
3. Results
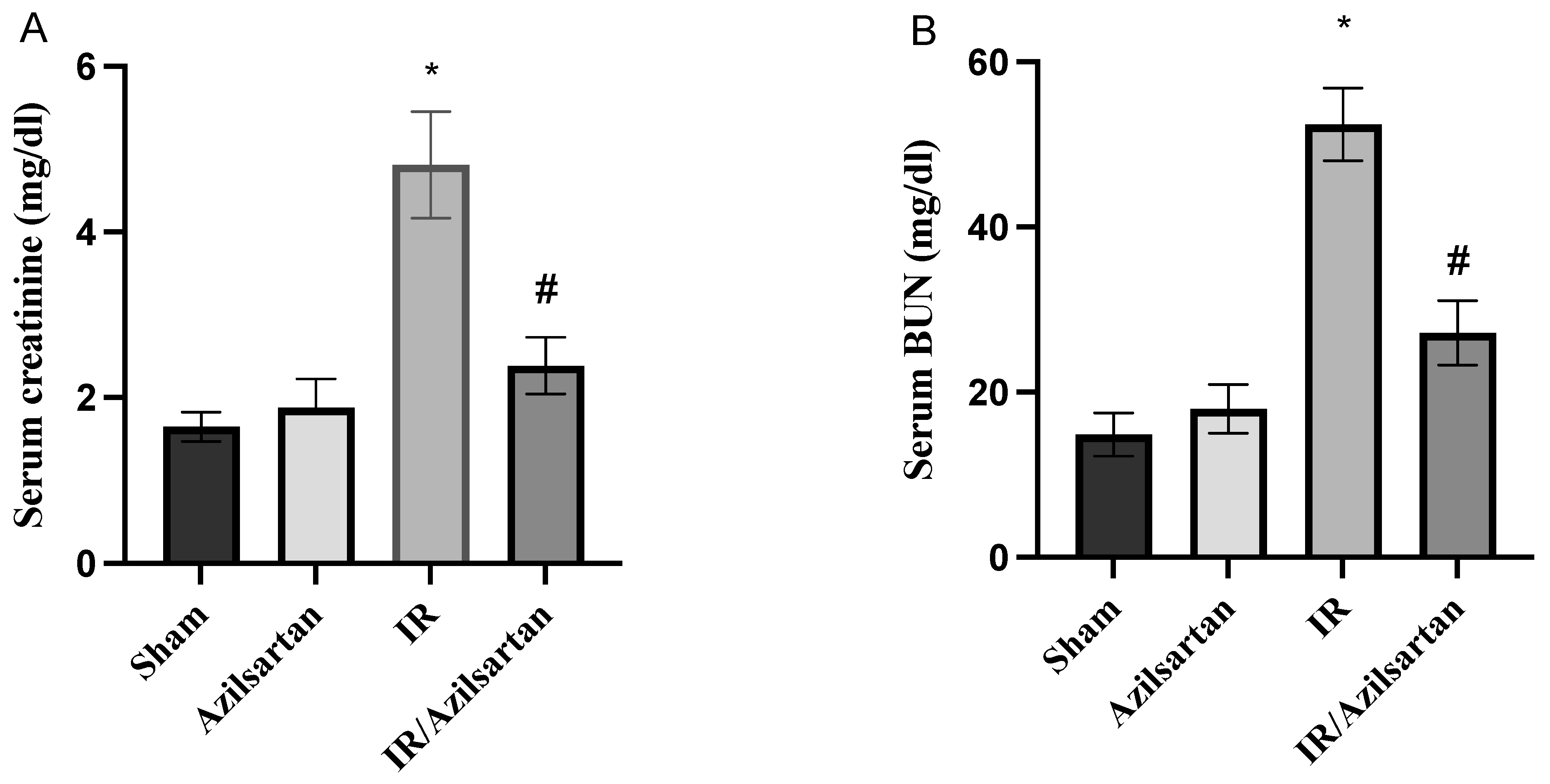
3.1. Creatinine and BUN Serum Levels
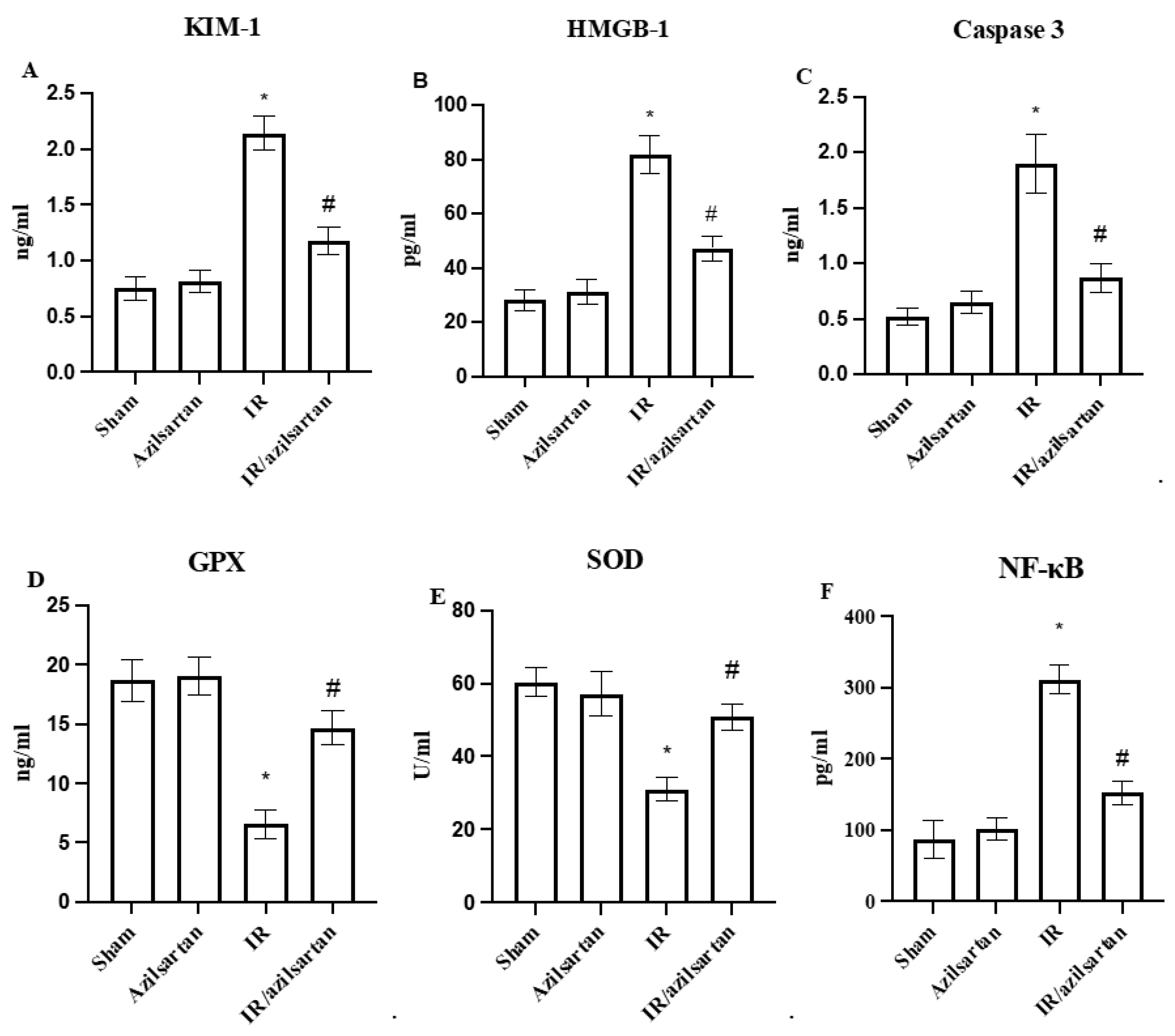
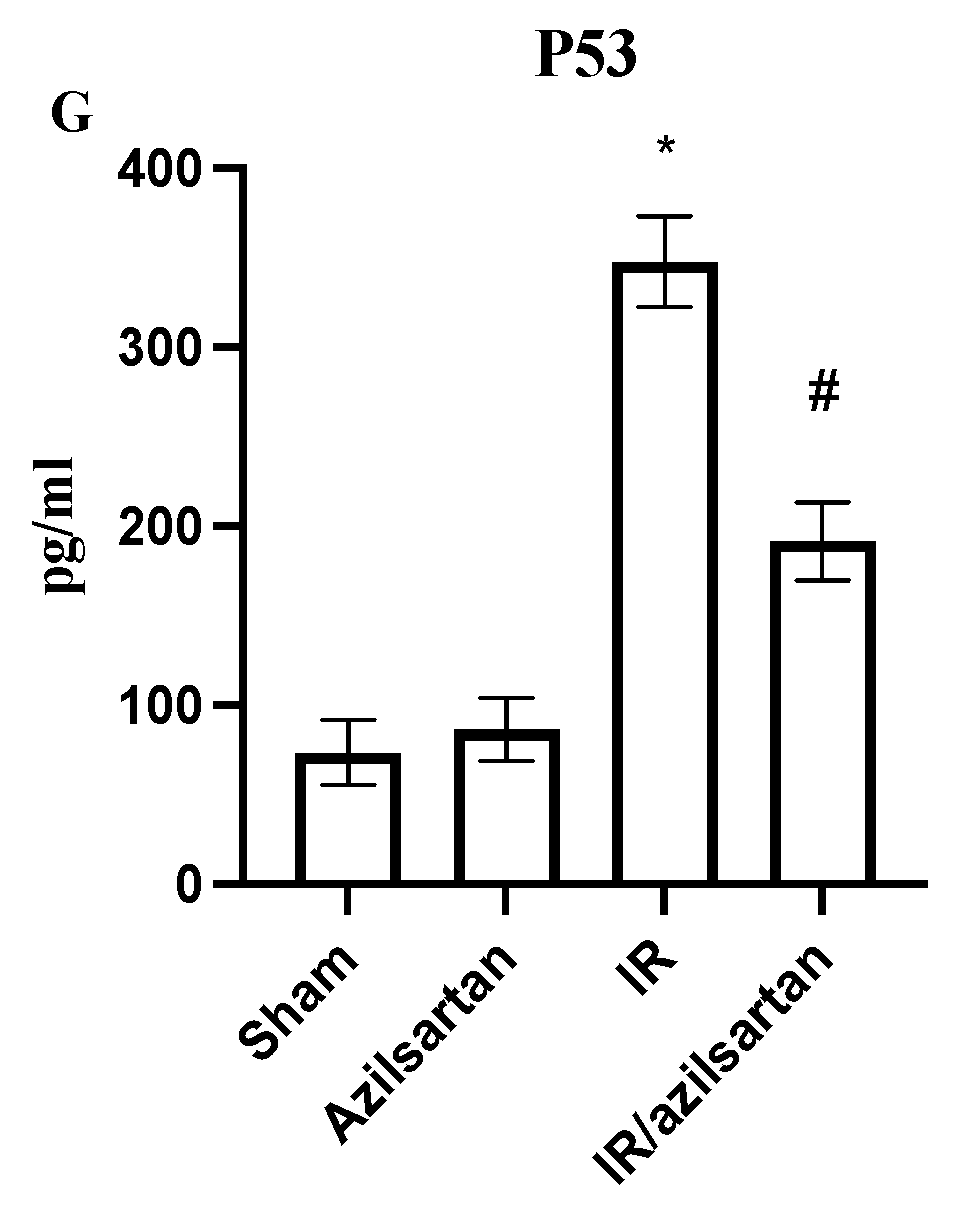
3.2. Levels of KIM-1, HMGB-1, Caspase 3, GPX, SOD, NF-κB and p53
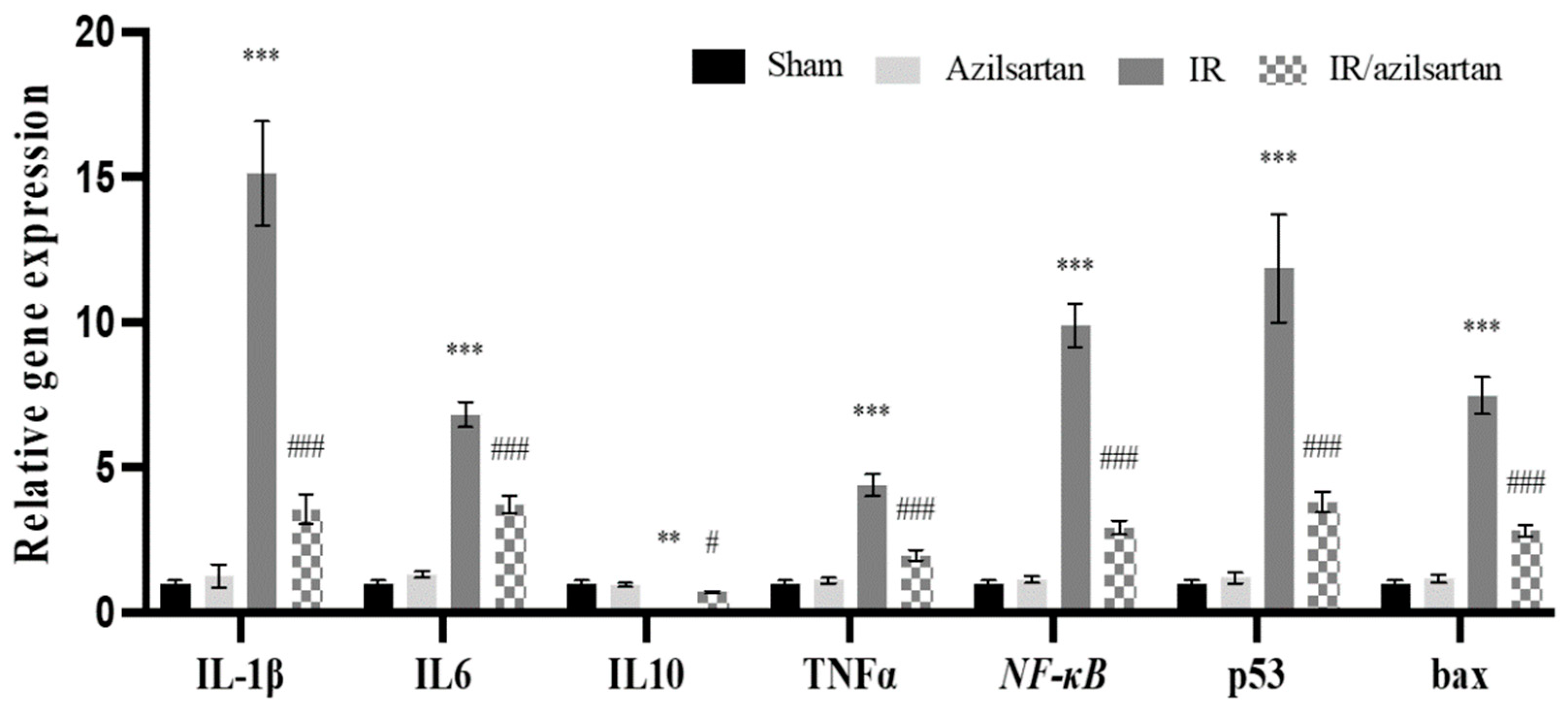
3.3. Expression of IL-1β, IL6, IL10, TNF-α, NF-κB, p53, and Bax Genes
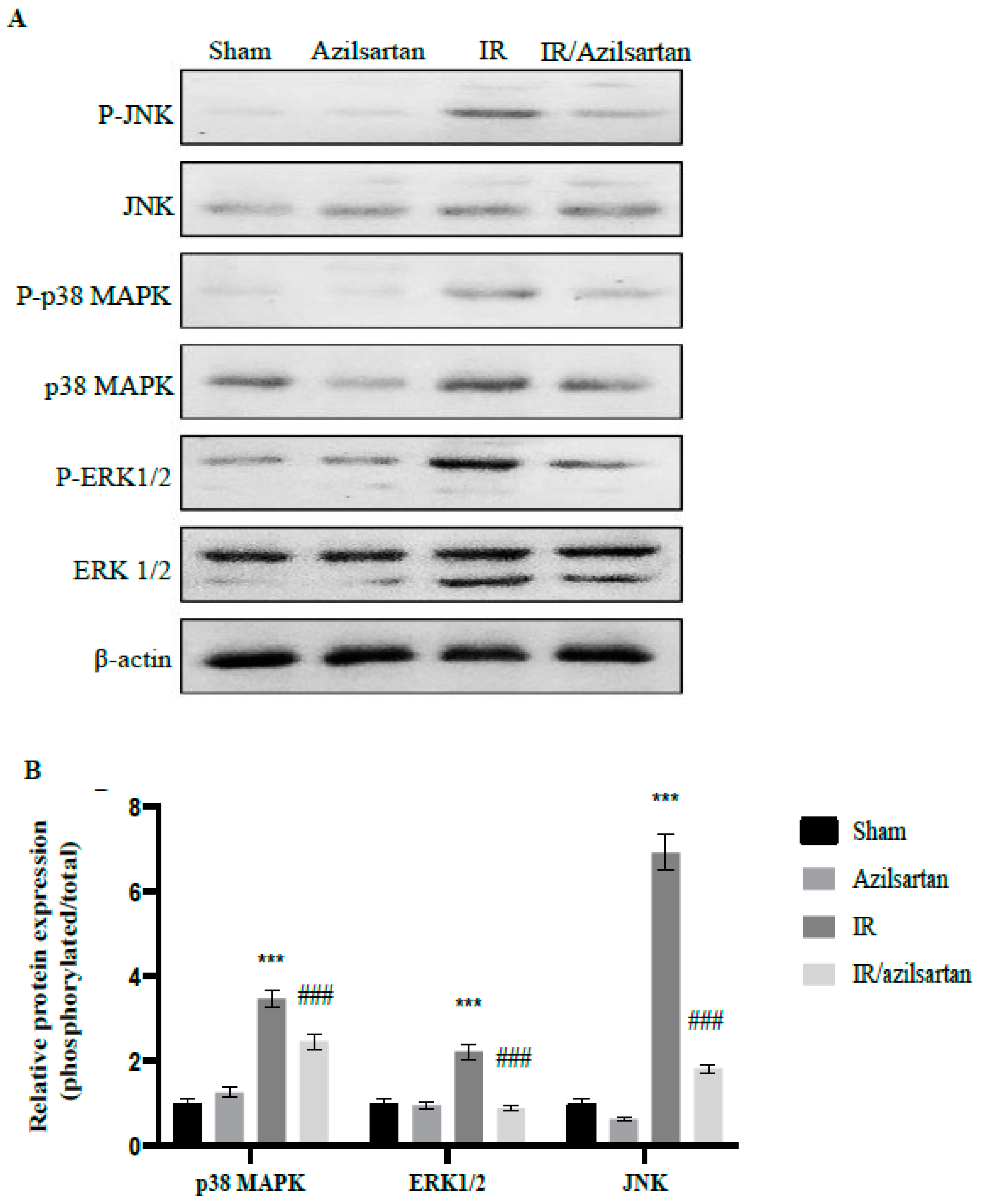
3.4. Expression of p38 MAPK, ERK1/2, and JNK Proteins
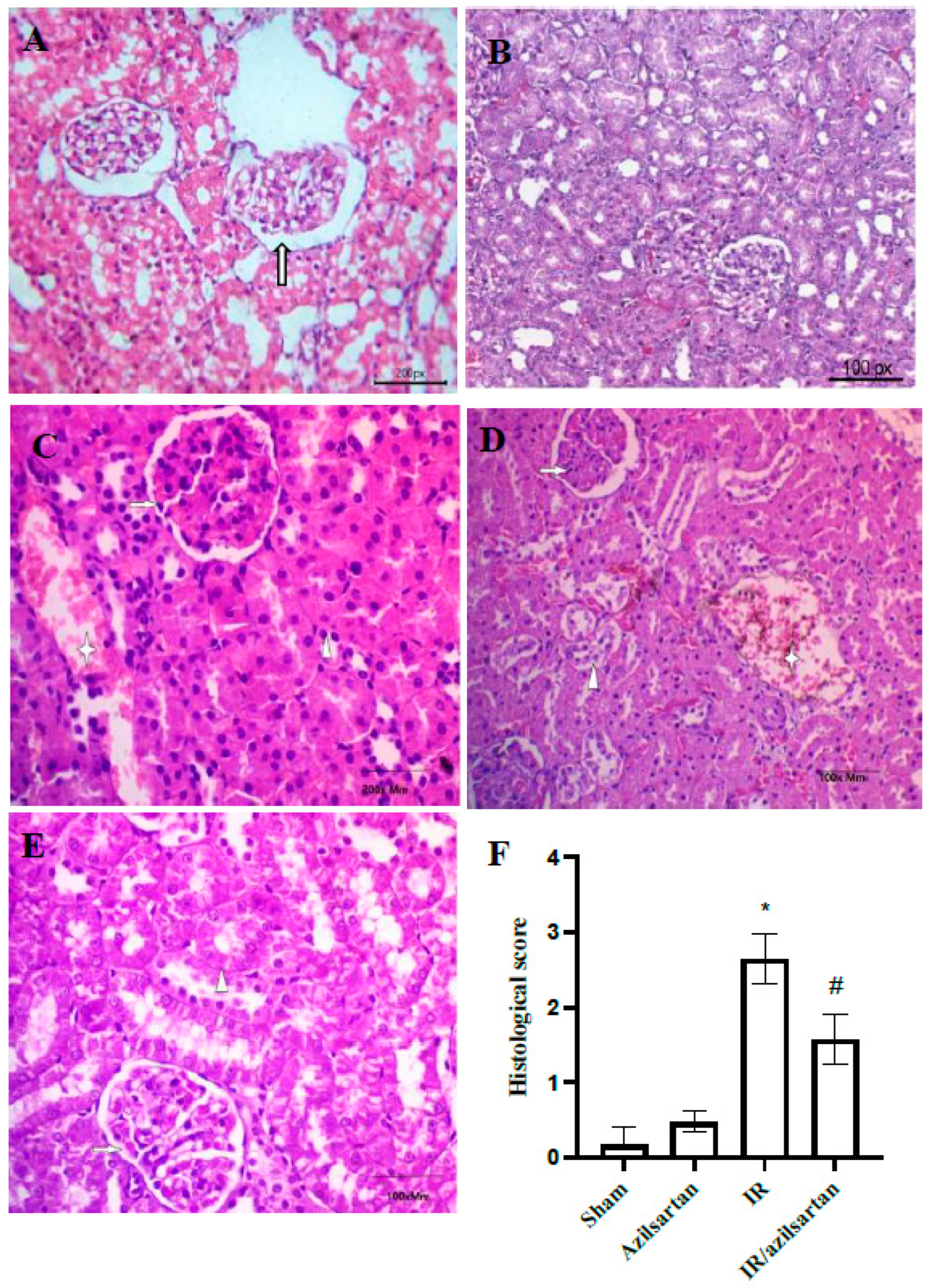
3.5. Histopathological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tammaro, A.; Kers, J.; Scantlebery, A.M.L.; Florquin, S. Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration. Front. Immunol. 2020, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Weinberg, J.M. Postischemic renal injury due to oxygen radicals. Curr. Opin. Nephrol. Hypertens. 1993, 2, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shi, H.; Liu, D. Chrysin protects against renal ischemia reperfusion induced tubular cell apoptosis and inflammation in mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Awale, S.; Nikaido, T. Phosphorylated Akt Protein at Ser473 Enables HeLa Cells to Tolerate Nutrient-Deprived Conditions. Asian Pac. J. Cancer Prev. 2017, 18, 3255–3260. [Google Scholar] [CrossRef]
- Fathy, M.; Khalifa, E.M.; Fawzy, M.A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci. 2019, 216, 207–214. [Google Scholar] [CrossRef]
- Fathy, M.; Nikaido, T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ. Health Prev. Med. 2013, 18, 377–385. [Google Scholar] [CrossRef]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef]
- Abdel-Latif, R.; Fathy, M.; Anwar, H.A.; Naseem, M.; Dandekar, T.; Othman, E.M. Cisplatin-Induced Reproductive Toxicity and Oxidative Stress: Ameliorative Effect of Kinetin. Antioxidants 2022, 11, 863. [Google Scholar] [CrossRef]
- Fathy, M.; Darwish, M.A.; Abdelhamid, A.-S.M.; Alrashedy, G.M.; Othman, O.A.; Naseem, M.; Dandekar, T.; Othman, E.M. Kinetin Ameliorates Cisplatin-Induced Hepatotoxicity and Lymphotoxicity via Attenuating Oxidative Damage, Cell Apoptosis and Inflammation in Rats. Biomedicines 2022, 10, 1620. [Google Scholar] [CrossRef]
- Fathy, M.; Abdel-Latif, R.; Abdelgwad, Y.M.; Othman, O.A.; Abdel-Razik, A.-R.H.; Dandekar, T.; Othman, E.M. Nephroprotective potential of eugenol in a rat experimental model of chronic kidney injury; targeting NOX, TGF-β, and Akt signaling. Life Sci. 2022, 308, 120957. [Google Scholar] [CrossRef]
- El-Baky, R.M.A.; Hetta, H.F.; Koneru, G.; Ammar, M.; Shafik, E.A.; A Mohareb, D.; El-Masry, M.A.; Ramadan, H.K.; Abu Rahma, M.Z.; Fawzy, M.A.; et al. Impact of interleukin IL-6 rs-1474347 and IL-10 rs-1800896 genetic polymorphisms on the susceptibility of HCV-infected Egyptian patients to hepatocellular carcinoma. Immunol. Res. 2020, 68, 118–125. [Google Scholar] [CrossRef]
- Eldafashi, N.; Darlay, R.; Shukla, R.; McCain, M.; Watson, R.; Liu, Y.; McStraw, N.; Fathy, M.; Fawzy, M.; Zaki, M.; et al. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers 2021, 13, 1412. [Google Scholar] [CrossRef]
- Fathy, M.; Sun, S.; Zhao, Q.-L.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Awale, S.; Nikaido, T. A New Ciprofloxa-cin-derivative Inhibits Proliferation and Suppresses the Migration Ability of HeLa Cells. Anticancer Res. 2020, 40, 5025–5033. [Google Scholar] [CrossRef]
- Naseem, M.; Othman, E.M.; Fathy, M.; Iqbal, J.; Howari, F.M.; AlRemeithi, F.A.; Kodandaraman, G.; Stopper, H.; Bencurova, E.; Vlachakis, D.; et al. Integrated structural and functional analysis of the protective effects of kinetin against oxidative stress in mammalian cellular systems. Sci. Rep. 2020, 10, 13330. [Google Scholar] [CrossRef]
- Voss, A.; Bode, G.; Kerkhoff, C. Double-Stranded RNA Induces IL-8 and MCP-1 Gene Expression via TLR3 in HaCaT-Keratinocytes. Inflamm. Allergy-Drug Targets 2012, 11, 397–405. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Li, G.S.; Wang, J. The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats. Ren. Fail. 2018, 40, 680–686. [Google Scholar] [CrossRef]
- Abdellatef, A.A.; Fathy, M.; Mohammed, A.E.-S.I.; Abu Bakr, M.S.; Ahmed, A.H.; Abbass, H.S.; El-Desoky, A.H.; Morita, H.; Nikaido, T.; Hayakawa, Y. Inhibition of cell-intrinsic NF-κB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J. Nat. Med. 2021, 75, 840–853. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Z.; Zeng, R.; Yao, Y. HMGB1 in kidney diseases. Life Sci. 2020, 259, 118203. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, X.; Zuo, X.; Wang, J.; Yin, W. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm. Sin. B 2016, 6, 183–188. [Google Scholar] [CrossRef]
- Zaki, M.Y.W.; Fathi, A.M.; Samir, S.; Eldafashi, N.; William, K.Y.; Nazmy, M.H.; Fathy, M.; Gill, U.S.; Shetty, S. Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma. Cancers 2022, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, S.; Zhang, W.; Liu, X.; Li, Y.; Zhang, C.; Zeng, Y.; Xu, M.; Rong, R.; Yang, T.; et al. High-mobility group box 1 protein antagonizes the immunosuppressive capacity and therapeutic effect of mesenchymal stem cells in acute kidney injury. J. Transl. Med. 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Kunduzova, O.R.; Bianchi, P.; Pizzinat, N.; Escourrou, G.; Seguelas, M.H.; Parini, A.; Cambon, C. Regulation of JNK/ERK activation, cell apoptosis, and tissue regeneration by monoamine oxidases after renal ischemia-reperfusion. FASEB J. 2002, 16, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A.; Maher, S.A.; El-Rehany, M.A.; Welson, N.N.; Albezrah, N.K.A.; Batiha, G.E.-S.; Fathy, M. Vincamine Modulates the Effect of Pantoprazole in Renal Ischemia/Reperfusion Injury by Attenuating MAPK and Apoptosis Signaling Pathways. Molecules 2022, 27, 1383. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Salom, M.G.; Arregui, B.; Valero, F.; Fenoy, F.J. Role of Superoxide in Modulating the Renal Effects of Angiotensin II. Hypertension 2003, 42, 1150–1156. [Google Scholar] [CrossRef]
- Pazoki-Toroudi, H.R.; Hesami, A.; Vahidi, S.; Sahebjam, F.; Seifi, B.; Djahanguiri, B. The preventive effect of captopril or enalapril on reperfusion injury of the kidney of rats is independent of angiotensin II AT1 receptors. Fundam. Clin. Pharmacol. 2003, 17, 595–598. [Google Scholar] [CrossRef]
- Ali, A.N.; Altimimi, M.L.; Al-Ardi, H.M.; Hadi, N.R. Nephroprotective Potential Effect of Azilsartan in Renal Ischemia Reperfusion Injury/role VEGF Pathway. Syst. Rev. Pharm. 2019, 10, 90–99. [Google Scholar]
- Alaaeldin, R.; Abuo-Rahma, G.E.-D.A.; Zhao, Q.-L.; Fathy, M. Modulation of Apoptosis and Epithelial-Mesenchymal Transition E-cadherin/TGF-β/Snail/TWIST Pathways by a New Ciprofloxacin Chalcone in Breast Cancer Cells. Anticancer. Res. 2021, 41, 2383–2395. [Google Scholar] [CrossRef]
- Nagura, S.; Otaka, S.; Koike, C.; Okabe, M.; Yoshida, T.; Fathy, M.; Fukahara, K.; Yoshimura, N.; Misaki, T.; Nikaido, T. Effect of Exogenous Oct4 Overexpression on Cardiomyocyte Differentiation of Human Amniotic Mesenchymal Cells. Cell. Reprogramming 2013, 15, 471–480. [Google Scholar] [CrossRef]
- Oba, J.; Okabe, M.; Yoshida, T.; Soko, C.; Fathy, M.; Amano, K.; Kobashi, D.; Wakasugi, M.; Okudera, H. Hyperdry human amniotic membrane application as a wound dressing for a full-thickness skin excision after a third-degree burn injury. Burn. Trauma 2020, 8, tkaa014. [Google Scholar] [CrossRef]
- Okabe, M.; Yoshida, T.; Suzuki, M.; Goto, M.; Omori, M.; Taguchi, M.; Toda, A.; Nakagawa, K.; Hiramoto, F.; Ushijima, T.; et al. Hyperdry Human Amniotic Membrane (HD-AM) is Supporting Aciclovir Included Device of Poly-N-p-Vinyl-Benzyl-D-Lactonamide (PVLA) Sphere for Treatment of HSV-1 Infected Rabbit Keratitis Model. J. Biotechnol. Biomater. 2017, 7, 251. [Google Scholar] [CrossRef]
- Otaka, S.; Nagura, S.; Koike, C.; Okabe, M.; Yoshida, T.; Fathy, M.; Yanagi, K.; Misaki, T.; Nikaido, T. Selective Isolation of Nanog-Positive Human Amniotic Mesenchymal Cells and Differentiation into Cardiomyocytes. Cell. Reprogramming 2013, 15, 80–91. [Google Scholar] [CrossRef]
- Zhou, K.; Koike, C.; Yoshida, T.; Okabe, M.; Fathy, M.; Kyo, S.; Kiyono, T.; Saito, S.; Nikaido, T. Establishment and Characterization of Immortalized Human Amniotic Epithelial Cells. Cell. Reprogramming 2013, 15, 55–67. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Wang, H.; Zhang, Y.; Duan, X.; Xue, Y.; He, H.; Huang, Y.; Chen, Z.; Ren, H.; et al. Gastrin Attenuates Renal Ischemia/Reperfusion Injury by a PI3K/Akt/Bad-Mediated Anti-apoptosis Signaling. Front. Pharmacol. 2020, 11, 540479. [Google Scholar] [CrossRef]
- AL-SHIBANI, B.I.M.; KAHALEQ, M.A.A.; ABOSAOODA, M.; MOSA, A.K.; ABDULHUSSEIN, M.A.; HADI, N.R. Potential Nephroprotective Effect of Valsartan in Renal Ischemia Reperfusion Injury Role of NF-KBP65 Pathway in Rat. Int. J. Pharm. Res. 2020, 12, 928–935. [Google Scholar]
- Morsy, M.A.; Abdel-Gaber, S.A.; Rifaai, R.A.; Mohammed, M.M.; Nair, A.B.; Abdelzaher, W.Y. Protective mechanisms of telmisartan against hepatic ischemia/reperfusion injury in rats may involve PPARγ-induced TLR4/NF-κB suppression. Biomed. Pharmacother. 2022, 145, 112374. [Google Scholar] [CrossRef]
- Garg, S.; Khan, S.I.; Malhotra, R.K.; Sharma, M.K.; Kumar, M.; Kaur, P.; Nag, T.C.; Ray, R.; Bhatia, J.; Arya, D.S. Cardiopro-tective effects of azilsartan compared with that of telmisartan on an in vivo model of myocardial ischemia-reperfusion injury. J. Biochem. Mol. Toxicol. 2021, 35, e22785. [Google Scholar] [CrossRef]
- Gupta, V.; Dhull, D.K.; Joshi, J.; Kaur, S.; Kumar, A. Neuroprotective potential of azilsartan against cerebral ischemic injury: Possible involvement of mitochondrial mechanisms. Neurochem. Int. 2019, 132, 104604. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Yang, X. Azilsartan attenuates lipopolysaccharide-induced acute lung injury via the Nrf2/HO-1 signaling pathway. Immunol. Res. 2022, 70, 97–105. [Google Scholar] [CrossRef]
- Dong, Q.; Li, Y.; Chen, J.; Wang, N. Azilsartan Suppressed LPS-Induced Inflammation in U937 Macrophages through Sup-pressing Oxidative Stress and Inhibiting the TLR2/MyD88 Signal Pathway. ACS Omega 2020, 6, 113–118. [Google Scholar] [CrossRef]
- Baumann, P.Q.; Zaman, A.T.; McElroy-Yaggy, K.; Sobel, B.E. The efficacy and tolerability of azilsartan in mice with left ven-tricular pressure overload or acute myocardial infarction. J. Cardiovasc. Pharmacol. 2013, 61, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A.; Beshay, O.N.; Bekhit, A.A.; Abdel-Hafez, S.M.N.; Batiha, G.E.-S.; Bin Jardan, Y.A.; Fathy, M. Nephroprotective effect of AT-MSCs against cisplatin-induced EMT is improved by azilsartan via attenuating oxidative stress and TGF-β/Smad signaling. Biomed. Pharmacother. 2023, 158, 114097. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, E.A. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2013. [Google Scholar]
- Ozbilgin, S.; Ozkardesler, S.; Akan, M.; Boztas, N.; Ozbilgin, M.; Ergur, B.U.; Derici, S.; Guneli, M.E.; Meseri, R. Renal ische-mia/reperfusion injury in diabetic rats: The role of local ischemic preconditioning. BioMed Res. Int. 2016, 2016, 8580475. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, E.A.; Galceran, J.M.; Raij, L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998, 54, 775–784. [Google Scholar] [CrossRef]
- Haugen, E.N.; Croatt, A.J.; Nath, K.A. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int. 2000, 58, 144–152. [Google Scholar] [CrossRef][Green Version]
- Kontogiannis, J.; Burns, K.D. Role of AT1angiotensin II receptors in renal ischemic injury. Am. J. Physiol. Physiol. 1998, 274, F79–F90. [Google Scholar] [CrossRef]
- Cho, J.-H.; Choi, S.-Y.; Ryu, H.-M.; Oh, E.-J.; Yook, J.-M.; Ahn, J.-S.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D. Fimasartan attenuates renal ischemia-reperfusion injury by modulating inflammation-related apoptosis. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2018, 22, 661. [Google Scholar] [CrossRef]
- Safari, T.; Shahraki, M.R.; Miri, S.; Bakhshani, N.M.; Niazi, A.A.; Komeili, G.R.; Bagheri, H. The effect of angiotensin 1-7 and losartan on renal ischemic/reperfusion injury in male rats. Res. Pharm. Sci. 2019, 14, 441–447. [Google Scholar] [CrossRef]
- Fouad, A.A.; Qureshi, H.A.; Al-Sultan, A.I.; Yacoubi, M.T.; Al-Melhim, W.N. Nephroprotective Effect of Telmisartan in Rats with Ischemia/Reperfusion Renal Injury. Pharmacology 2010, 85, 158–167. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.-L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. [Google Scholar] [CrossRef]
- Othman, E.; Fathy, M.; Bekhit, A.; Abdel-Razik, A.-R.; Jamal, A.; Nazzal, Y.; Shams, S.; Dandekar, T.; Naseem, M. Modulatory and Toxicological Perspectives on the Effects of the Small Molecule Kinetin. Molecules 2021, 26, 670. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Abdel-Rahman, I.M.; Ali, F.E.M.; Bekhit, A.A.; Elhamadany, E.Y.; Zhao, Q.-L.; Cui, Z.-G.; Fathy, M. Dual Topoisomerase I/II Inhibition-Induced Apoptosis and Necro-Apoptosis in Cancer Cells by a Novel Ciprofloxacin Derivative via RIPK1/RIPK3/MLKL Activation. Molecules 2022, 27, 7993. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Hassan, H.A.; Abdel-Rahman, I.M.; Mohyeldin, R.H.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.-L.; Fathy, M. A New EGFR Inhibitor from Ficus benghalensis Exerted Potential Anti-Inflammatory Activity via Akt/PI3K Pathway Inhibition. Curr. Issues Mol. Biol. 2022, 44, 2967–2981. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Mustafa, M.; Abuo-Rahma, G.E.A.; Fathy, M. In vitro inhibition and molecular docking of a new ciprofloxacin-chalcone against SARS-CoV-2 main protease. Fundam. Clin. Pharmacol. 2021, 36, 160–170. [Google Scholar] [CrossRef]
- Fathy, M.; Okabe, M.; Othman, E.M.; Eldien, H.M.S.; Yoshida, T. Preconditioning of Adipose-Derived Mesenchymal Stem-Like Cells with Eugenol Potentiates Their Migration and Proliferation In Vitro and Therapeutic Abilities in Rat Hepatic Fibrosis. Molecules 2020, 25, 2020. [Google Scholar] [CrossRef]
- Shytaj, I.L.; Fares, M.; Gallucci, L.; Lucic, B.; Tolba, M.M.; Zimmermann, L.; Adler, J.M.; Xing, N.; Bushe, J.; Gruber, A.D.; et al. The FDA-Approved Drug Cobicistat Synergizes with Remdesivir To Inhibit SARS-CoV-2 Replication In Vitro and Decreases Viral Titers and Disease Progression in Syrian Hamsters. Mbio 2022, 13, e0370521. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Nazmy, M.H.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Fathy, M. Cell Cycle Arrest and Apoptotic Effect of 7-(4-(N-substituted carbamoylmethyl) piperazin-1-yl) Ciprofloxacin-derivative on HCT 116 and A549 Cancer Cells. Anticancer. Res. 2020, 40, 2739–2749. [Google Scholar] [CrossRef]
- Eisa, M.A.; Fathy, M.; Nazmy, M.H. Potential COX2 mediated Therapeutic effect of Ciprofloxacin. Minia J. Med. Res. 2021, 32, 47–57. [Google Scholar] [CrossRef]
- Fathy, M.; Eldin, S.M.S.; Naseem, M.; Dandekar, T.; Othman, E.M. Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential. Nutrients 2022, 14, 1495. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Necka, J.; Haines, J.; Imig, J.D.; Khan, A.H.; Neckar, J. Azilsartan Improves Glycemic Status and Reduces Kidney Damage in Zucker Diabetic Fatty Rats. Am. J. Hypertens. 2014, 27, 1087–1095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alaaeldin, R.; Ali, F.E.; Bekhit, A.A.; Zhao, Q.-L.; Fathy, M. Inhibition of NF-kB/IL-6/JAK2/STAT3 Pathway and Epitheli-al-Mesenchymal Transition in Breast Cancer Cells by Azilsartan. Molecules 2022, 27, 7825. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Investig. 2008, 68, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhou, X. The Nrf2/HO-1 pathway mediates the antagonist effect of L-arginine on renal ischemia/reperfusion injury in rats. Kidney Blood Press. Res. 2017, 42, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Saad El dien, H.M.; Bakhaat, G.A.; Rashwan, E.K.; Alaaeldin, R.; Fathy, M. Bone marrow-derived mesenchymal stem cells modulate apoptosis and angiogenesis in cyclophosphamide-induced spleen injury in rats. Egypt. J. Histol. 2022, 45. [Google Scholar] [CrossRef]
- Hou, N.; Li, L.-R.; Shi, Y.-Y.; Yuan, W.-C.; Zhao, G.-J.; Liu, X.-W.; Cai, S.-A.; Huang, Y.; Zhan, H.-X.; Pan, W.-B.; et al. Azilsartan ameliorates ventricular hypertrophy in rats suffering from pressure overload-induced cardiac hypertrophy by activating the Keap1–Nrf2 signalling pathway. J. Pharm. Pharmacol. 2021, 73, 1715–1725. [Google Scholar] [CrossRef]
- Qu, D.; Ling, Z.; Tan, X.; Chen, Y.; Huang, Q.; Li, M.; Liu, T.; Hou, C.; Chen, Y. High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF-κB pathways. Int. J. Clin. Exp. Pathol. 2019, 12, 3268–3278. [Google Scholar]
- Jeong, S.-R.; Park, H.-Y.; Kim, Y.; Lee, K.-W. Methylglyoxal-derived advanced glycation end products induce matrix metalloproteinases through activation of ERK/JNK/NF-κB pathway in kidney proximal epithelial cells. Food Sci. Biotechnol. 2019, 29, 675–682. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.M.; Ramadan, M.F.; Amgad, S.W. Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am. J. Cancer Prev. 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Liles, J.T.; Corkey, B.K.; Notte, G.T.; Budas, G.R.; Lansdon, E.B.; Hinojosa-Kirschenbaum, F.; Badal, S.S.; Lee, M.; Schultz, B.E.; Wise, S.; et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Investig. 2018, 128, 4485–4500. [Google Scholar] [CrossRef]
- Sabra, R.T.; Abdellatef, A.A.; Abdel-Sattar, E.; Fathy, M.; Meselhy, M.R.; Hayakawa, Y. Russelioside A, a Pregnane Glycoside from Caralluma tuberculate, Inhibits Cell-Intrinsic NF-κB Activity and Metastatic Ability of Breast Cancer Cells. Biol. Pharm. Bull. 2022, 45, 1564–1571. [Google Scholar] [CrossRef]
- di Mari, J.F.; Davis, R.; Safirstein, R.L. MAPK activation determines renal epithelial cell survival during oxidative injury. Am. J. Physiol. Physiol. 1999, 277, F195–F203. [Google Scholar] [CrossRef]
- Feliers, D.; Kasinath, B.S. Erk in Kidney Diseases. J. Signal Transduct. 2011, 2011, 768512. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Maher, S.A.; Bakkar, S.M.; El-Rehany, M.A.; Fathy, M. Pantoprazole Attenuates MAPK (ERK1/2, JNK, p38)–NF-κB and Apoptosis Signaling Pathways after Renal Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 10669. [Google Scholar] [CrossRef]
- Brown, L.; Benchimol, S. The involvement of MAPK signaling pathways in determining the cellular response to p53 activation: Cell cycle arrest or apoptosis. J. Biol. Chem. 2006, 281, 3832–3840. [Google Scholar] [CrossRef]
- Sun, A.; Zou, Y.; Wang, P.; Xu, D.; Gong, H.; Wang, S.; Qin, Y.; Zhang, P.; Chen, Y.; Harada, M.; et al. Mitochondrial Aldehyde Dehydrogenase 2 Plays Protective Roles in Heart Failure After Myocardial Infarction via Suppression of the Cytosolic JNK/p53 Pathway in Mice. J. Am. Hear. Assoc. 2014, 3, e000779. [Google Scholar] [CrossRef]
- Liu, J.; Mao, W.; Ding, B.; Liang, C.-S. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am. J. Physiol. Circ. Physiol. 2008, 295, H1956–H1965. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
| Primer | Sequence of the Primer |
|---|---|
| IL-1β | Forward: 5′-CCTATGTCTTGCCCGTGGAG-3’ Reverse: 5′-CACACACTAGCAGGTCGTCA-3′. |
| IL6 | Forward: 5′-CCTACCCCAACTTCCAATGCT-3′ Reverse: 5′-GGTCTTGGTCCTTAGCCACT-3′. |
| IL10 | Forward: 5′-CTGGCTCAGCACTGCTATGT-3′ Reverse: 5′-GCAGTTATTGTCACCCCGGA-3′. |
| TNFα | Forward: 5′-GGAGGGAGAACAGCAACTCC-3′ Reverse: 5′-GCCAGTGTATGAGAGGGACG-3′. |
| NF-κB | Forward: 5′-TCAACATGGCAGACGACGATCC-3′ Reverse: 5′-GAAGGTATGGGCCATCTGTTGAC-3′. |
| P53 | Forward: 5′-AGCGACTACAGTTAGGGGGT-3′ Reverse: 5′-ACAGTTATCCAGTCTTCAGGGG-3′. |
| Bax | Forward: 5′-CGTCTGCGGGGAGTCAC-3′ Reverse: 5′-AGCCATCCTCTCTGCTCGAT-3′. |
| GAPDH | Forward: 5′-AACCTGCCAAGTATGATGACATCA-3′ Reverse: 5′-TTCCACTGATATCCCAGCTGCT-3′. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaaeldin, R.; Bakkar, S.M.; Mohyeldin, R.H.; Ali, F.E.M.; Abdel-Maqsoud, N.M.R.; Fathy, M. Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury. Cells 2023, 12, 185. https://doi.org/10.3390/cells12010185
Alaaeldin R, Bakkar SM, Mohyeldin RH, Ali FEM, Abdel-Maqsoud NMR, Fathy M. Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury. Cells. 2023; 12(1):185. https://doi.org/10.3390/cells12010185
Chicago/Turabian StyleAlaaeldin, Rania, Sally M. Bakkar, Reham H. Mohyeldin, Fares E. M. Ali, Nehad M. Reda Abdel-Maqsoud, and Moustafa Fathy. 2023. "Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury" Cells 12, no. 1: 185. https://doi.org/10.3390/cells12010185
APA StyleAlaaeldin, R., Bakkar, S. M., Mohyeldin, R. H., Ali, F. E. M., Abdel-Maqsoud, N. M. R., & Fathy, M. (2023). Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury. Cells, 12(1), 185. https://doi.org/10.3390/cells12010185









