Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque
Abstract
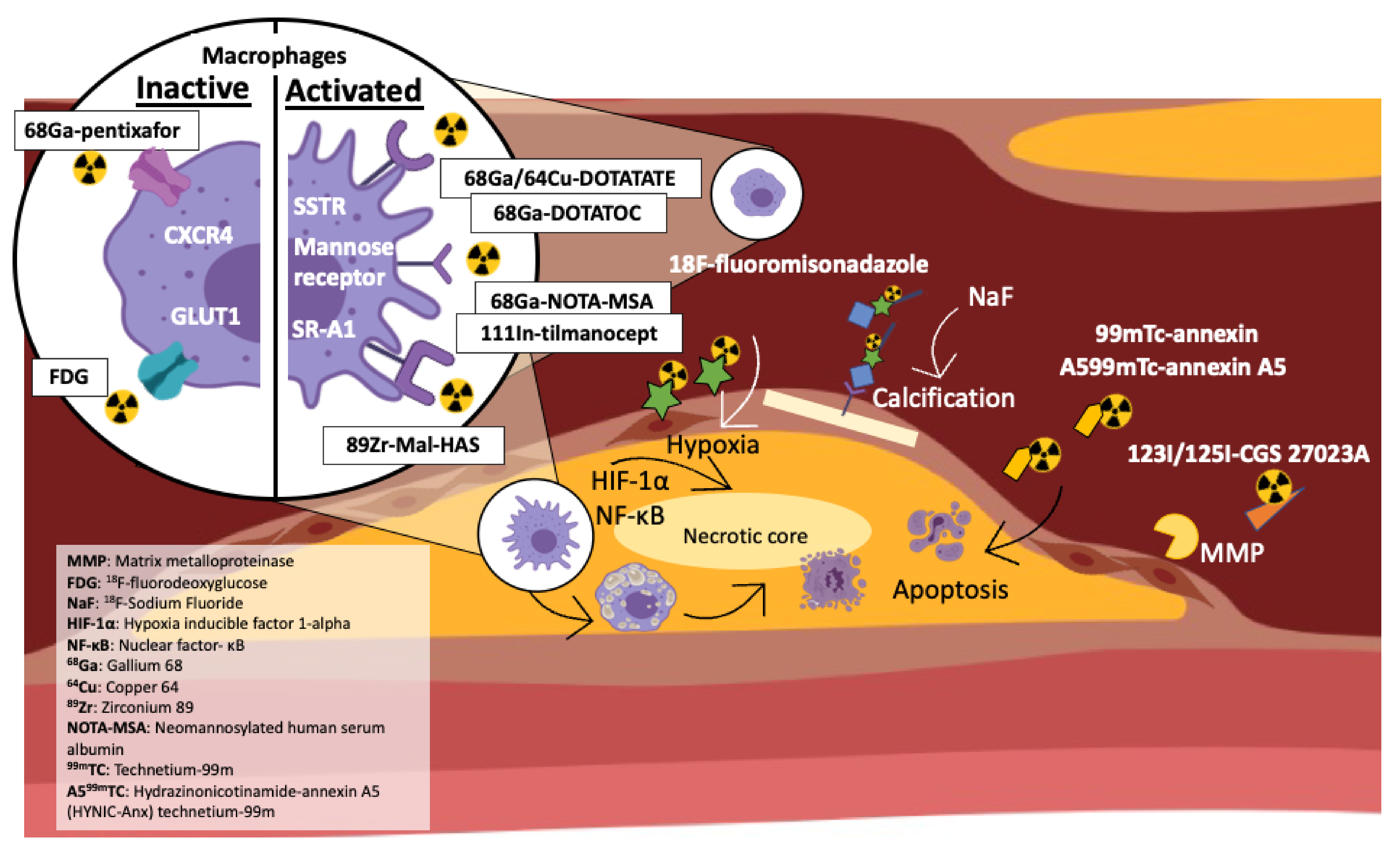
:1. Introduction
2. Lipid Accumulation & Inflammation in Plaque Development
3. 18F-Fluorodeoxyglucose (FDG) -PET Detects Plaque Development and Inflammatory Cell Infiltrate
4. 18F-Sodium Fluoride (18F-NaF) PET Predicts Plaque Calcification
5. Somatostatin 2 Receptor (SSTR) Imaging
5.1. Gallium DOTATATE (68Ga-DOTATATE) and Plaque Identification
5.2. Dota Derived Somatostatin Analogue 68Ga-DOTATOC
5.3. 64Cu-DOTATATE
6. Chemokine Imaging
68Ga-Pentixafor
7. Experimental/Novel PET Imaging Radiotracers including Their Studies in Animals
7.1. Hypoxia
7.2. MMP and Degradation
7.3. Activated Macrophages via Mannose Receptor
7.4. Chemokine Receptor Targeting in Atherosclerosis
7.5. Macrophage Scavenger Receptor (SR-A1)
7.6. Challenges in Animal PET Imaging
8. ApoE-/- Mouse PET Imaging
9. General Considerations in PET Imaging
10. Future Direction and Imaging Strategies
11. Clinical Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A. Preclinical PET/CT 18F-FDG Imaging Protocol
References
- Ramjattan, N.A.; Lala, V.; Kousa, O.; Makaryus, A.N. Coronary CT Angiography; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Matter, C.M.; Stuber, M.; Nahrendorf, M. Imaging of the unstable plaque: How far have we got? Eur. Heart J. 2009, 30, 2566–2574. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Ćorović, A.; Wall, C.; Gopalan, D.; Rudd, J.H. Positron emission tomography imaging in cardiovascular disease. Heart 2020, 106, 1712–1718. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Fayad, Z.A.; Mulder, W.J. Atherosclerosis Immunoimaging by Positron Emission Tomography. Arter. Thromb. Vasc. Biol. 2020, 40, 865–873. [Google Scholar] [CrossRef]
- Lee, S.; Bartlett, B.; Dwivedi, G. Adaptive Immune Responses in Human Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 9322. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.; Ludewick, H.P.; Misra, A.; Lee, S.; Dwivedi, G. Macrophages and T cells in atherosclerosis: A translational perspective. Am. J. Physiol. Circ. Physiol. 2019, 317, H375–H386. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. 18Fluorodeoxyglucose Positron Emission Tomography Imaging of Atherosclerotic Plaque Inflammation Is Highly Reproducible: Implications for Atherosclerosis Therapy Trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Rogers, I.S.; Nasir, K.; Figueroa, A.L.; Cury, R.C.; Hoffmann, U.; Vermylen, D.A.; Brady, T.J.; Tawakol, A. Feasibility of FDG Imaging of the Coronary Arteries: Comparison Between Acute Coronary Syndrome and Stable Angina. JACC Cardiovasc. Imaging 2010, 3, 388–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, P.; Tawakol, A. Imaging atherosclerosis with positron emission tomography. Eur. Heart J. 2016, 37, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Borja, A.J.; Hancin, E.C.; Auslander, T.; Revheim, M.-E.; Moghbel, M.C.; Werner, T.J.; Alavi, A.; Rajapakse, C.S. Imaging Atherosclerosis by PET, With Emphasis on the Role of FDG and NaF as Potential Biomarkers for This Disorder. Front. Physiol. 2020, 11, 511391. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Short, J.D.; Downs, K.; Nguyen, H.N.; Lai, Y.; Zhang, W.; Jerabek, P.; Goins, B.; Sadeghi, M.M.; Asmis, R. Differential Regulation of Macrophage Glucose Metabolism by Macrophage Colony-stimulating Factor and Granulocyte-Macrophage Colony-stimulating Factor: Implications for 18F FDG PET Imaging of Vessel Wall Inflammation. Radiology 2017, 283, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Dilsizian, V.; Jadvar, H. Science to Practice: Does FDG Differentiate Morphologically Unstable from Stable Atherosclerotic Plaque? Radiology 2017, 283, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.; Millon, A.; Fayad, Z.A. Molecular imaging in atherosclerosis: FDG PET. Curr. Atheroscler. Rep. 2012, 14, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, F.M.; Verweij, S.L.; Zwinderman, K.A.; Strang, A.C.; Kaiser, Y.; Marquering, H.A.; Nederveen, A.J.; Stroes, E.S.; Verberne, H.J.; Rudd, J.H. Thresholds for Arterial Wall Inflammation Quantified by 18F-FDG PET Imaging: Implications for Vascular Interventional Studies. JACC Cardiovasc. Imaging 2016, 9, 1198–1207. [Google Scholar] [CrossRef]
- Gewirtz, H.; Dilsizian, V. Defining Inflammatory Levels of Carotid Artery and Aortic 18FDG Uptake: Implications for Clinical Trial Design and Individual Patient Management*. JACC Cardiovasc. Imaging 2016, 9, 1208–1210. [Google Scholar] [CrossRef]
- Minamimoto, R. Series of myocardial FDG uptake requiring considerations of myocardial abnormalities in FDG-PET/CT. Jpn. J. Radiol. 2021, 39, 540–557. [Google Scholar] [CrossRef]
- Kaneta, T.; Hakamatsuka, T.; Takanami, K.; Yamada, T.; Takase, K.; Sato, A.; Higano, S.; Kinomura, S.; Fukuda, H.; Takahashi, S.; et al. Evaluation of the relationship between physiological FDG uptake in the heart and age, blood glucose level, fasting period, and hospitalization. Ann. Nucl. Med. 2006, 20, 203–208. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; Calvert, P.A.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, S.J.; Tuzcu, E.M.; Wolski, K.; Sipahi, I.; Schoenhagen, P.; Crowe, T.; Kapadia, S.R.; Hazen, S.L.; Nissen, S.E. Coronary Artery Calcification and Changes in Atheroma Burden in Response to Established Medical Therapies. J. Am. Coll. Cardiol. 2007, 49, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, Y.; Wolski, K.; Uno, K.; Puri, R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Spotty Calcification as a Marker of Accelerated Progression of Coronary Atherosclerosis: Insights from Serial Intravascular Ultrasound. J. Am. Coll. Cardiol. 2012, 59, 1592–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høilund-Carlsen, P.F.; Sturek, M.; Alavi, A.; Gerke, O. Atherosclerosis imaging with 18F-sodium fluoride PET: State-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1538–1551. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis Associates with Inflammation in Early-Stage Atherosclerosis Evaluated by Molecular Imaging In Vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef] [Green Version]
- Vengrenyuk, Y.; Carlier, S.; Xanthos, S.; Cardoso, L.; Ganatos, P.; Virmani, R.; Einav, S.; Gilchrist, L.; Weinbaum, S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl. Acad. Sci. USA 2006, 103, 14678–14683. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, L.; Weinbaum, S. Microcalcifications, Their Genesis, Growth, and Biomechanical Stability in Fibrous Cap Rupture. Chem. Biol. Pteridines Folates 2018, 1097, 129–155. [Google Scholar] [CrossRef]
- Puri, R.; Nicholls, S.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of Statins on Serial Coronary Calcification During Atheroma Progression and Regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef]
- Akers, E.J.; Nicholls, S.J.; Di Bartolo, B.A. Plaque Calcification: Do lipoproteins have a role? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1902–1910. [Google Scholar] [CrossRef]
- Arani, L.; Gharavi, M.; Saboury, B.; Al-Zaghal, A.; Jahangiri, P.; Khosravi, M.; Pournazari, K.; Werner, T.; Hoilund-Carlsen, P.F.; Alavi, A. Assessment of the role of age and cardiovascular risk factors on 18F-Fluorodeoxyglucose (18F-FDG) and 18F-Sodium Fluoride (NaF) uptake in abdominal aortic artery. J. Nucl. Med. 2018, 59, 1539. [Google Scholar]
- Fiz, F.; Morbelli, S.; Bauckneht, M.; Piccardo, A.; Ferrarazzo, G.; Nieri, A.; Artom, N.; Cabria, M.; Marini, C.; Canepa, M.; et al. Correlation between thoracic aorta 18F-natrium fluoride uptake and cardiovascular risk. World J. Radiol. 2016, 8, 82–89. [Google Scholar] [CrossRef]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, e008564. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira-Santos, M.; Castelo-Branco, M.; Silva, R.; Gomes, A.; Chichorro, N.; Abrunhosa, A.; Donato, P.; de Lima, J.P.; Pego, M.; Gonçalves, L.; et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects—A subclinical atherosclerosis imaging study with 18F-NaF PET-CT. Atherosclerosis 2017, 260, 41–46. [Google Scholar] [CrossRef]
- Lee, J.M.; Bang, J.I.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Yaliang, T.; Suh, M.; Paeng, J.C.; Shiono, Y.; et al. Clinical Relevance of 18F-Sodium Fluoride Positron-Emission Tomography in Noninvasive Identification of High-Risk Plaque in Patients with Coronary Artery Disease. Circ. Cardiovasc. Imaging 2017, 10, e006704. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, T.; Yamamoto, H.; Toshimitsu, S.; Sasaki, K.; Senoo, A.; Kubo, Y.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. 18F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis 2017, 263, 385–392. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yamamoto, H.; Nakamoto, Y.; Sasaki, K.; Toshimitsu, S.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. Predictive Value of 18F-Sodium Fluoride Positron Emission Tomography in Detecting High-Risk Coronary Artery Disease in Combination with Computed Tomography. J. Am. Heart Assoc. 2018, 7, e010224. [Google Scholar] [CrossRef] [Green Version]
- Kwiecinski, J.; Tzolos, E.; Adamson, P.D.; Cadet, S.; Moss, A.J.; Joshi, N.; Williams, M.C.; van Beek, E.J.; Dey, D.; Berman, D.S.; et al. Coronary 18F-Sodium Fluoride Uptake Predicts Outcomes in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 3061–3074. [Google Scholar] [CrossRef]
- Dalm, V.A.S.H.; van Hagen, P.M.; van Koetsveld, P.M.; Achilefu, S.; Houtsmuller, A.B.; Pols, D.; van der Lely, A.-J.; Lamberts, S.W.J.; Hofland, L.J. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am. J. Physiol. Metab. 2003, 285, E344–E353. [Google Scholar] [CrossRef]
- Armani, C.; Catalani, E.; Balbarini, A.; Bagnoli, P.; Cervia, D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J. Leukoc. Biol. 2006, 81, 845–855. [Google Scholar] [CrossRef]
- Evans, N.R.; Tarkin, J.M.; Chowdhury, M.M.; Warburton, E.A.; Rudd, J.H.F. PET Imaging of Atherosclerotic Disease: Advancing Plaque Assessment from Anatomy to Pathophysiology. Curr. Atheroscler. Rep. 2016, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.-G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of Somatostatin Receptor 2-Targeting PET Tracers in the Detection of Mouse Atherosclerotic Plaques. Mol. Imaging Biol. 2015, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bauer, W.; Kreissl, M.C.; Weirather, J.; Bauer, E.; Israel, I.; Richter, D.; Riehl, G.; Buck, A.; Samnick, S. Specific somatostatin receptor II expression in arterial plaque: 68Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis 2013, 230, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Wan, M.Y.S.; Endozo, R.; Michopoulou, S.; Shortman, R.; Rodriguez-Justo, M.; Menezes, L.; Yusuf, S.; Richards, T.; Wild, D.; Waser, B.; et al. PET/CT Imaging of Unstable Carotid Plaque with68Ga-Labeled Somatostatin Receptor Ligand. J. Nucl. Med. 2016, 58, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Bucerius, J.; Dijkgraaf, I.; Mottaghy, F.M.; Schurgers, L.J. Target identification for the diagnosis and intervention of vulnerable atherosclerotic plaques beyond 18F-fluorodeoxyglucose positron emission tomography imaging: Promising tracers on the horizon. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC Versus 68Ga-DOTATATE PET/CT in Functional Imaging of Neuroendocrine Tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.; Loft, A.; Hag, A.M.; Kjaer, A. 64Cu-DOTATATE for Noninvasive Assessment of Atherosclerosis in Large Arteries and Its Correlation with Risk Factors: Head-to-Head Comparison with 68Ga-DOTATOC in 60 Patients. J. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Kim, J.; Paeng, J.C.; Byun, J.W.; Cheon, G.J.; Lee, D.S.; Chung, J.-K.; Kang, K.W. Measurement of 68Ga-DOTATOC Uptake in the Thoracic Aorta and Its Correlation with Cardiovascular Risk. Nucl. Med. Mol. Imaging 2018, 52, 279–286. [Google Scholar] [CrossRef]
- Li, X.; Samnick, S.; Lapa, C.; Israel, I.; Buck, A.K.; Kreissl, M.C.; Bauer, W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with 18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, S.F.; Sandholt, B.V.; Keller, S.H.; Hansen, A.E.; Clemmensen, A.E.; Sillesen, H.; Højgaard, L.; Ripa, R.S.; Kjær, A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rominger, A.; Saam, T.; Vogl, E.; Übleis, C.; la Fougère, C.; Förster, S.; Haug, A.; Cumming, P.; Reiser, M.F.; Nikolaou, K.; et al. In Vivo Imaging of Macrophage Activity in the Coronary Arteries Using 68Ga-DOTATATE PET/CT: Correlation with Coronary Calcium Burden and Risk Factors. J. Nucl. Med. 2010, 51, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Boyle, J.J.; Harrington, H.A.; Piper, E.; Elderfield, K.; Stark, J.; Landis, R.C.; Haskard, D.O. Coronary Intraplaque Hemorrhage Evokes a Novel Atheroprotective Macrophage Phenotype. Am. J. Pathol. 2009, 174, 1097–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kircher, M.; Tran-Gia, J.; Kemmer, L.; Zhang, X.; Schirbel, A.; Werner, R.A.; Buck, A.K.; Wester, H.-J.; Hacker, M.; Lapa, C.; et al. Imaging Inflammation in Atherosclerosis with CXCR4-Directed 68Ga-Pentixafor PET/CT: Correlation with 18F-FDG PET/CT. J. Nucl. Med. 2019, 61, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.M.; Megens, R.T.; Zernecke, A.; Bidzhekov, K.; Akker, N.M.V.D.; Rademakers, T.; van Zandvoort, M.A.; Hackeng, T.M.; Koenen, R.R.; Weber, C. Endothelial Junctional Adhesion Molecule-A Guides Monocytes into Flow-Dependent Predilection Sites of Atherosclerosis Clinical Perspective. Circulation 2014, 129, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Döring, Y.; Pawig, L.; Weber, C.; Noels, H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front. Physiol. 2014, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Merckelbach, S.; van der Vorst, E.P.C.; Kallmayer, M.; Rischpler, C.; Burgkart, R.; Döring, Y.; de Borst, G.-J.; Schwaiger, M.; Eckstein, H.-H.; Weber, C.; et al. Expression and Cellular Localization of CXCR4 and CXCL12 in Human Carotid Atherosclerotic Plaques. Thromb. Haemost. 2018, 118, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; van der Vorst, E.P.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the Cytokine Receptor CXCR4 in Atherosclerotic Plaques with the Radiotracer 68Ga-Pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernecke, A.; Weber, C. Chemokines in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 742–750. [Google Scholar] [CrossRef]
- Li, X.; Heber, D.; Leike, T.; Beitzke, D.; Lu, X.; Zhang, X.; Wei, Y.; Mitterhauser, M.; Wadsak, W.; Kropf, S.; et al. [68Ga]Pentixafor-PET/MRI for the detection of Chemokine receptor 4 expression in atherosclerotic plaques. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, D.; Thackeray, J.T.; Daum, G.; Sohns, J.S.; Kropf, S.; Wester, H.-J.; Ross, T.L.; Bengel, F.; Derlin, T. Clinical Molecular Imaging of Chemokine Receptor CXCR4 Expression in Atherosclerotic Plaque Using 68Ga-Pentixafor PET: Correlation with Cardiovascular Risk Factors and Calcified Plaque Burden. J. Nucl. Med. 2017, 59, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Döring, Y.; Noels, H.; van der Vorst, E.P.; Neideck, C.; Egea, V.; Drechsler, M.; Mandl, M.; Pawig, L.; Jansen, Y.; Schröder, K.; et al. Vascular CXCR4 Limits Atherosclerosis by Maintaining Arterial Integrity: Evidence from Mouse and Human Studies. Circulation 2017, 136, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Meester, E.J.; Krenning, B.J.; De Swart, J.; Segbers, M.; Barrett, H.E.; Bernsen, M.R.; van der Heiden, K.; De Jong, M. Perspectives on Small Animal Radionuclide Imaging; Considerations and Advances in Atherosclerosis. Front. Med. 2019, 6. [Google Scholar] [CrossRef]
- Tarbell, J.; Mahmoud, M.; Corti, A.; Cardoso, L.; Caro, C. The role of oxygen transport in atherosclerosis and vascular disease. J. R. Soc. Interface 2020, 17, 20190732. [Google Scholar] [CrossRef] [Green Version]
- Marsch, E.; Sluimer, J.; Daemen, M. Hypoxia in atherosclerosis and inflammation. Curr. Opin. Lipidol. 2013, 24, 393–400. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Bandarra, D.; Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016, 283, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Bowden, N.; Fragiadaki, M.; Souilhol, C.; Hsiao, S.; Mahmoud, M.; Allen, S.; Pirri, D.; Ayllon, B.T.; Akhtar, S.; et al. Mechanical Activation of Hypoxia-Inducible Factor 1α Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2087–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Huang, R.-T.; Hamanaka, R.B.; Krause, M.; Oh, M.-J.; Kuo, C.-H.; Nigdelioglu, R.; Meliton, A.Y.; Witt, L.; Dai, G.; et al. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. eLife 2017, 6, e25217. [Google Scholar] [CrossRef]
- Xu, X.; Tan, X.; Tampe, B.; Sanchez, E.; Zeisberg, M.; Zeisberg, E.M. Snail Is a Direct Target of Hypoxia-inducible Factor 1α (HIF1α) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells. J. Biol. Chem. 2015, 290, 16653–16664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Niu, W.; Dong, H.; Liu, M.; Luo, Y.; Li, Z. Hypoxia induces endothelial-mesenchymal transition in pulmonary vascular remodeling. Int. J. Mol. Med. 2018, 42, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.M.; Kim, H.R.; Xing, R.; Hsiao, S.; Mammoto, A.; Chen, J.; Serbanovic-Canic, J.; Feng, S.; Bowden, N.P.; Maguire, R.; et al. TWIST1 Integrates Endothelial Responses to Flow in Vascular Dysfunction and Atherosclerosis. Circ. Res. 2016, 119, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Qin, L.; Baeyens, N.; Li, G.; Afolabi, T.; Budatha, M.; Tellides, G.; Schwartz, M.A.; Simons, M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef] [Green Version]
- Mateo, J.; Izquierdo-Garcia, D.; Badimon, J.J.; Fayad, Z.A.; Fuster, V. Noninvasive Assessment of Hypoxia in Rabbit Advanced Atherosclerosis Using 18F-fluoromisonidazole Positron Emission Tomographic Imaging. Circ. Cardiovasc. Imaging 2014, 7, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.; Safarova, M.; Afanasieva, O.; Mitroshkin, M.; Matchin, Y.; Pokrovsky, S. Matrix Metalloproteinase 9 as a Predictor of Coronary Atherosclerotic Plaque Instability in Stable Coronary Heart Disease Patients with Elevated Lipoprotein(a) Levels. Biomolecules 2019, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahdentausta, L.; Leskelä, J.; Winkelmann, A.; Tervahartiala, T.; Sorsa, T.; Pesonen, E.; Pussinen, P.J. Serum MMP-9 Diagnostics, Prognostics, and Activation in Acute Coronary Syndrome and Its Recurrence. J. Cardiovasc. Transl. Res. 2018, 11, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Liu, Y.; Li, W.; Deng, F.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Hu, C.; Chen, L. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima–media thickness. Atherosclerosis 2014, 232, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, M.; Riemann, B.; Kopka, K.; Breyholz, H.-J.; Wagner, S.; Schäfers, K.P.; Law, M.P.; Schober, O.; Levkau, B. Scintigraphic Imaging of Matrix Metalloproteinase Activity in the Arterial Wall In Vivo. Circulation 2004, 109, 2554–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, S.; Hartung, D.; Ohshima, S.; Edwards, D.S.; Zhou, J.; Yalamanchili, P.; Azure, M.; Fujimoto, A.; Isobe, S.; Matsumoto, Y.; et al. Molecular Imaging of Matrix Metalloproteinase in Atherosclerotic Lesions: Resolution with Dietary Modification and Statin Therapy. J. Am. Coll. Cardiol. 2008, 52, 1847–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshima, S.; Petrov, A.; Fujimoto, S.; Zhou, J.; Azure, M.; Edwards, D.S.; Murohara, T.; Narula, N.; Tsimikas, S.; Narula, J. Molecular Imaging of Matrix Metalloproteinase Expression in Atherosclerotic Plaques of Mice Deficient in Apolipoprotein E or Low-Density-Lipoprotein Receptor. J. Nucl. Med. 2009, 50, 612–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varasteh, Z.; Hyafil, F.; Anizan, N.; Diallo, D.; Aid-Launais, R.; Mohanta, S.; Li, Y.; Braeuer, M.; Steiger, K.; Vigne, J.; et al. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using 111In-tilmanocept. EJNMMI Res. 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Kim, S.; Seo, H.S.; Lee, Y.J.; Eo, J.S.; Jeong, J.M.; Lee, B.; Kim, J.Y.; Park, Y.M.; Jeong, M. Novel PET Imaging of Atherosclerosis with 68Ga-Labeled NOTA-Neomannosylated Human Serum Albumin. J. Nucl. Med. 2016, 57, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luehmann, H.P.; Detering, L.; Fors, B.P.; Pressly, E.D.; Woodard, P.K.; Randolph, G.J.; Gropler, R.J.; Hawker, C.J.; Liu, Y. PET/CT Imaging of Chemokine Receptors in Inflammatory Atherosclerosis Using Targeted Nanoparticles. J. Nucl. Med. 2016, 57, 1124–1129. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Tegnebratt, T.; Tran, T.A.; Lu, L.; Damberg, P.; Gisterå, A.; Tarnawski, L.; Bone, D.; Hedin, U.; Eriksson, P.; et al. Molecular Imaging of Inflammation in a Mouse Model of Atherosclerosis Using a Zirconium-89-Labeled Probe. Int. J. Nanomed. 2020, 15, 6137–6152. [Google Scholar] [CrossRef]
- Cal-Gonzalez, J.; Vaquero, J.J.; Herraiz, J.L.; Liva, M.P.; Soto-Montenegro, M.L.; Peña-Zalbidea, S.; Desco, M.; Udias, J. Improving PET Quantification of Small Animal [68Ga]DOTA-Labeled PET/CT Studies by Using a CT-Based Positron Range Correction. Mol. Imaging Biol. 2018, 20, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, S.K.; Vacek, J.L. Imaging Techniques in Acute Coronary Syndromes: A Review. ISRN Cardiol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Świątkiewicz, I.; Magielski, P.; Kubica, J.; Zadourian, A.; DeMaria, A.N.; Taub, P.R. Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure. Int. J. Mol. Sci. 2020, 21, 807. [Google Scholar] [CrossRef] [Green Version]
- Nahrendorf, M.; Swirski, F.K. Innate immune cells in ischaemic heart disease: Does myocardial infarction beget myocardial infarction? Eur. Heart J. 2016, 37, 868–872. [Google Scholar] [CrossRef]
- Joshi, N.V.; Toor, I.; Shah, A.S.V.; Carruthers, K.; Vesey, A.T.; Alam, S.R.; Sills, A.; Hoo, T.Y.; Melville, A.J.; Langlands, S.P.; et al. Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J. Am. Heart Assoc. 2015, 4, e001956. [Google Scholar] [CrossRef] [Green Version]
- Afrasyab, A.; Qu, P.; Zhao, Y.; Peng, K.; Wang, H.; Lou, D.; Niu, N.; Yuan, D. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessel. 2015, 31, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, I.; Kozinski, M.; Magielski, P.; Fabiszak, T.; Sukiennik, A.; Navarese, E.P.; Odrowaz-Sypniewska, G.; Kubica, J. Value of C-Reactive Protein in Predicting Left Ventricular Remodelling in Patients with a First ST-Segment Elevation Myocardial Infarction. Mediat. Inflamm. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment–Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, I.; Taub, P.R. The usefulness of C-reactive protein for the prediction of post-infarct left ventricular systolic dysfunction and heart failure. Kardiol. Pol. 2018, 76, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Toldo, S.; Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018, 15, 203–214. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, B.; Ludewick, H.P.; Lee, S.; Verma, S.; Francis, R.J.; Dwivedi, G. Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells 2021, 10, 2573. https://doi.org/10.3390/cells10102573
Bartlett B, Ludewick HP, Lee S, Verma S, Francis RJ, Dwivedi G. Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells. 2021; 10(10):2573. https://doi.org/10.3390/cells10102573
Chicago/Turabian StyleBartlett, Benjamin, Herbert P. Ludewick, Silvia Lee, Shipra Verma, Roslyn J. Francis, and Girish Dwivedi. 2021. "Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque" Cells 10, no. 10: 2573. https://doi.org/10.3390/cells10102573
APA StyleBartlett, B., Ludewick, H. P., Lee, S., Verma, S., Francis, R. J., & Dwivedi, G. (2021). Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells, 10(10), 2573. https://doi.org/10.3390/cells10102573





