Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels
Abstract
1. Introduction
2. The Association between OA and CVD
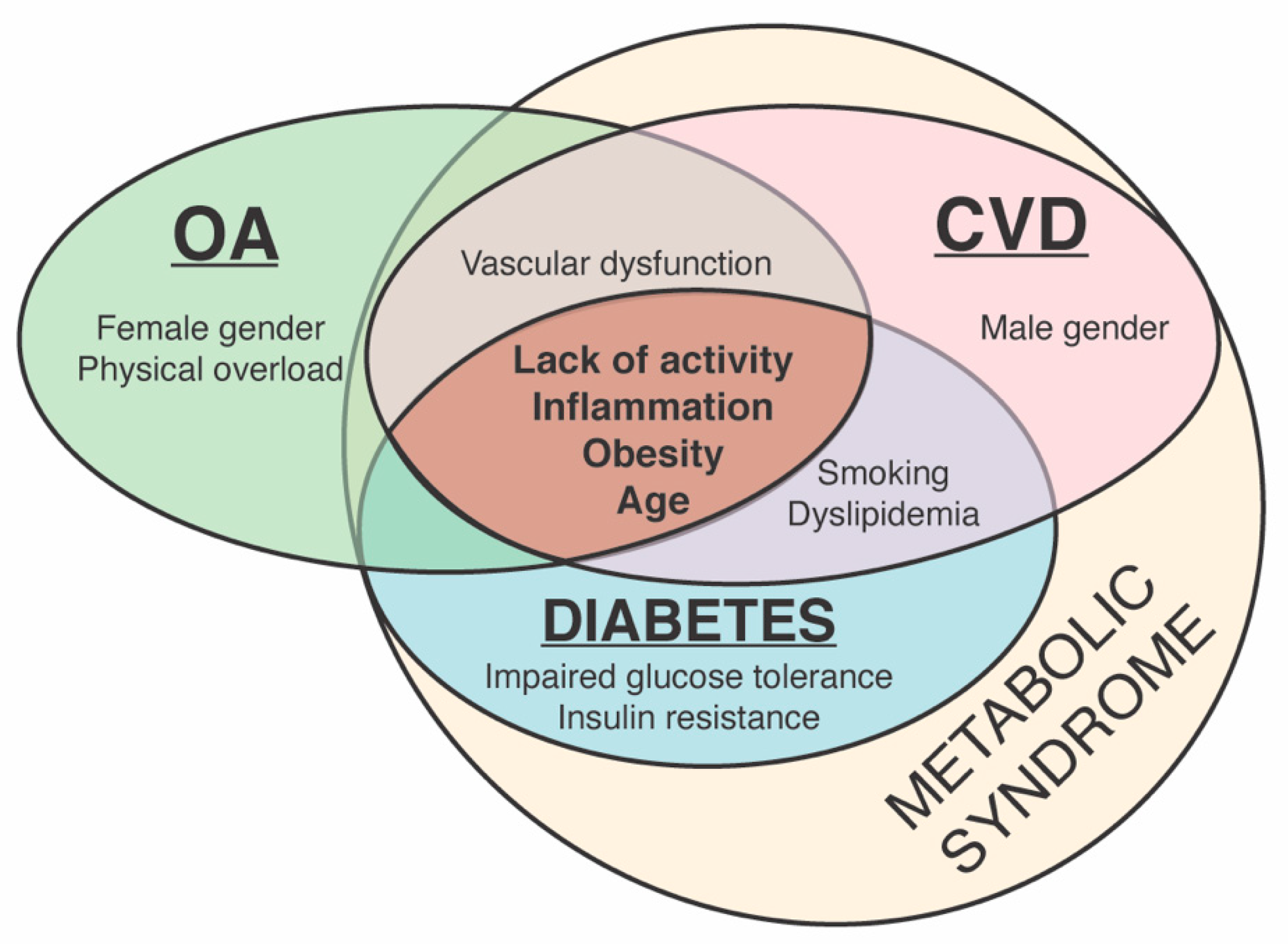
2.1. Risk Factors for OA and CVD
2.1.1. Common Risk Factors for OA and CVD
2.1.2. Distinct Risk Factors for CVD and OA
2.2. Oxidative Stress and Other Common Molecular Mechanisms in CVD and OA
3. Ion Channel Regulators for the Treatment of CVD
4. Cardiovascular Drugs Directly and Indirectly Regulating Ion Channels
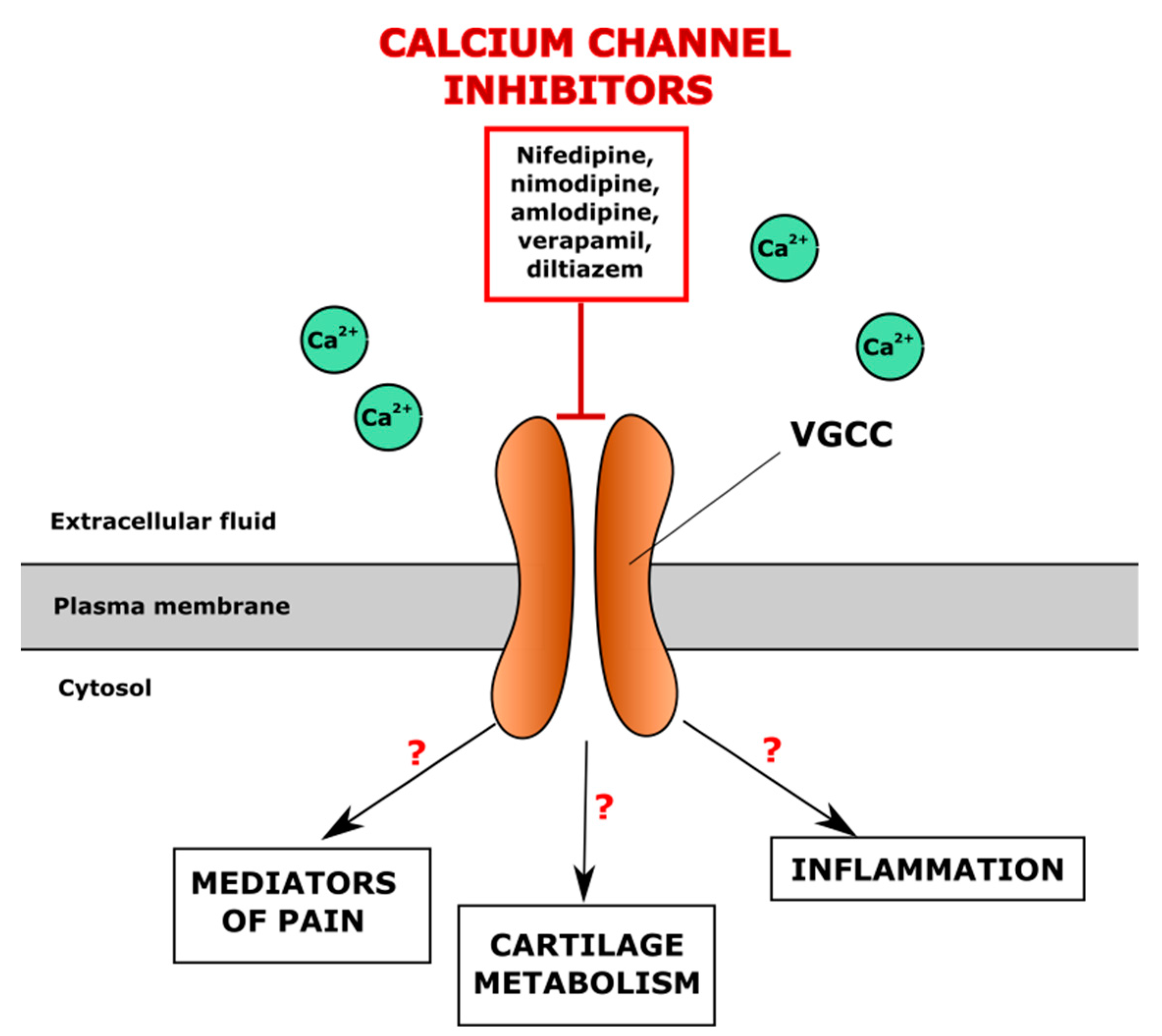
4.1. Inhibitors of Voltage-Gated Calcium (Ca2+) Channels in Chondrogenesis
4.2. Voltage-Gated Sodium (Na+) Channels in Chondrogenesis
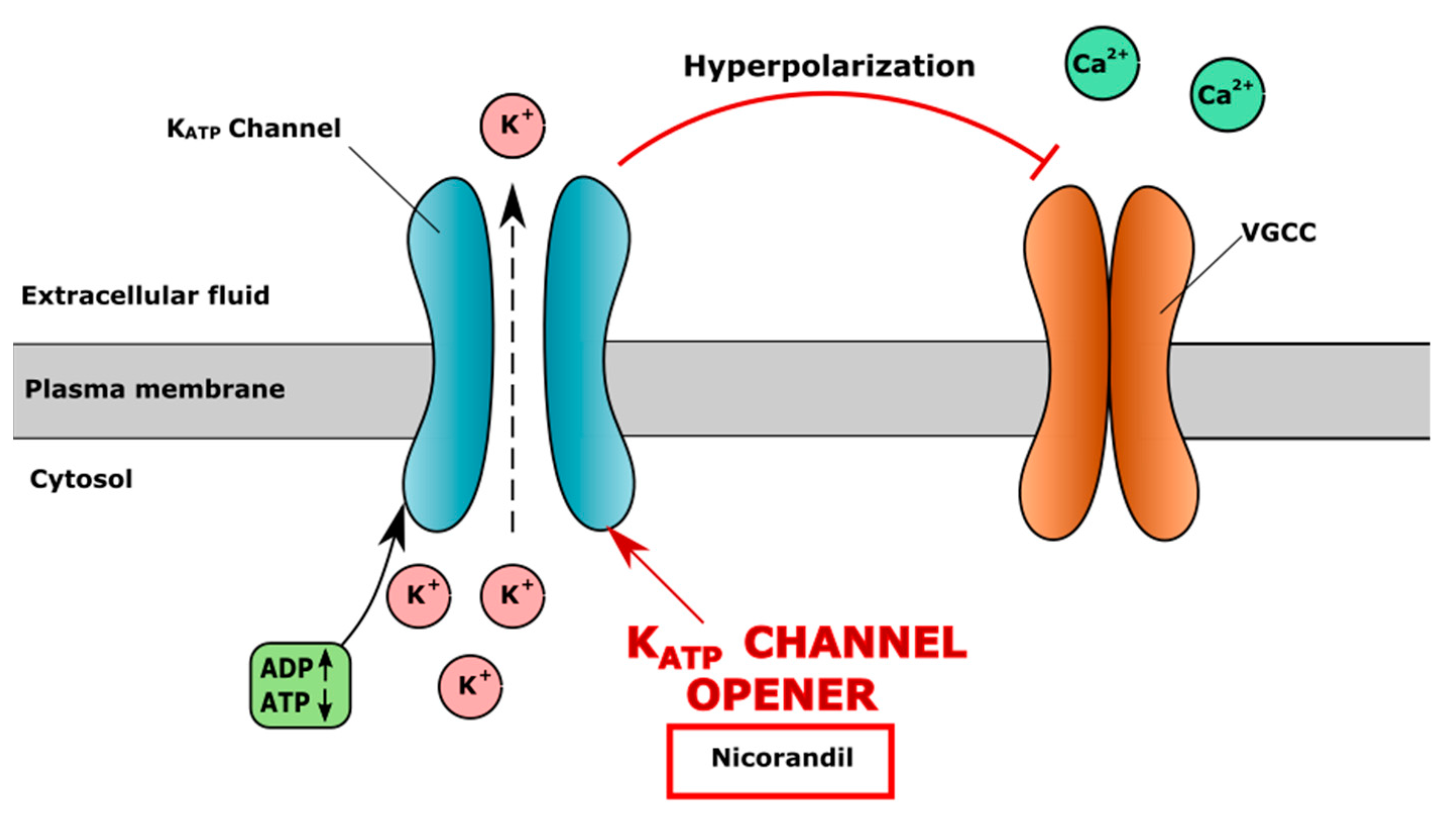
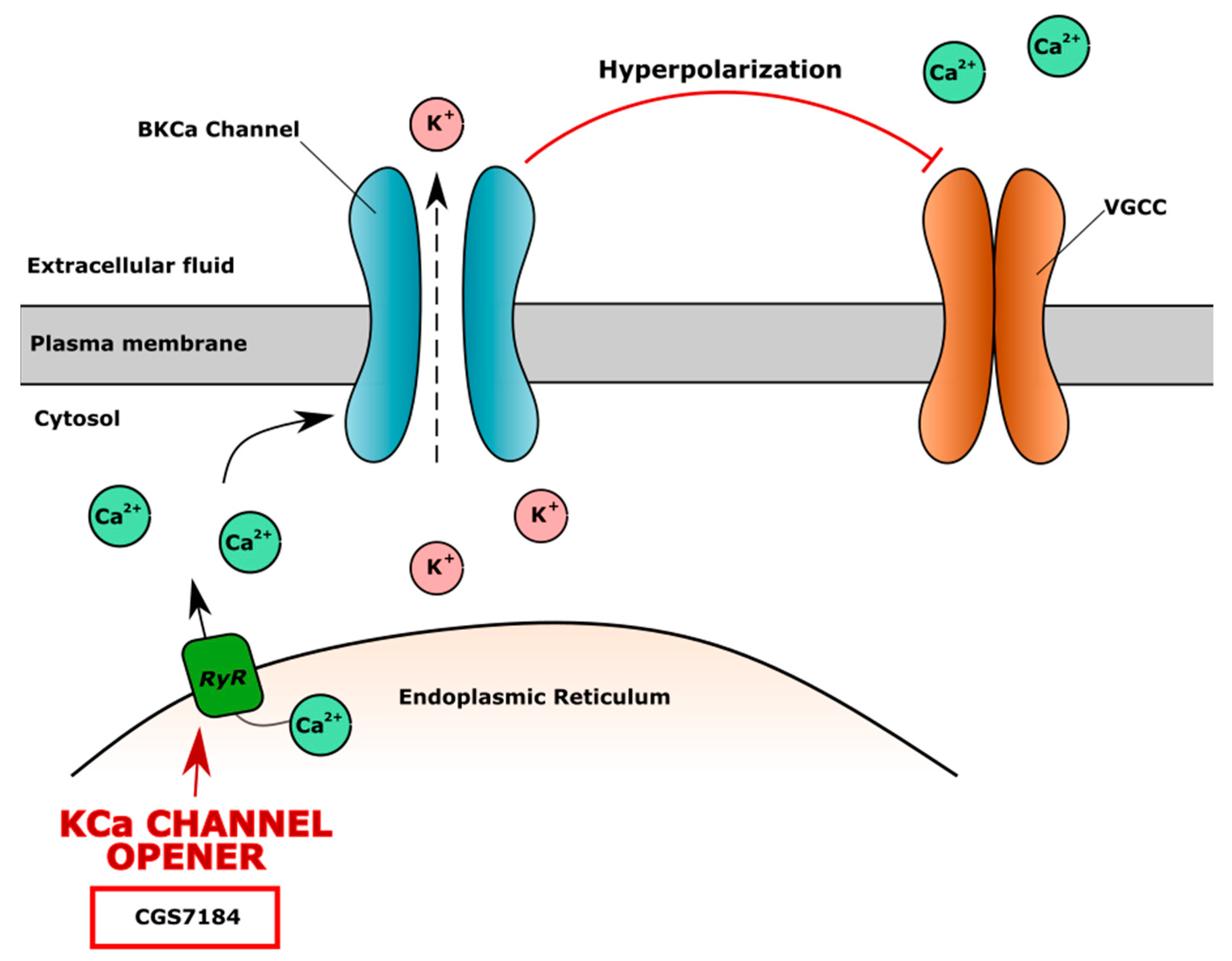
4.3. Potassium (K+) Channels in CVD and Chondrocytes Metabolism
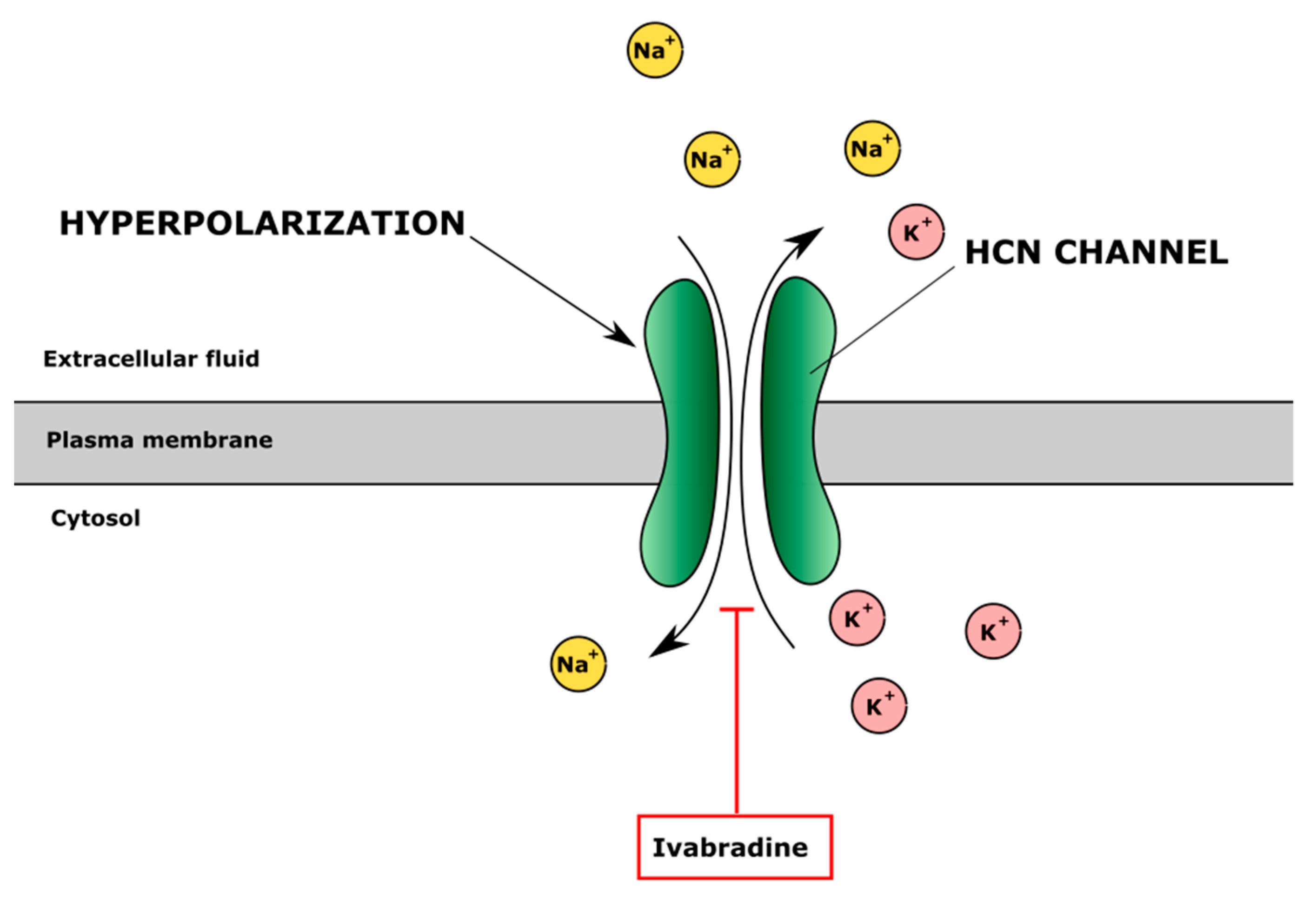
4.4. Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels
4.5. Complex Activity of Ion Channel Regulators
5. CVD Drugs Indirectly Regulating Ion Channels
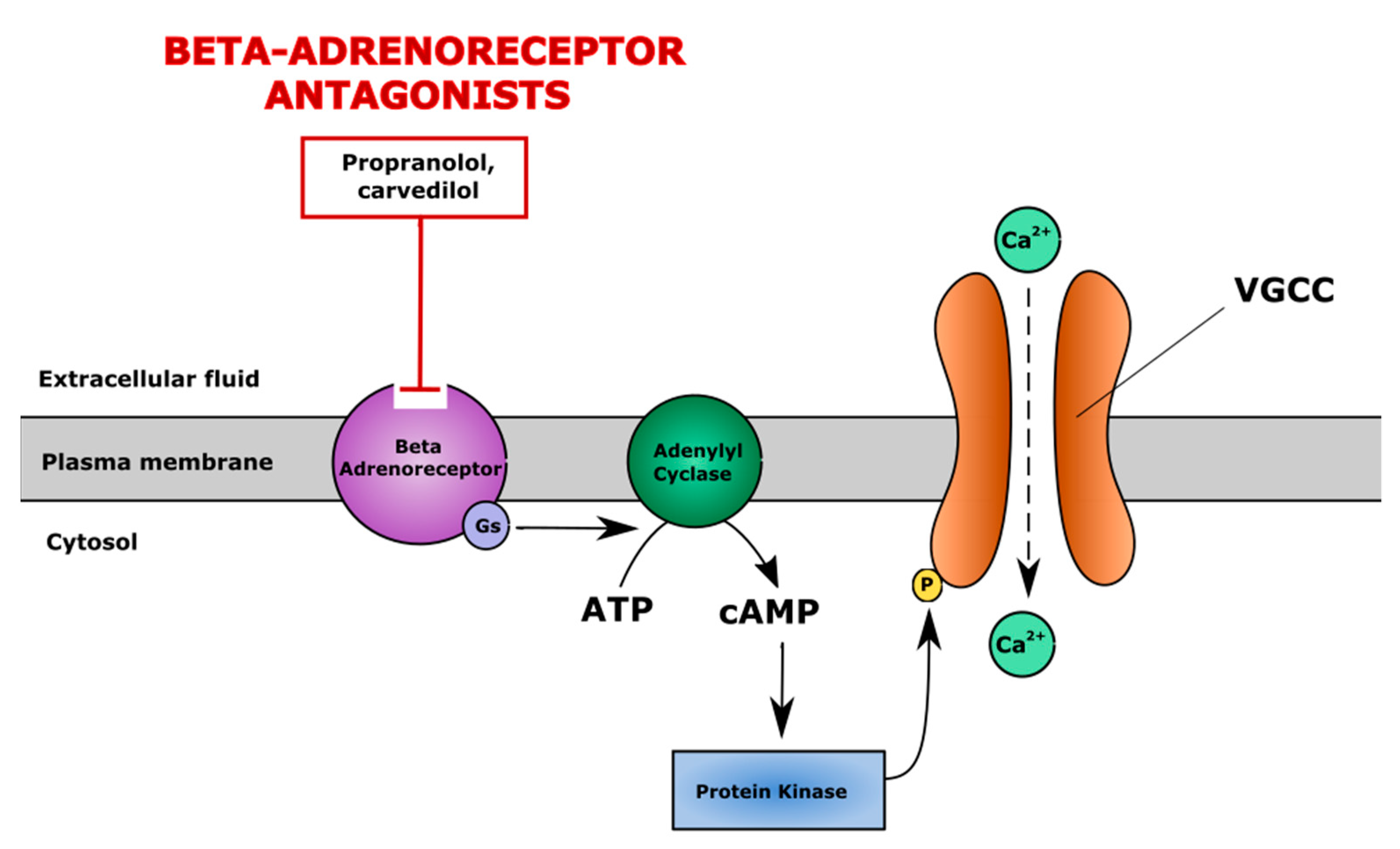
5.1. Non-Selective Inhibitors of β-Adrenergic System
5.2. Modulators of Renin-Angiotensin-Aldosterone System (RAAS)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Martin, J.; Mankin, H.J. Synovial joint degeneration and the syndrome of osteoarthritis. Instr. Course Lect 2000, 49, 481–489. [Google Scholar]
- Mahjoub, M.; Berenbaum, F.; Houard, X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos. Int. 2012, 23 (Suppl. 8), S841–S846. [Google Scholar] [CrossRef]
- Goldring, S.R. The role of bone in osteoarthritis pathogenesis. Rheum. Dis. Clin. N. Am. 2008, 34, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. Role of bone in osteoarthritis pathogenesis. Med. Clin. N. Am. 2009, 93, 25–35. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 2013, 15, 323–325. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Zeng, N.; Yan, Z.P.; Chen, X.Y.; Ni, G.X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef]
- Weber, J.; Koch, M.; Angele, P.; Zellner, J. The role of meniscal repair for prevention of early onset of osteoarthritis. J. Exp. Orthop. 2018, 5, 10. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Intraarticular Ligament Degeneration Is Interrelated with Cartilage and Bone Destruction in Osteoarthritis. Cells 2019, 8, 990. [Google Scholar] [CrossRef]
- Roemer, F.W.; Eckstein, F.; Hayashi, D.; Guermazi, A. The role of imaging in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 31–60. [Google Scholar] [CrossRef]
- Braun, H.J.; Gold, G.E. Diagnosis of osteoarthritis: Imaging. Bone 2012, 51, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Hunter, D.J.; Roemer, F.W. Plain radiography and magnetic resonance imaging diagnostics in osteoarthritis: Validated staging and scoring. J. Bone Jt. Surg. Am. 2009, 91 (Suppl. 1), 54–62. [Google Scholar] [CrossRef]
- Choi, J.A.; Gold, G.E. MR imaging of articular cartilage physiology. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 249–282. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.J.; Jin, X.; Ding, C. The importance of synovial inflammation in osteoarthritis: Current evidence from imaging assessments and clinical trials. Osteoarthr. Cartil. 2018, 26, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chuckpaiwong, B.; Charles, H.C.; Kraus, V.B.; Guilak, F.; Nunley, J.A. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J. Orthop. Res. 2010, 28, 1149–1154. [Google Scholar] [CrossRef]
- Mobasheri, A.; Saarakkala, S.; Finnilä, M.; Karsdal, M.A.; Bay-Jensen, A.C.; van Spil, W.E. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Creamer, P.; Lethbridge-Cejku, M.; Hochberg, M.C. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxf.) 2000, 39, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M.; Arden, N.; Bannwarth, B.; Bijlsma, J.; Gunther, K.-P.; Hauselmann, H.J.; Herrero-Beaumont, G.; Jordan, K.; Kaklamanis, P.; et al. EULAR evidence based recommendations for the management of hip osteoarthritis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2005, 64, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Hunter, D.J. What is the selection process for osteoarthritis pharmacotherapy? Expert Opin Pharm. 2020, 21, 1393–1397. [Google Scholar] [CrossRef]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging Technologies and Platforms for the Immunodetection of Multiple Biochemical Markers in Osteoarthritis Research and Therapy. Front. Med. (Lausanne) 2020, 7, 572977. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Yamaoka, K.; Sakata, K.; Sonomoto, K.; Lin, L.; Nakano, K.; Tanaka, Y. Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Arthritis Rheumatol. 2015, 67, 1250–1260. [Google Scholar] [CrossRef]
- Frazer, A.; Bunning, R.A.D.; Thavarajah, M.; Seid, J.M.; Russell, R.G.G. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-β and interleukin-1β. Osteoarthr. Cartil. 1994, 2, 235–245. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype-Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 1–13. [Google Scholar] [CrossRef]
- Steward, A.J.; Kelly, D.J.; Wagner, D.R. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. eCM 2014, 28, 358–371. [Google Scholar] [CrossRef]
- Lewis, R.; Asplin, K.E.; Bruce, G.; Dart, C.; Mobasheri, A.; Barrett-Jolley, R. The Role of the Membrane Potential in Chondrocyte Volume Regulation. J. Cell. Physiol. 2011, 226, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Ramalingam, S.; Zelenski, N.A.; Benefield, H.C.; Rigo, I.; Little, D.; Wu, C.-L.; Chen, D.; Liedtke, W.; McNulty, A.L.; et al. Cartilage-Specific Knockout of the Mechanosensory Ion Channel TRPV4 Decreases Age-Related Osteoarthritis. Sci. Rep. 2016, 6, 29053. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Chen, X.; Han, L.; Wang, L.; Lu, X.L. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2018, 36, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Mobasheri, R.; Francis, M.J.; Trujillo, E.; Alvarez de la Rosa, D.; Martín-Vasallo, P. Ion transport in chondrocytes: Membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol. Histopathol. 1998, 13, 893–910. [Google Scholar] [CrossRef]
- Jahr, H.; Matta, C.; Mobasheri, A. Physicochemical and Biomechanical Stimuli in Cell-Based Articular Cartilage Repair. Curr. Rheumatol. Rep. 2015, 17, 12. [Google Scholar] [CrossRef]
- Bertram, K.L.; Banderali, U.; Tailor, P.; Krawetz, R.J. Ion channel expression and function in normal and osteoarthritic human synovial fluid progenitor cells. Channels 2016, 10, 148–157. [Google Scholar] [CrossRef]
- Staessen, J.A.; Thijs, L.; Fagard, R.H.; Birkenhäger, W.H.; Arabidze, G.; Babeanu, S.; Gil-Extremera, B.; Bulpitt, C.J.; Davidson, C.; de Leeuw, P.W.; et al. Calcium channel blockade and cardiovascular prognosis in the European trial on isolated systolic hypertension. Hypertension 1998, 32, 410–416. [Google Scholar] [CrossRef]
- Hearon, C.M.; Richards, J.C.; Racine, M.L.; Luckasen, G.J.; Larson, D.G.; Dinenno, F.A. Amplification of endothelium-dependent vasodilatation in contracting human skeletal muscle: Role of KIR channels. J. Physiol. 2019, 597, 1321–1335. [Google Scholar] [CrossRef]
- Freedman, R.A.; Steinberg, J.S. Selective prolongation of QRS late potentials by sodium channel blocking antiarrhythmic drugs: Relation to slowing of ventricular tachycardia. Electrophysiologic Study Versus Electrocardiographic Monitoring Trial (ESVEM) Investigators. J. Am. Coll. Cardiol. 1991, 17, 1017–1025. [Google Scholar] [CrossRef]
- European Society of Cardiology. ESC/EASD Guidelines on Diabetes, Pre-Diabetes and Cardiovascular Diseases; ESC: Brussels, Belgium, 2019. [Google Scholar]
- Wang, H.; Bai, J.; He, B.; Hu, X.; Liu, D. Osteoarthritis and the risk of cardiovascular disease: A meta-analysis of observational studies. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Montecucco, F.; Tardif, J.-C.; Libby, P.; Camici, G.G. Inflamm-ageing: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef]
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C.; Gram, H.; Thuren, T.; Roubenoff, R.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement. Ann. Intern. Med. 2020, 173, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R. Revealed aspect of metabolic osteoarthritis. J. Orthop. 2016, 13, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Calders, P.; Van Ginckel, A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 47, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.A.; Jordan, K.P.; Kadam, U.T. Associations between cardiovascular disease severity, osteoarthritis co-morbidity and physical health: A population-based study. Rheumatology (Oxf.) 2014, 53, 1794–1802. [Google Scholar] [CrossRef]
- van den Oever, I.A.M.; Visman, I.M.; de Rooij, M.; Roorda, L.D.; Lems, W.F.; Nurmohamed, M.T.; Dekker, J.; van der Leeden, M.; van der Esch, M. Cardiovascular disease is associated with activity limitations in osteoarthritis patients. Int. J. Clin. Rheumatol. 2019, 14, 99. [Google Scholar]
- Rahman, M.M.; Kopec, J.A.; Cibere, J.; Goldsmith, C.H.; Anis, A.H. The relationship between osteoarthritis and cardiovascular disease in a population health survey: A cross-sectional study. BMJ Open 2013, 3, e002624. [Google Scholar] [CrossRef]
- Moghimi, N.; Rahmani, K.; Delpisheh, A.; Saidi, A.; Azadi, N.A.; Afkhamzadeh, A. Risk factors of knee osteoarthritis: A case-control study. Pak. J. Med. Sci 2019, 35, 636–640. [Google Scholar] [CrossRef]
- van Tunen, J.A.C.; Peat, G.; Bricca, A.; Larsen, L.B.; Søndergaard, J.; Thilsing, T.; Roos, E.M.; Thorlund, J.B. Association of osteoarthritis risk factors with knee and hip pain in a population-based sample of 29–59 year olds in Denmark: A cross-sectional analysis. BMC Musculoskelet. Disord. 2018, 19, s12891. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome—What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29. [Google Scholar] [CrossRef]
- Cunha-Miranda, L.; Faustino, A.; Alves, C.; Vicente, V.; Barbosa, S.; Cunha-Miranda, L.; Faustino, A.; Alves, C.; Vicente, V.; Barbosa, S. Assessing the magnitude of osteoarthritis disadvantage on people’s lives: The MOVES study. Rev. Bras. Reumatol. 2015, 55, 22–30. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Yuan, Y.; Zheng, M.; Pan, A.; Wang, M.; Zhao, M.; Li, Y.; Yao, S.; Chen, S.; Wu, S.; et al. Association of Age of Onset of Hypertension with Cardiovascular Diseases and Mortality. J. Am. Coll. Cardiol. 2020, 75, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Manninen, P.; Riihimäki, H.; Heliövaara, M.; Suomalainen, O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxf.) 2001, 40, 432–437. [Google Scholar] [CrossRef]
- Roos, E.M.; Dahlberg, L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: A four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005, 52, 3507–3514. [Google Scholar] [CrossRef]
- Fu, S.; Thompson, C.L.; Ali, A.; Wang, W.; Chapple, J.P.; Mitchison, H.M.; Beales, P.L.; Wann, A.K.T.; Knight, M.M. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthr. Cartil. 2019, 27, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Stotter, C.; Stojanović, B.; Bauer, C.; Rodríguez Ripoll, M.; Franek, F.; Klestil, T.; Nehrer, S. Effects of Loading Conditions on Articular Cartilage in a Metal-on-Cartilage Pairing. J. Orthop. Res. 2019, 37, 2531–2539. [Google Scholar] [CrossRef]
- Lachman, S.; Boekholdt, S.M.; Luben, R.N.; Sharp, S.J.; Brage, S.; Khaw, K.-T.; Peters, R.J.G.; Wareham, N.J. Impact of physical activity on the risk of cardiovascular disease in middle-aged and older adults: EPIC Norfolk prospective population study. Eur. J. Prev. Cardiol. 2018, 25, 200–208. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Xu, D. Aerobic exercise reduces triglycerides by targeting apolipoprotein C3 in patients with coronary heart disease. Clin. Cardiol. 2019, 42, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef]
- Franks, P.W. Obesity, inflammatory markers and cardiovascular disease: Distinguishing causality from confounding. J. Hum. Hypertens. 2006, 20, 837–840. [Google Scholar] [CrossRef]
- Pearson, M.J.; Herndler-Brandstetter, D.; Tariq, M.A.; Nicholson, T.A.; Philp, A.M.; Smith, H.L.; Davis, E.T.; Jones, S.W.; Lord, J.M. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 2017, 7, s41598. [Google Scholar] [CrossRef]
- Virdis, A.; Schiffrin, E.L. Vascular inflammation: A role in vascular disease in hypertension? Curr. Opin. Nephrol. Hypertens. 2003, 12, 181–187. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Vanharanta, H.; Dieppe, P.A. Is progressive osteoarthritis an atheromatous vascular disease? Ann. Rheum. Dis. 2005, 64, 1539–1541. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, s11906. [Google Scholar] [CrossRef] [PubMed]
- Wilson Peter, W.F.; D’Agostino Ralph, B.; Parise, H.; Sullivan, L.; Meigs James, B. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Wei, J.; Zeng, C.; Yang, T.; Li, H.; Wang, Y.L.; Long, H.Z.; Wu, Z.Y.; Qian, Y.X.; Li, K.H.; et al. Association between metabolic syndrome and knee osteoarthritis: A cross-sectional study. BMC Musculoskelet. Disord. 2017, 18, s12891. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Forrester, K.; Hart, D.A.; Leonard, C.; Salo, P.; Bray, R.C. Endothelial dysfunction and decreased vascular responsiveness in the anterior cruciate ligament-deficient model of osteoarthritis. J. Appl. Physiol. 2007, 102, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R.S.; Palfrey, A.J. A histological study of human femoral condylar articular cartilage. J. Anat. 1987, 155, 77–85. [Google Scholar] [PubMed]
- Kinlay, S.; Behrendt, D.; Wainstein, M.; Beltrame, J.; Fang James, C.; Creager Mark, A.; Selwyn Andrew, P.; Ganz, P. Role of Endothelin-1 in the Active Constriction of Human Atherosclerotic Coronary Arteries. Circulation 2001, 104, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Sin, A.; Tang, W.; Wen, C.Y.; Chung, S.K.; Chiu, K.Y. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthr. Cartil. 2015, 23, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Celletti, F.L.; Waugh, J.M.; Amabile, P.G.; Brendolan, A.; Hilfiker, P.R.; Dake, M.D. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Zupan, J.; Vrtačnik, P.; Cör, A.; Haring, G.; Weryha, G.; Visvikis-Siest, S.; Marc, J. VEGF-A is associated with early degenerative changes in cartilage and subchondral bone. Growth Factors 2018, 36, 263–273. [Google Scholar] [CrossRef]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef]
- Allport, S.A.; Kikah, N.; Abu Saif, N.; Ekokobe, F.; Atem, F.D. Parental Age of Onset of Cardiovascular Disease as a Predictor for Offspring Age of Onset of Cardiovascular Disease. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Dubé, C.E.; Liu, S.-H.; Driban, J.B.; McAlindon, T.E.; Eaton, C.B.; Lapane, K.L. The relationship between smoking and knee osteoarthritis in the Osteoarthritis Initiative. Osteoarthr. Cartil. 2016, 24, 465–472. [Google Scholar] [CrossRef]
- Gao, K.; Shi, X.; Wang, W. The life-course impact of smoking on hypertension, myocardial infarction and respiratory diseases. Sci. Rep. 2017, 7, 4330. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y. Smoking and osteoarthritis: A review of the evidence and its implications. Osteoarthr. Cartil. 2015, 23, 331–333. [Google Scholar] [CrossRef]
- Kwon, H.M.; Yang, I.H.; Park, K.K.; Cho, B.W.; Byun, J.; Lee, W.S. Cigarette smoking and knee osteoarthritis in the elderly: Data from the Korean National Health and Nutritional Examination Survey. Exp. Gerontol. 2020, 133, 110873. [Google Scholar] [CrossRef]
- Lee, Y.H. Causal association between smoking behavior and the decreased risk of osteoarthritis: A Mendelian randomization. Z. Rheumatol. 2019, 78, 461–466. [Google Scholar] [CrossRef]
- Gu, Q.; Li, D.; Wei, B.; Guo, Y.; Yan, J.; Mao, F.; Zhang, X.; Wang, L. Effects of nicotine on a rat model of early stage osteoarthritis. Int. J. Clin. Exp. Pathol. 2015, 8, 3602–3612. [Google Scholar]
- Johnsen, M.B.; Pihl, K.; Nissen, N.; Sørensen, R.R.; Jørgensen, U.; Englund, M.; Thorlund, J.B. The association between smoking and knee osteoarthritis in a cohort of Danish patients undergoing knee arthroscopy. BMC Musculoskelet. Disord. 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Hui, M.; Doherty, M.; Zhang, W. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann. Rheum. Dis. 2011, 70, 1231–1237. [Google Scholar] [CrossRef]
- Swärd, P.; Frobell, R.; Englund, M.; Roos, H.; Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)--a cross-sectional analysis. Osteoarthr. Cartil. 2012, 20, 1302–1308. [Google Scholar] [CrossRef]
- Paccou, J.; D’Angelo, S.; Rhodes, A.; Curtis, E.M.; Raisi-Estabragh, Z.; Edwards, M.; Walker-Bone, K.; Cooper, C.; Petersen, S.E.; Harvey, N.C. Prior fragility fracture and risk of incident ischaemic cardiovascular events: Results from UK Biobank. Osteoporos. Int. 2018, 29, 1321–1328. [Google Scholar] [CrossRef]
- Sung, M.M.; Dyck, J.R. Age-related cardiovascular disease and the beneficial effects of calorie restriction. Heart Fail. Rev. 2012, 17, 707–719. [Google Scholar] [CrossRef]
- Cardillo, C.; Panza, J.A. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc. Med. 1998, 3, 138–144. [Google Scholar] [CrossRef]
- Ashmeik, W.; Joseph, G.B.; Nevitt, M.C.; Lane, N.E.; McCulloch, C.E.; Link, T.M. Association of blood pressure with knee cartilage composition and structural knee abnormalities: Data from the osteoarthritis initiative. Skelet. Radiol. 2020, 49, 1359–1368. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Zeng, L.; He, D.; Wei, X. Nutrition and degeneration of articular cartilage. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1751–1762. [Google Scholar] [CrossRef]
- Westerhof, B.E.; van Lieshout, J.J.; Parati, G.; van Montfrans, G.A.; Guelen, I.; Spaan, J.A.E.; Westerhof, N.; Karemaker, J.M.; Bos, W.J.W. Cardiac oxygen supply is compromised during the night in hypertensive patients. Med. Biol. Eng. Comput. 2011, 49, 1073–1081. [Google Scholar] [CrossRef][Green Version]
- Elahi, M.M.; Kong, Y.X.; Matata, B.M. Oxidative stress as a mediator of cardiovascular disease. Oxidative Med. Cell. Longev. 2009, 2, 259–269. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef]
- Pawluk, H.; Pawluk, R.; Robaczewska, J.; Kędziora-Kornatowska, K.; Kędziora, J. Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep. 2017, 22, 542–546. [Google Scholar] [CrossRef][Green Version]
- Olszewska-Slonina, D.M.; Jung, S.; Olszewski, K.J.; Cwynar, A.; Drewa, G. Evaluation of Selected Parameters of Lipid Peroxidation and Paraoxonase Activity in Blood of Patients with Joint Osteoarthritis. Protein Pept. Lett. 2018, 25, 853–861. [Google Scholar] [CrossRef]
- El-barbary, A.M.; Khalek, M.A.A.; Elsalawy, A.M.; Hazaa, S.M. Assessment of lipid peroxidation and antioxidant status in rheumatoid arthritis and osteoarthritis patients. Egypt. Rheumatol. 2011, 33, 179–185. [Google Scholar] [CrossRef][Green Version]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of Disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Hu, K.-Z.; Yin, Z.-S. Inhibition of the p38-MAPK signaling pathway suppresses the apoptosis and expression of proinflammatory cytokines in human osteoarthritis chondrocytes. Cytokine 2017, 90, 135–143. [Google Scholar] [CrossRef]
- He, T.; Wu, D.; He, L.; Wang, X.; Yang, B.; Li, S.; Chen, Y.; Wang, K.; Chen, R.; Liu, B.; et al. Casein kinase 1 epsilon facilitates cartilage destruction in osteoarthritis through JNK pathway. FASEB J. 2020, 34, 6466–6478. [Google Scholar] [CrossRef]
- Wu, J.; Zou, M.; Ping, A.; Deng, Z.; Cai, L. MicroRNA-449a upregulation promotes chondrocyte extracellular matrix degradation in osteoarthritis. Biomed. Pharmacother. 2018, 105, 940–946. [Google Scholar] [CrossRef]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Neseem, N.O.; Metwally, S.S.; Dein M Farag, S.E. IL-17 in primary knee osteoarthritis and its relation with severity of the disease. Int. J. Clin. Rheumatol. 2018, 13. [Google Scholar] [CrossRef]
- de Boer, T.N.; van Spil, W.E.; Huisman, A.M.; Polak, A.A.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Mastbergen, S.C. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012, 20, 846–853. [Google Scholar] [CrossRef]
- Ding, W.; Cheng, H.; Chen, F.; Yan, Y.; Zhang, M.; Zhao, X.; Hou, D.; Mi, J. Adipokines are Associated with Hypertension in Metabolically Healthy Obese (MHO) Children and Adolescents: A Prospective Population-Based Cohort Study. J. Epidemiol. 2018, 28, 19–26. [Google Scholar] [CrossRef]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- Hawker, G.A.; Croxford, R.; Bierman, A.S.; Harvey, P.J.; Ravi, B.; Stanaitis, I.; Lipscombe, L.L. All-Cause Mortality and Serious Cardiovascular Events in People with Hip and Knee Osteoarthritis: A Population Based Cohort Study. PLoS ONE 2014, 9, e91286. [Google Scholar] [CrossRef]
- Haugen, I.K.; Ramachandran, V.S.; Misra, D.; Neogi, T.; Niu, J.; Yang, T.; Zhang, Y.; Felson, D.T. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: Data from the Framingham heart study. Ann. Rheum. Dis. 2015, 74, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Israel, C.W. Mechanisms of sudden cardiac death. Indian Heart J. 2014, 66, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-F.; Liu, C.-J.; Tuan, T.-C.; Chen, S.-J.; Chen, T.-J.; Lip, G.Y.H.; Chen, S.-A. Risk and Prediction of Sudden Cardiac Death and Ventricular Arrhythmias for Patients with Atrial Fibrillation—A Nationwide Cohort Study. Sci. Rep. 2017, 7, 46445. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M. Safe and Effective Pharmacologic Management of Arrhythmias. Tex. Heart Inst. J. 2005, 32, 209–211. [Google Scholar] [PubMed]
- Yildiz, A.; Yigit, Z.; Okcun, B.; Baskurt, M.; Ortak, K.; Kaya, A.; Kucukoglu, S. Comparison of rate and rhythm control in hypertension patients with atrial fibrillation. Circ. J. 2008, 72, 705–708. [Google Scholar] [CrossRef]
- Turi, Z.G.; Braunwald, E. The use of beta-blockers after myocardial infarction. JAMA 1983, 249, 2512–2516. [Google Scholar] [CrossRef]
- Urban, M.K.; Markowitz, S.M.; Gordon, M.A.; Urquhart, B.L.; Kligfield, P. Postoperative prophylactic administration of beta-adrenergic blockers in patients at risk for myocardial ischemia. Anesth. Analg. 2000, 90, 1257–1261. [Google Scholar] [CrossRef]
- Sato, T.; Li, Y.; Saito, T.; Nakaya, H. Minoxidil opens mitochondrial KATP channels and confers cardioprotection. Br. J. Pharm. 2004, 141, 360–366. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, X.; Fang, X.; Zheng, J.; Chen, T.; Huang, J. Effects of mitochondrial ATP-sensitive potassium channel activation (nicorandil) in patients with angina pectoris undergoing elective percutaneous coronary interventions. Medicine (Baltim.) 2019, 98. [Google Scholar] [CrossRef]
- Melgari, D.; Brack, K.E.; Zhang, C.; Zhang, Y.; El Harchi, A.; Mitcheson, J.S.; Dempsey, C.E.; Ng, G.A.; Hancox, J.C. hERG Potassium Channel Blockade by the HCN Channel Inhibitor Bradycardic Agent Ivabradine. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Nie, J.; Duan, Q.; He, M.; Li, X.; Wang, B.; Zhou, C.; Wu, L.; Wen, Z.; Chen, C.; Wang, D.W.; et al. Ranolazine Prevents Pressure Overload-Induced Cardiac Hypertrophy and Heart Failure by Restoring Aberrant Na+ and Ca2+ Handling. J. Cell. Physiol. 2019, 234, 11587–11601. [Google Scholar] [CrossRef]
- Mancilla, E.E.; Galindo, M.; Fertilio, B.; Herrera, M.; Salas, K.; Gatica, H.; Goecke, A. L-type calcium channels in growth plate chondrocytes participate in endochondral ossification. J. Cell. Biochem. 2007, 101, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, K.; Georges, P.; Tibesku, C.O.; Prehm, P. Positive side effects of Ca antagonists for osteoarthritic joints—Results of an in vivo pilot study. J. Orthop. Surg. Res. 2015, 10, s13018. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, A.; Ohkawara, B.; Ito, M.; Masuda, A.; Sakai, T.; Ishiguro, N.; Ohno, K. Verapamil Protects against Cartilage Degradation in Osteoarthritis by Inhibiting Wnt/β-Catenin Signaling. PLoS ONE 2014, 9, e92699. [Google Scholar] [CrossRef] [PubMed]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; Porvaneckas, N.; Kvederas, G.; Mobasheri, A. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front. Endocrinol. (Lausanne) 2019, 10. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kobayashi, S.; Takeno, K.; Yayama, T.; Meir, A.; Baba, H. Lidocaine cytotoxicity to the bovine articular chondrocytes in vitro: Changes in cell viability and proteoglycan metabolism. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1198–1205. [Google Scholar] [CrossRef]
- Park, J.; Sutradhar, B.C.; Hong, G.; Choi, S.H.; Kim, G. Comparison of the cytotoxic effects of bupivacaine, lidocaine, and mepivacaine in equine articular chondrocytes. Vet. Anaesth. Analg. 2011, 38, 127–133. [Google Scholar] [CrossRef]
- Reid, M.C.; Shengelia, R.; Parker, S.J. Pharmacologic Management of Osteoarthritis-Related Pain in Older Adults. HSS J. 2012, 8, 159–164. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, Y.; Sun, H.; Tan, S.; Lu, Z.; Huang, L.; Wang, W. Ivabradine abrogates TNF-α-induced degradation of articular cartilage matrix. Int. Immunopharmacol. 2019, 66, 347–353. [Google Scholar] [CrossRef]
- Jiao, K.; Niu, L.-N.; Li, Q.-h.; Ren, G.-t.; Zhao, C.-m.; Liu, Y.-d.; Tay, F.R.; Wang, M.-q. β2-adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 2015, 5, 12593. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Hojo, H.; Iemata, M.; Sahara, K.; Kodama, A.; Nakamura, N.; Hinoi, E.; Yoneda, Y. Interference by adrenaline with chondrogenic differentiation through suppression of gene transactivation mediated by Sox9 family members. Bone 2009, 45, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, M.; Takasuna, K.; Tamura, K.; Yamaguchi, K.; Maekawa, K.; Yamada, S.; Iwasaki, S.; Yoshida, M.; Nomura, M.; Taguchi, K. General pharmacological profiles of the new beta-adrenoceptor antagonist carvedilol. Arzneimittelforschung 1990, 40, 735–746. [Google Scholar]
- Li, Z.; Liu, B.; Wang, B.; Liu, Y. Carvedilol suppresses cartilage matrix destruction. Biochem. Biophys. Res. Commun. 2016, 480, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.K.; Karvonen, K.E.; Kerola, T.V.; Ryhänen, L.J. Inhibitory effect of the angiotensin converting enzyme inhibitors captopril and enalapril on the conversion of procollagen to collagen. J. Hypertens. 2001, 19, 1835–1839. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, X.; Lu, X. Captopril, an angiotensin-converting enzyme inhibitor, possesses chondroprotective efficacy in a rat model of osteoarthritis through suppression local renin-angiotensin system. Int. J. Clin. Exp. Med. 2015, 8, 12584–12592. [Google Scholar] [PubMed]
- de Sá, G.A.; dos Santos, A.C.P.M.; Nogueira, J.M.; dos Santos, D.M.; Amaral, F.A.; Jorge, E.C.; Caliari, M.V.; Queiroz-Junior, C.M.; Ferreira, A.J. Angiotensin II triggers knee joint lesions in experimental osteoarthritis. Bone 2021, 145, 115842. [Google Scholar] [CrossRef]
- Chan, P.; Au, M.; Yang, W.; Yan, C.; Chiu, K.; Wen, C. Role of systemic hypertension in cell senescence and subchondral bone disturbance of knee joint. Osteoarthr. Cartil. 2018, 26, S118. [Google Scholar] [CrossRef]
- Thomas, M.; Fronk, Z.; Gross, A.; Willmore, D.; Arango, A.; Higham, C.; Nguyen, V.; Lim, H.; Kale, V.; McMillan, G.; et al. Losartan attenuates progression of osteoarthritis in the synovial temporomandibular and knee joints of a chondrodysplasia mouse model through inhibition of TGF-β1 signaling pathway. Osteoarthr. Cartil. 2019, 27, 676–686. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Scibetta, A.; Ravuri, S.; Lowe, W.R.; Philippon, M.J.; Alliston, T.; Huard, J. Improvement of Cartilage Repair with Biologically Regulated Marrow Stimulation by Blocking TGF-β1 in A Rabbit Osteochondral Defect Model. Orthop. J. Sports Med. 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Nakama, G.; Scibetta, A.C.; Ravuri, S.K.; Goldman, J.L.; Lowe, W.R.; Rodkey, W.G.; et al. Biologically Regulated Marrow Stimulation by Blocking TGF-β1 With Losartan Oral Administration Results in Hyaline-like Cartilage Repair: A Rabbit Osteochondral Defect Model. Am. J. Sports Med. 2020, 48, 974–984. [Google Scholar] [CrossRef]
- Chen, S.; Grover, M.; Sibai, T.; Black, J.; Rianon, N.; Rajagopal, A.; Munivez, E.; Bertin, T.; Dawson, B.; Chen, Y.; et al. Losartan increases bone mass and accelerates chondrocyte hypertrophy in developing skeleton. Mol. Genet. Metab. 2015, 115, 53–60. [Google Scholar] [CrossRef]
- Brice, N.L.; Dolphin, A.C. Differential plasma membrane targeting of voltage-dependent calcium channel subunits expressed in a polarized epithelial cell line. J. Physiol. 1999, 515, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-Gated Calcium Channels. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, K.V.; Hennessey, J.A.; Barnett, A.S.; Yin, X.; Stadt, H.A.; Foster, E.; Shah, R.A.; Yazawa, M.; Dolmetsch, R.E.; Kirby, M.L.; et al. Calcium influx through L-type CaV1.2 Ca2+ channels regulates mandibular development. J. Clin. Investig. 2013, 123, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef]
- Khaksari, M.; Mahani, S.E.; Mahmoodi, M. Calcium channel blockers reduce inflammatory edema in the rat: Involvement of the hypothalamus-pituitary-adrenal axis. Indian J. Pharmacol. 2004, 36, 351–354. [Google Scholar]
- Lipskaia, L.; Keuylian, Z.; Blirando, K.; Mougenot, N.; Jacquet, A.; Rouxel, C.; Sghairi, H.; Elaib, Z.; Blaise, R.; Adnot, S.; et al. Expression of Sarco (Endo) plasmic Reticulum Calcium ATPase (SERCA) system in normal mouse cardiovascular tissues, heart failure and atherosclerosis. Biochim. Biophys. Acta 2014, 1843, 2705–2718. [Google Scholar] [CrossRef]
- Clark, C.C.; Iannotti, J.P.; Misra, S.; Richards, C.F. Effects of thapsigargin, an intracellular calcium-mobilizing agent, on synthesis and secretion of cartilage collagen and proteoglycan. J. Orthop. Res. 1994, 12, 601–611. [Google Scholar] [CrossRef]
- Hamamura, K.; Goldring, M.B.; Yokota, H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes in response to surviving stress to endoplasmic reticulum. Arch. Oral Biol. 2009, 54, 279–286. [Google Scholar] [CrossRef]
- Asmar, A.; Barrett-Jolley, R.; Werner, A.; Kelly, R.; Stacey, M. Membrane channel gene expression in human costal and articular chondrocytes. Organogenesis 2016, 12, 94–107. [Google Scholar] [CrossRef]
- Sugimoto, T.; Yoshino, M.; Nagao, M.; Ishii, S.; Yabu, H. Voltage-gated ionic channels in cultured rabbit articular chondrocytes. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 115, 223–232. [Google Scholar] [CrossRef]
- Gammaitoni, A.R.; Galer, B.S.; Onawola, R.; Jensen, M.P.; Argoff, C.E. Lidocaine patch 5% and its positive impact on pain qualities in osteoarthritis: Results of a pilot 2-week, open-label study using the Neuropathic Pain Scale. Curr. Med. Res. Opin. 2004, 20 (Suppl. 2), S13–S19. [Google Scholar] [CrossRef]
- McCann, G. Pharmacological treatment of significant cardiac arrhythmias. Br. J. Sports Med. 2000, 34, 401–402. [Google Scholar] [CrossRef][Green Version]
- Asad, Z.; Sachidanandan, C. Chemical screens in a zebrafish model of CHARGE syndrome identifies small molecules that ameliorate disease-like phenotypes in embryo. Eur. J. Med. Genet. 2020, 63, 103661. [Google Scholar] [CrossRef]
- Park, J.F.; Luo, Z.D. Calcium channel functions in pain processing. Channels 2010, 4, 510–517. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lewis, R.; Ferreira-Mendes, A.; Rufino, A.; Dart, C.; Barrett-Jolley, R. Potassium channels in articular chondrocytes. Channels 2012, 6, 416–425. [Google Scholar] [CrossRef]
- Aliot, E.; Capucci, A.; Crijns, H.J.; Goette, A.; Tamargo, J. Twenty-five years in the making: Flecainide is safe and effective for the management of atrial fibrillation. Europace 2011, 13, 161–173. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Kaski, J.C. Nicorandil and Long-acting Nitrates: Vasodilator Therapies for the Management of Chronic Stable Angina Pectoris. Eur. Cardiol. 2018, 13, 23–28. [Google Scholar] [CrossRef]
- Mauerer, U.R.; Boulpaep, E.L.; Segal, A.S. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J. Gen. Physiol. 1998, 111, 161–180. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Wright, M.O.; Flatman, P.W.; Salter, D.M. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 2004, 41, 567–575. [Google Scholar]
- Clark, R.B.; Hatano, N.; Kondo, C.; Belke, D.D.; Brown, B.S.; Kumar, S.; Votta, B.J.; Giles, W.R. Voltage-gated K currents in mouse articular chondrocytes regulate membrane potential. Channels 2010, 4, 179–191. [Google Scholar] [CrossRef]
- Karbat, I.; Altman-Gueta, H.; Fine, S.; Szanto, T.; Hamer-Rogotner, S.; Dym, O.; Frolow, F.; Gordon, D.; Panyi, G.; Gurevitz, M.; et al. Pore-modulating toxins exploit inherent slow inactivation to block K+ channels. Proc. Natl. Acad. Sci. USA 2019, 116, 18700–18709. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated Potassium Channels as Therapeutic Drug Targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Ponce, A. Expression of Voltage Dependent Potassium Currents in Freshly Dissociated Rat Articular Chondrocytes. Cell. Physiol. Biochem. 2006, 18, 35–46. [Google Scholar] [CrossRef]
- Trohman, R.G.; Sharma, P.S.; McAninch, E.A.; Bianco, A.C. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc. Med. 2019, 29, 285–295. [Google Scholar] [CrossRef]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef]
- Xu, X.; Urban, J.P.G.; Tirlapur, U.K.; Cui, Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthr. Cartil. 2010, 18, 433–439. [Google Scholar] [CrossRef]
- Cordeiro, B.; Terentyev, D.; Clements, R.T. BKCa channel activation increases cardiac contractile recovery following hypothermic ischemia/reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H625–H633. [Google Scholar] [CrossRef]
- Goswami, S.K.; Ponnalagu, D.; Hussain, A.T.; Shah, K.; Karekar, P.; Gururaja Rao, S.; Meredith, A.L.; Khan, M.; Singh, H. Expression and Activation of BKCa Channels in Mice Protects Against Ischemia-Reperfusion Injury of Isolated Hearts by Modulating Mitochondrial Function. Front. Cardiovasc. Med. 2019, 5. [Google Scholar] [CrossRef]
- Wrzosek, A.; Tomaskova, Z.; Ondrias, K.; Łukasiak, A.; Szewczyk, A. The potassium channel opener CGS7184 activates Ca2+ release from the endoplasmic reticulum. Eur. J. Pharmacol. 2012, 690, 60–67. [Google Scholar] [CrossRef]
- Kase, D.; Imoto, K. The Role of HCN Channels on Membrane Excitability in the Nervous System. J. Signal. Transduct. 2012, 2012, 619747. [Google Scholar] [CrossRef] [PubMed]
- Mączewski, M.; Mackiewicz, U. Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc. Res. 2008, 79, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Abdelsayed, M.; Ruben, P.C. Effects of Amiodarone and N-desethylamiodarone on Cardiac Voltage-Gated Sodium Channels. Front. Pharm. 2016, 7. [Google Scholar] [CrossRef]
- Lubic, S.P.; Nguyen, K.P.; Dave, B.; Giacomini, J.C. Antiarrhythmic agent amiodarone possesses calcium channel blocker properties. J. Cardiovasc. Pharmacol. 1994, 24, 707–714. [Google Scholar] [CrossRef]
- Turker, I.; Yu, C.-C.; Chang, P.-C.; Chen, Z.; Sohma, Y.; Lin, S.-F.; Chen, P.-S.; Ai, T. Amiodarone Inhibits Apamin-Sensitive Potassium Currents. PLoS ONE 2013, 8, e70450. [Google Scholar] [CrossRef] [PubMed]
- Kodirov, S.A.; Zhuravlev, V.L.; Brachmann, J. Prevailing Effects of Ibutilide on Fast Delayed Rectifier K+ Channel. J. Membr. Biol. 2019, 252, 609–616. [Google Scholar] [CrossRef] [PubMed]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; O’Leary, M.E. State-dependent trapping of flecainide in the cardiac sodium channel. J. Physiol. 2004, 560, 37–49. [Google Scholar] [CrossRef]
- Mehra, D.; Imtiaz, M.S.; van Helden, D.F.; Knollmann, B.C.; Laver, D.R. Multiple Modes of Ryanodine Receptor 2 Inhibition by Flecainide. Mol. Pharm. 2014, 86, 696–706. [Google Scholar] [CrossRef]
- Wang, S.; Morales, M.J.; Qu, Y.-J.; Bett, G.C.L.; Strauss, H.C.; Rasmusson, R.L. Kv1.4 channel block by quinidine: Evidence for a drug-induced allosteric effect. J. Physiol. 2003, 546, 387–401. [Google Scholar] [CrossRef]
- Shibata, K.; Hirasawa, A.; Foglar, R.; Ogawa, S.; Tsujimoto, G. Effects of Quinidine and Verapamil on Human Cardiovascular α1-Adrenoceptors. Circulation 1998, 97, 1227–1230. [Google Scholar] [CrossRef]
- Haechl, N.; Ebner, J.; Hilber, K.; Todt, H.; Koenig, X. Pharmacological Profile of the Bradycardic Agent Ivabradine on Human Cardiac Ion Channels|Cell Physiol Biochem. Cell. Physiol. Biochem. 2019, 53, 36–48. [Google Scholar]
- Johnson, M. Beta2-adrenoceptors: Mechanisms of action of beta2-agonists. Paediatr. Respir. Rev. 2001, 2, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.P.; Mitchell, J. Beta2-adrenergic receptors expressed on murine chondrocytes stimulate cellular growth and inhibit the expression of Indian hedgehog and collagen type X. J. Cell. Biochem. 2008, 104, 545–553. [Google Scholar] [CrossRef]
- Mitchell, J.; Lai, L.P.; Peralta, F.; Xu, Y.; Sugamori, K. β2-Adrenergic receptors inhibit the expression of collagen type II in growth plate chondrocytes by stimulating the AP-1 factor Jun-B. Am. J. Physiol.-Endocrinol. Metab. 2010, 300, E633–E639. [Google Scholar] [CrossRef]
- Hernandez, D.; Millard, R.; Sivakumaran, P.; Kong, A.; Mitchell, G.; PÈbay, A.; Shepherd, R.; Dusting, G.; Lim, S. Influence of continuous electrical stimulation on development of human cardiomyocytes from induced pluripotent stem cells. Cond. Med. 2018, 1, 306–312. [Google Scholar]
- Rybin, V.O.; Xu, X.; Lisanti, M.P.; Steinberg, S.F. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000, 275, 41447–41457. [Google Scholar] [CrossRef]
- Galaz-Montoya, M.; Wright, S.J.; Rodriguez, G.J.; Lichtarge, O.; Wensel, T.G. β(2)-Adrenergic receptor activation mobilizes intracellular calcium via a non-canonical cAMP-independent signaling pathway. J. Biol. Chem. 2017, 292, 9967–9974. [Google Scholar] [CrossRef]
- Shen, J.X. Isoprenaline enhances local Ca2+ release in cardiac myocytes1. Acta Pharmacol. Sin. 2006, 27, 927–932. [Google Scholar] [CrossRef]
- Takemura, H.; Hatta, S.; Yamada, K.; Ohshika, H. β-Adrenergic receptor-mediated calcium mobilization in the human Jurkat T cell line. Life Sci. 1995, 56, 1443–1454. [Google Scholar] [CrossRef]
- Koitabashi, N.; Arai, M.; Tomaru, K.; Takizawa, T.; Watanabe, A.; Niwano, K.; Yokoyama, T.; Wuytack, F.; Periasamy, M.; Nagai, R.; et al. Carvedilol effectively blocks oxidative stress-mediated downregulation of sarcoplasmic reticulum Ca2+-ATPase 2 gene transcription through modification of Sp1 binding. Biochem. Biophys. Res. Commun. 2005, 328, 116–124. [Google Scholar] [CrossRef]
- Lorenz, J.; Schäfer, N.; Bauer, R.; Jenei-Lanzl, Z.; Springorum, R.H.; Grässel, S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr. Cartil. 2016, 24, 325–334. [Google Scholar] [CrossRef]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014, 2014, 689360. [Google Scholar] [CrossRef]
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef]
- Henrotin, Y.; Pesesse, L.; Lambert, C. Targeting the synovial angiogenesis as a novel treatment approach to osteoarthritis. Adv. Musculoskelet. Dis. 2014, 6, 20–34. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Zhou, L.; Liu, Y. An essential role for Wnt/β-catenin signaling in mediating hypertensive heart disease. Sci. Rep. 2018, 8, 8996. [Google Scholar] [CrossRef]
- Corada, M.; Nyqvist, D.; Orsenigo, F.; Caprini, A.; Giampietro, C.; Taketo, M.M.; Iruela-Arispe, M.L.; Adams, R.H.; Dejana, E. The Wnt/β-Catenin Pathway Modulates Vascular Remodeling and Specification by Upregulating Dll4/Notch Signaling. Dev. Cell 2010, 18, 938–949. [Google Scholar] [CrossRef]
- Wang, H.; Charles, P.C.; Wu, Y.; Ren, R.; Pi, X.; Moser, M.; Barshishat-Kupper, M.; Rubin, J.S.; Perou, C.; Bautch, V.; et al. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ. Res. 2006, 98, 1331–1339. [Google Scholar] [CrossRef]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharm. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Williams, R.N.; Parsons, S.L.; Morris, T.M.; Rowlands, B.J.; Watson, S.A. Inhibition of matrix metalloproteinase activity and growth of gastric adenocarcinoma cells by an angiotensin converting enzyme inhibitor in in vitro and murine models. Eur. J. Surg. Oncol. 2005, 31, 1042–1050. [Google Scholar] [CrossRef]
- Garcia, P.; Schwenzer, S.; Slotta, J.E.; Scheuer, C.; Tami, A.E.; Holstein, J.H.; Histing, T.; Burkhardt, M.; Pohlemann, T.; Menger, M.D. Inhibition of angiotensin-converting enzyme stimulates fracture healing and periosteal callus formation—Role of a local renin-angiotensin system. Br. J. Pharm. 2010, 159, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.B.; Alvarenga, É.C.; Siqueira, P.D.; Paredes-Gamero, E.J.; Sabatini, R.A.; Morais, R.L.T.; Reis, R.I.; Santos, E.L.; Teixeira, L.G.D.; Casarini, D.E.; et al. Angiotensin II Binding to Angiotensin I–Converting Enzyme Triggers Calcium Signaling. Hypertension 2011, 57, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chapman, D.; Dhalla, N.S. Partial prevention of changes in SK gene expression in congestive heart failure due to myocardial infarction by enalapril or losartan. Mol. Cell. Biochem. 2003, 254, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
| Drug | Role in CVD | Effect on Cartilage In Vitro | Effect on Cartilage in Animal Models In Vivo | Effect on Patients | References |
|---|---|---|---|---|---|
| Direct ion channel modulators | |||||
| Voltage-Gated Calcium Channel Blockers | |||||
| Verapamil | Inhibits VGCC in vascular smooth muscle and myocardial tissue, reduces peripheral vascular resistance and heart contractility. Indications: hypertension, chest angina, arrhythmia | Inhibition of chondrocyte proliferation, decreased number of hypertrophic chondrocytes, upregulation of chondrogenic markers (ACAN, COL2A1, SOX9), downregulation of AXIN2 and MMP3 in human osteoarthritic chondrocytes | - | Worsened OA patients’ condition based on Lequesne scoring system | [123,124,125] |
| Nifedipine | Inhibits VGCC in vascular smooth muscle cells, causing smooth muscle relaxation and decrease of blood pressure. Indications: hypertension, chest angina | Inhibition of chondrocyte maturation and proliferation in bone marrow-mesenchymal stem cells and chondrocytes; upregulated production of GAGs and collagen type II in human chondrocytes | - | No effects in OA patients according to Lequesne scoring system | [124,126] |
| Amlodipine, lercanidipine, felodipine, nitrendipine | - | - | Improved Lequesne index in OA patients | [124] | |
| Voltage-Gated Sodium Channel Blockers | |||||
| Lidocaine | Inhibits VGSC, decreases the depolarization, automaticity and excitability in the ventricles Indication: arrhythmia | Chondrotoxic effect; necrosis in equine and bovine articular chondrocytes | - | Reduced pain in OA patients | [127,128,129] |
| Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Inhibitors | |||||
| Ivabradine | Lowers heart rate by selectively inhibiting If channels (“funny channels”) in the heart and prolonging diastolic depolarization Indication: heart failure | Reduced expression of matrix metalloproteinases (MMP-3 and MMP-13), ADAMTS-4 and ADAMTS-5 in primary human chondrocytes | - | - | [130] |
| Indirect ion channel modulators | |||||
| β-adrenoreceptor Inhibitors | |||||
| Propranolol | Nonselective β-adrenergic receptor antagonist Indications: hypertension, angina, arrhythmia | Promoted chondrogenic differentiation to hypertrophic chondrocytes by increasing Col I and Col X gene expression, decreasing SOX6 expression in murine pre-chondrogenic ATDC5 cells; suppressed subchondral bone loss in rat model. | - | - | [131,132] |
| Carvedilol | Reversed IL-1β induced downregulation of aggrecan and Col II protein in murine pre-chondrogenic ATDC5 cells in SW1353 chondrocytes | - | - | [133,134] | |
| Angiotensin-Aldosterone System Modulators | |||||
| Captopril | Inhibitor of angiotensin-converting enzyme Indications: hypertension, heart failure | Inhibited reversion of procollagen to collagen in cartilage and tendon cell culture | Increased thickness of articular cartilage, decreased hypertrophic zone and increased proliferative zone in rat OA model | - | [135,136,137,138] |
| Enalapril | Inhibited reversion of procollagen to collagen in cartilage and tendon cell culture | - | - | [135] | |
| Losartan | Angiotensin II receptor inhibitor Indications: hypertension, heart failure | - | Increased OA progression according to histopathological scoring in murine OA model; increased Col10a1 expression in mice; diminished degradation of cartilage in mice; enhanced hyaline-like rabbit cartilage healing | - | [139,140,141,142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiciuleviciute, R.; Bironaite, D.; Uzieliene, I.; Mobasheri, A.; Bernotiene, E. Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels. Cells 2021, 10, 2572. https://doi.org/10.3390/cells10102572
Vaiciuleviciute R, Bironaite D, Uzieliene I, Mobasheri A, Bernotiene E. Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels. Cells. 2021; 10(10):2572. https://doi.org/10.3390/cells10102572
Chicago/Turabian StyleVaiciuleviciute, Raminta, Daiva Bironaite, Ilona Uzieliene, Ali Mobasheri, and Eiva Bernotiene. 2021. "Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels" Cells 10, no. 10: 2572. https://doi.org/10.3390/cells10102572
APA StyleVaiciuleviciute, R., Bironaite, D., Uzieliene, I., Mobasheri, A., & Bernotiene, E. (2021). Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels. Cells, 10(10), 2572. https://doi.org/10.3390/cells10102572








