Long-Term Risk of Hepatic and Extrahepatic-Related Events After Direct Antiviral Therapy for Chronic Hepatitis C: A Prospective Long-Term Study Cohort
Simple Summary
Abstract
1. Introduction
2. Methods
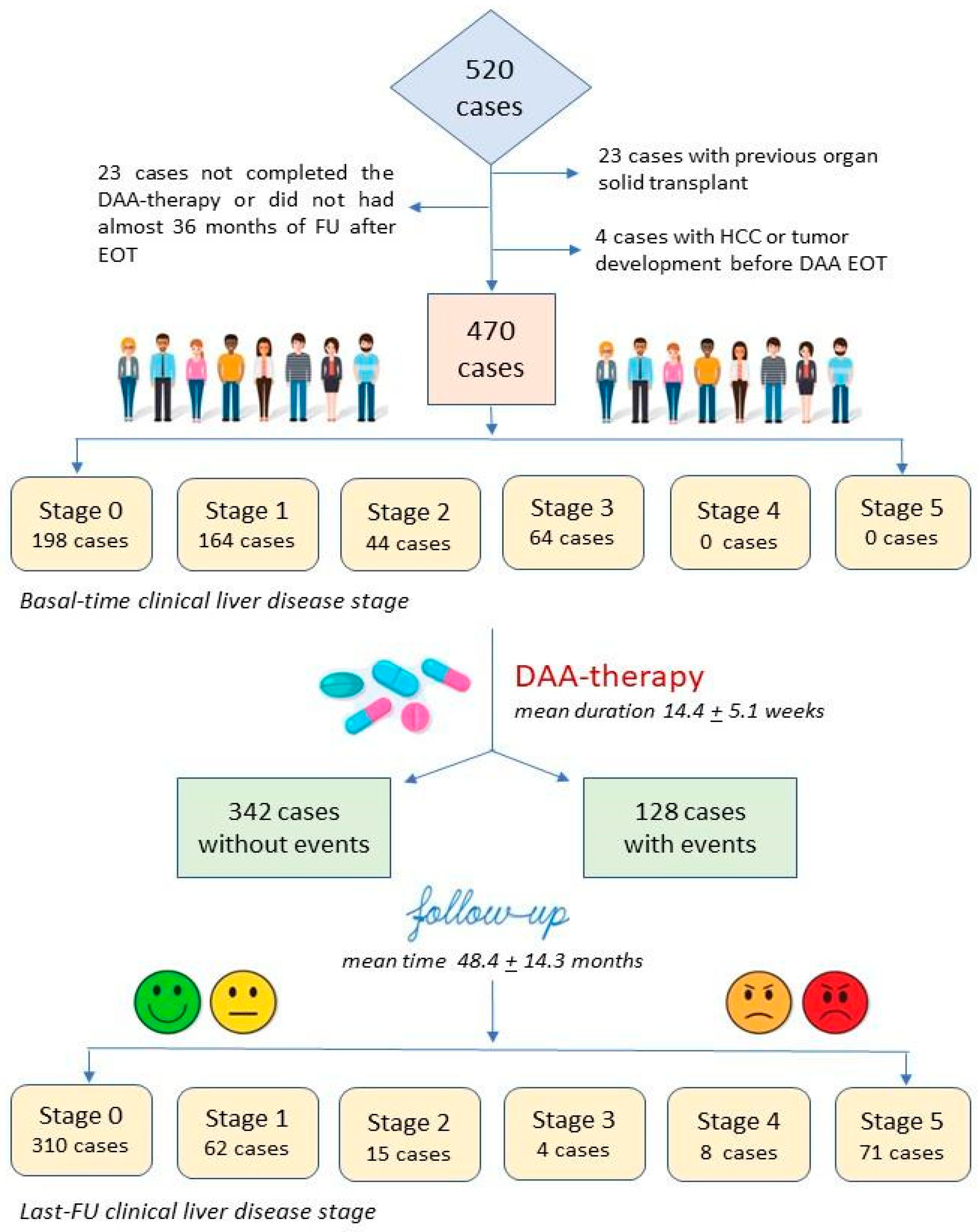
2.1. Study Design and Characteristics of Study Population
2.2. NIT for Estimation of Liver Fibrosis and Portal Hypertension
2.3. Assessment of Clinical Liver Disease Stage
2.4. Risk Stratification Assessment of Events
2.5. Statistical Analysis
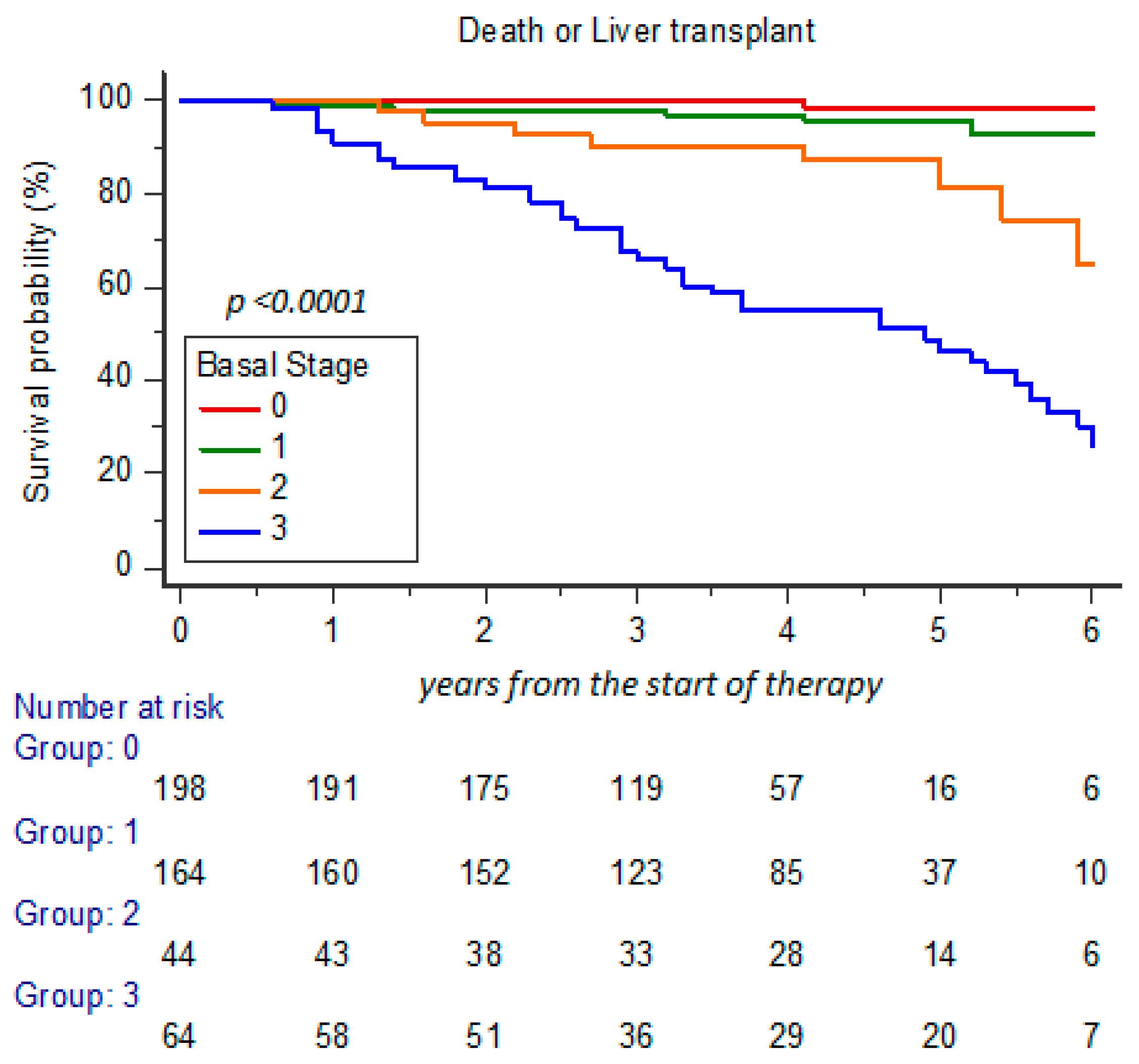
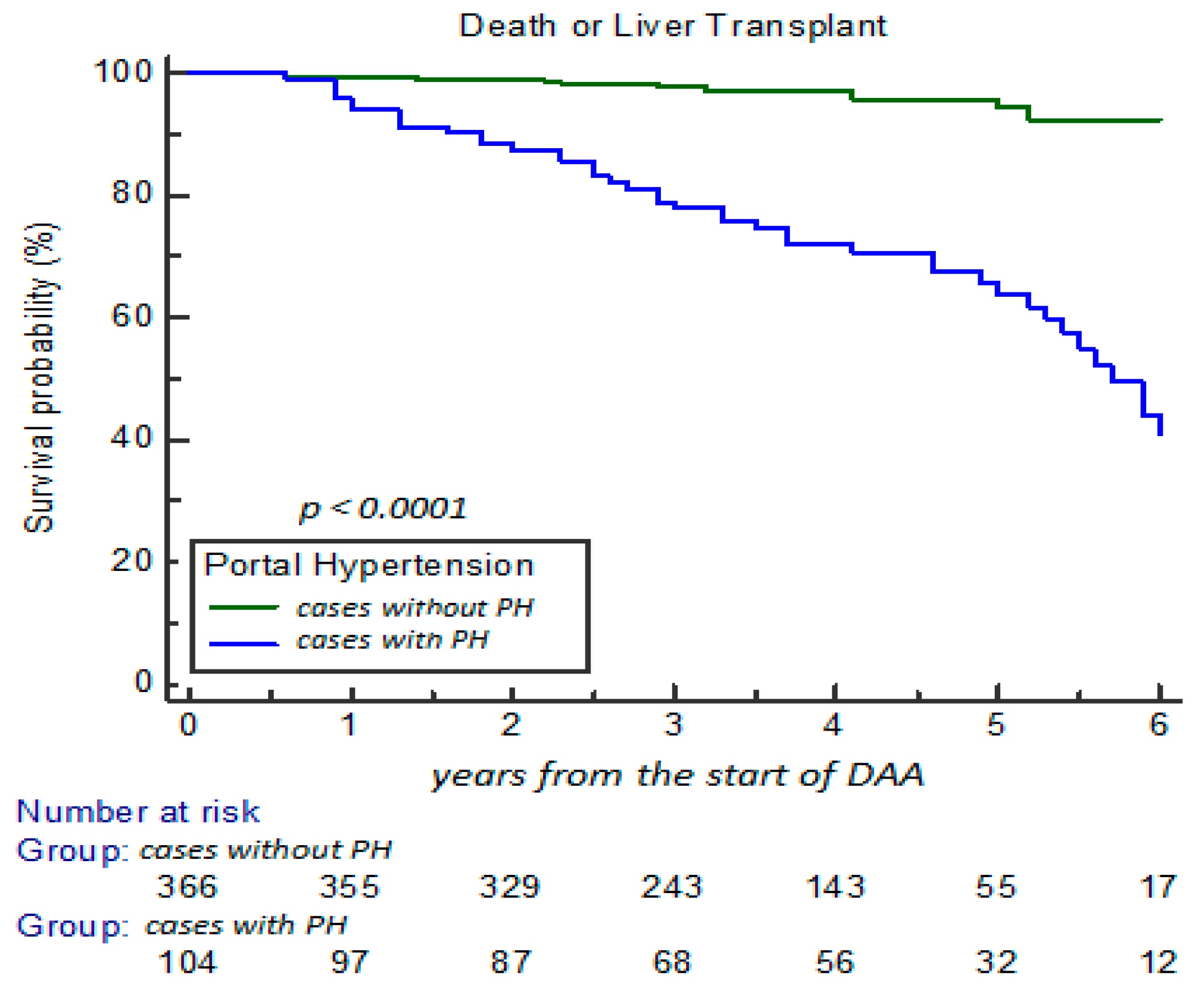
3. Results
3.1. Clinical Characteristics and Changes During Long-Term FU
3.2. Development of HE and EHE and the Impact on Clinical NIT Scores
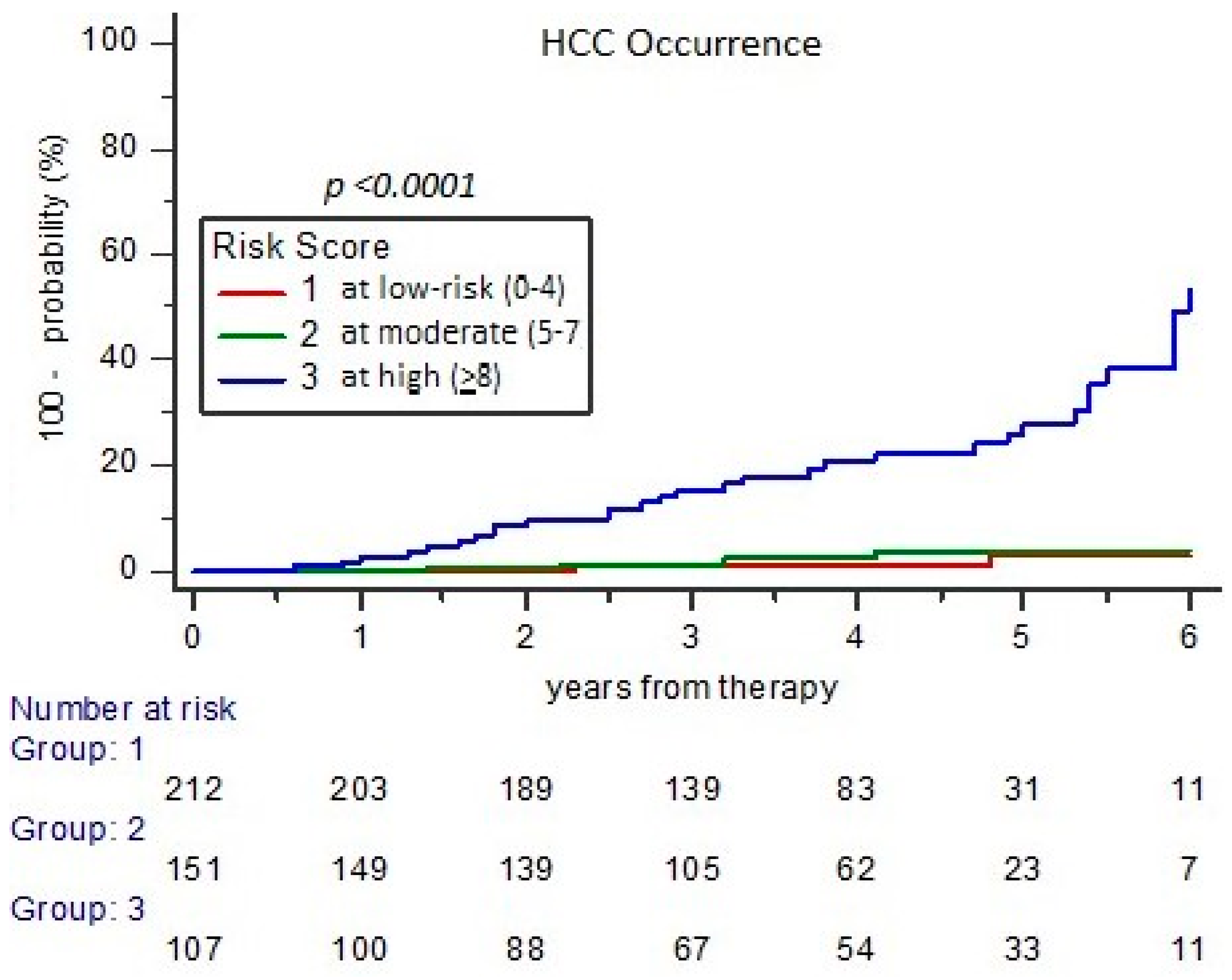
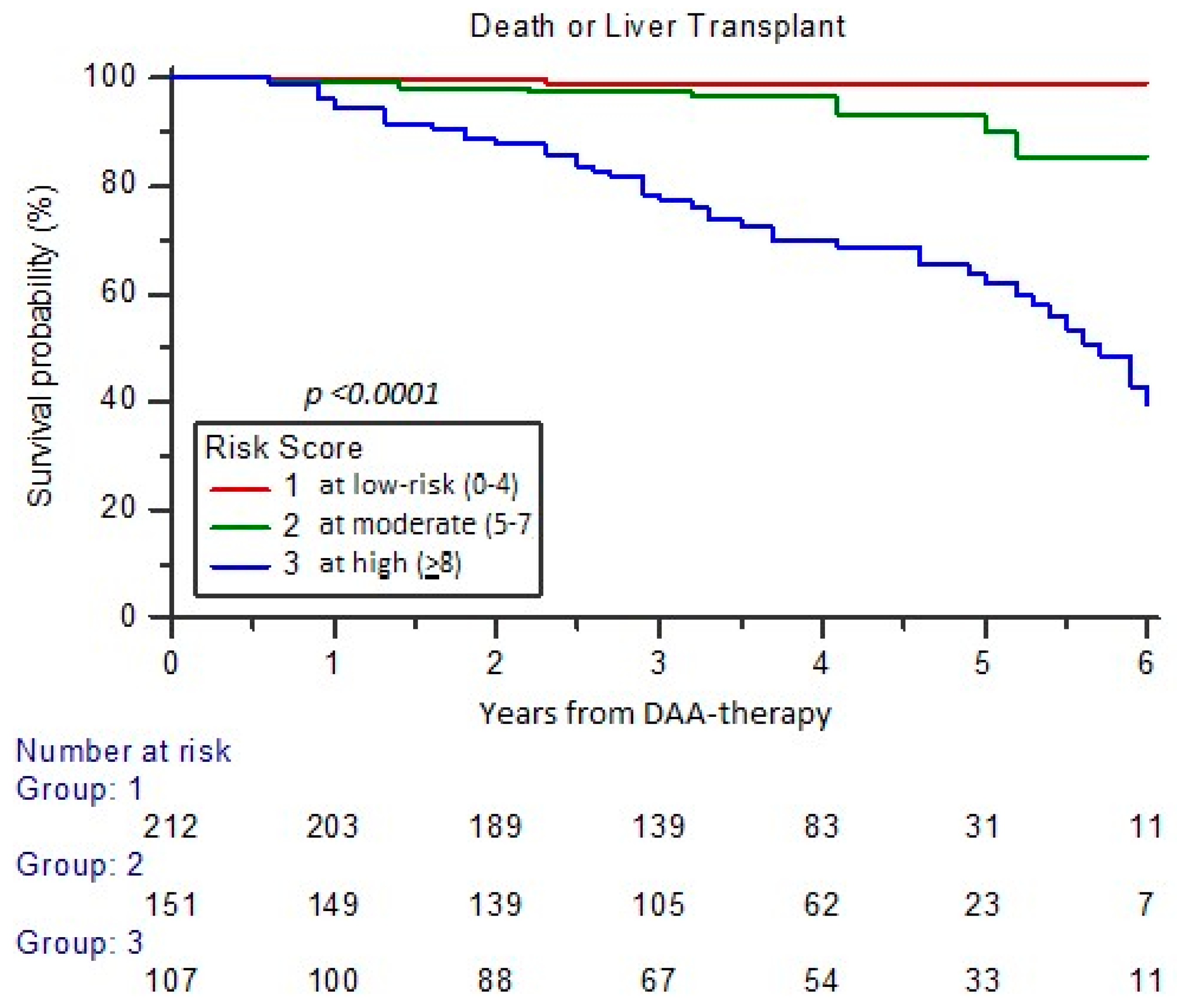
3.3. Assessment of Risk for Major Events Incidence at Long-Term FU (RISS Score)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| APRI | aspartate aminotransferase to platelet ratio index |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| cACLD | compensated advanced liver disease |
| CI | confidential interval |
| CHC | chronic hepatitis C |
| CLDS | clinical liver disease stage |
| RISS | Risk stratification system |
| DAA | direct-acting antiviral |
| DILI | drug-induced liver injury |
| EHE | extrahepatic-related events |
| EOT | end of therapy |
| EV | esophageal varices |
| FIB-4 | fibrosis-4 index for detection of liver fibrosis or cirrhosis |
| FORNS | staging of liver fibrosis or cirrhosis according to Forns’ formula |
| GGT | gamma-glutamil transferase |
| GE | gastro-esophageal |
| HCC | hepatocellular carcinoma |
| HCV | human hepatitis C virus |
| HE | hepatic-related events |
| HR | hazard ratio |
| LSM | liver stiffness measures |
| LSPS | liver stiffness platelet score |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NIT | non-invasive test |
| OLT | orthotopic liver transplantation |
| PH | portal hypertension |
| SD | standard deviation |
| SOF | sofosbuvir |
| SVR | sustained virologic response |
| TE | transient elastography |
References
- Zarębska-Michaluk, D.; Brzdęk, M.; Jaroszewicz, J.; Tudrujek-Zdunek, M.; Lorenc, B.; Klapaczyński, J.; Mazur, W.; Kazek, A.; Sitko, M.; Berak, H.; et al. Best therapy for the easiest to treat hepatitis C virus genotype 1b-infected patients. World J. Gastroenterol. 2022, 28, 6380–6396. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Terrault, N. Treatment of chronic hepatitis C in patients with cirrhosis. Curr. Opin. Gastroenterol. 2016, 32, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Aghemo, A.; Back, D.; Dusheiko, G.; Forns, X.; Puoti, M.; Sarrazin, C. EASL Recommendations on Treatment of Hepatitis C. J. Hepatol. 2015, 63, 199–236. [Google Scholar]
- Huang, C.F.; Yu, M.L. Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy. Clin. Mol. Hepatol. 2020, 26, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Villani, R.; Vendemiale, G.; Serviddio, G. Molecular Mechanisms Involved in HCC Recurrence after Direct-Acting Antiviral Therapy. Int. J. Mol. Sci. 2018, 20, 49. [Google Scholar] [CrossRef]
- Bersoff-Matcha, S.J.; Cao, K.; Jason, M.; Ajao, A.; Jones, S.C.; Meyer, T.; Brinker, A. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: A review of cases reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann. Intern. Med. 2017, 166, 792–798. [Google Scholar] [CrossRef]
- Néant, N.; Solas, C. Drug-drug interactions potential of direct acting antivirals for the treatment of chronic hepatitis C virus infection. Int. J. Antimicrob. Agents 2020, 56, 105–571. [Google Scholar] [CrossRef]
- Calvaruso, V.; Craxì, A. Hepatic benefits of HCV cure. J. Hepatol. 2020, 73, 1548–1556. [Google Scholar] [CrossRef]
- Laursen, T.L.; Sandahl, T.D.; Kazankov, K.; George, J.; Grønbæk, H. Liver-related effects of chronic hepatitis C antiviral treatment. World J. Gastroenterol. 2020, 26, 2931–2947. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Nielsen, E.E.; Feinberg, J.; Katakam, K.K.; Fobian, K.; Hauser, G.; Poropat, G.; Djurisic, S.; Weiss, K.H.; Bjelakovic, M.; et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst. Rev. 2017, 9, CD012143. [Google Scholar] [PubMed]
- Van der Meer, A.J.; Feld, J.J.; Hofer, H.; Almasio, P.L.; Calvaruso, V.; Fernandez-Rodriguez, C.M.; Aleman, S.; Ganne-Carrié, N.; D’Ambrosio, R.; Pol, S.; et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J. Hepatol. 2017, 66, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; FabioTinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef]
- Quaranta, M.G.; Cavalletto, L.; Russo, F.P.; Calvaruso, V.; Ferrigno, L.; Zanetto, A.; Mattioli, B.; D’Ambrosio, R.; Panetta, V.; Brancaccio, G.; et al. Reduction of the risk of hepatocellular carcinoma over time using direct-acting antivirals: A propensity score analysis of a real-life cohort (PITER-HCV). Viruses 2024, 16, 682. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, A.; Alimenti, E.; Gattai, R.; Filomia, R.; Parente, E.; Valenti, L.; Marzi, L.; Pellegatta, G.; Borgia, G.; Gambato, M.; et al. Undefined/non-malignant hepatic nodules are associated with early occurrence of HCC in DAA-treated patients with HCV-related cirrhosis. J. Hepatol. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; Cao, Y.; Li, L.; El Serag, H.B. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020, 71, 44–55. [Google Scholar] [CrossRef]
- Backus, L.I.; Belperio, P.S.; Shahoumian, T.A.; Mole, L.A. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with direct-acting antiviral treatment on mortality in patients with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology 2019, 69, 487–497. [Google Scholar]
- Giovannini, C.; Fornari, F.; Indio, V.; Trerè, D.; Renzulli, M.; Vasuri, F.; Cescon, M.; Ravaioli, M.; Perrucci, A.; Astolfi, A.; et al. Direct antiviral treatments for hepatitis C virus have off-target effects of oncologic relevance in hepatocellular carcinoma. Cancers 2020, 12, 2674. [Google Scholar] [CrossRef]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver angiopoietin-2 is a key predictor of de novo or recurrent HCC after hepatitis C virus direct-acting antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef]
- Nevola, R.; Tortorella, G.; Rosato, V.; Rinaldi, L.; Imbriani, S.; Perillo, P.; Mastrocinque, D.; La Montagna, M.; Russo, A.; Di Lorenzo, G.; et al. Gender differences in the pathogenesis and risk factors of hepatocellular carcinoma. Biology 2023, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C. J. Hepatol. 2016, 66, 153–194. [Google Scholar]
- EASL Clinical practice guideline on non-invasive test for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 2021, 75, 659–689. [CrossRef] [PubMed]
- Lok, A.S.; Ghany, M.G.; Godman, Z.D.; Wright, E.C.; Everson, G.T.; Sterling, R.K.; Everhart, J.E.; Lindsay, K.L.; Bonkovsky, H.L.; Di Bisceglie, A.M.; et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology 2005, 42, 282–292. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Shu-Hui Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase to platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Forns, X.; Ampurdanes, S.; Llovet, J.M.; Aponte, J.; Quinto, L.; Martinez-Bauer, E.; Bruguera, M.; Sanchez-Tapias, J.; Rode, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2020, 36, 986–992. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, D.Y.; Park, J.Y.; Ahn, S.H.; Chon, C.Y.; Kim, J.K.; Paik, Y.H.; Lee, K.S.; Park, Y.N.; Han, K.H. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting virus-infected patients. Liver Int. 2010, 30, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti ALSeijo, S.; Arena, U.; Abraldes, J.G.; Vizzutti, F.; Garcia-Pagàn, J.C.; Pinzani, M.; Bosch, J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013, 144, 102–111. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical states of cirrhosis and competing risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef]
- Ogawa, E.; Chien, N.; Kam, L.; Yeo, Y.H.; Ji, F.; Huang, D.Q.; Cheung, R.; Nguyen, M.H. Association of direct-acting antiviral therapy with liver and non-liver complications and long-term mortality in patients with chronic hepatitis C. JAMA Intern. Med. 2023, 183, 97–105. [Google Scholar] [CrossRef]
- Tahata, Y.; Hikita, H.; Mochida, S.; Enomoto, N.; Kawada, N.; Kurosaki, M.; Ido, A.; Miki, D.; Yoshiji, H.; Takikawa, Y.; et al. Liver-related events after direct-acting antiviral therapy in patients with hepatitis C virus-associated cirrhosis. J. Gastroenterol. 2022, 57, 120–132. [Google Scholar] [CrossRef] [PubMed]
- De Santis, A.; Maggi, D.; Lubrano Lobianco, F. Safety and efficacy of directly-acting antiviral therapy for chronic hepatitis C virus in elderly people. Aging Med. 2021, 4, 304–316. [Google Scholar] [CrossRef]
- ANRS Collaborative Study Group on Hepatocellular Carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J. Hepatol. 2016, 65, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Sapena, V.; Enea, M.; Torres, F.; Celsa, C.; Rios, J.; Rizzo, G.E.M.; Nahon, P.; Mariño, Z.; Tateishi, R.; Minami, T.; et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: An individual patient data meta-analysis. Gut 2022, 71, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; on behalf of the Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2021, 76, 959–974. [Google Scholar] [CrossRef]

| Cases Without Events n. 342 (72.7%) | p-Level 0 vs. 1 | Cases with HE n. 76 (16.2%) | p-Level 1 vs. 2 | Cases with EHE n. 52 (11.0%) | p-Level 0 vs. 2 | |
|---|---|---|---|---|---|---|
| Characteristics | Group 0 | Group 1 | Group 2 | |||
| N° males (%) | 164 (47.9%) | <0.001 | 53 (69.7%) | 0.01 | 24 (31.6%) | ns |
| Age (mean years ± SD) | 57.6 ± 12.9 | ns | 59.6 ± 11.4 | ns | 63.6 ± 12.9 | <0.005 |
| BMI (Kg/m2) | 25.1 ± 4.0 | <0.05 | 26.8 ± 4.3 | ns | 25.4 ± 4.1 | ns |
| Smoking habit | 155 (45.3%) | <0,05 | 46 (60.5%) | 0.05 | 22 (42.3%) | ns |
| Alcohol consumption | 175 (51.2%) | <0.05 | 50 (65.8%) | ns | 25 (48.1%) | ns |
| Comorbidities | 196 (57.3%) | <0.001 | 61 (80.3%) | ns | 39 (75.0%) | <0.05 |
| HCV genotype 1 infection | 212 (61.9%) | ns | 44 (57.9%) | ns | 32 (61.5%) | ns |
| DAA schedule SOF-based | 163 (47.7%) | <0.001 | 68 (89.5%) | 0.01 | 37 (71.1%) | <0.001 |
| Basal Time Clinical Liver Disease Stage | ||||||
| Mild fibrosis (stage 0) | 180 (52.6%) | <0.001 | 1 (1.3%) | <0.001 | 17 (32.7%) | <0.001 |
| Significant fibrosis or cirrhosis without EV (stage 1) | 134 (39.2%) | <0.001 | 8 (10.5%) | <0.001 | 22 (42.3%) | ns |
| Cirrhosis with EV (stage 2) | 24 (7.0%) | 0.01 | 13 (17.1%) | <0.005 | 7 (13.4%) | ns |
| Cirrhosis with only one known decompensation (stage 3) | 4 (1.2%) | <0.001 | 54 (71.1%) | <0.001 | 6 (11.5%) | 0.001 |
| Development of Major Events during FU | ||||||
| Cases with HCC | 0 | -- | 46 (60.5%) | -- | 0 | -- |
| Cases with OLT or/and dead | 0 | -- | 53 * (70.0%) | <0.001 | 9 (17.3%) | -- |
| Biochemical Laboratory Parameters | ||||||
| Hemoglobin (g/L) | 141.8 ± 17.7 | <0.001 | 129.3 ± 20.9 | 0.01 | 137.6 ± 16.6 | ns |
| White cell count (mm3) | 6.1 ± 1.9 | <0.001 | 4.5 ± 1.9 | <0.005 | 5.9 ± 1.8 | ns |
| PLTS count (mm3) | 204.8 ± 72.6 | <0.001 | 96.5 ± 68.4 | <0.001 | 179.7 ± 87.1 | 0.05 |
| INR ratio | 1.08 ± 0.25 | <0.001 | 1.22 ± 0.21 | <0.005 | 1.11 ± 0.26 | ns |
| Total bilirubin (μmol/L) | 11.5 ± 5.7 | <0.001 | 22.5 ± 9.9 | 0.001 | 11.1 ± 5.9 | ns |
| ALT (U/L) | 93.9 ± 89.5 | <0.005 | 70.5 ± 48.1 | ns | 90.7 ± 67.4 | ns |
| Gamma-GT (U/L) | 57.2 ± 60.4 | 0.001 | 99.6 ± 86.9 | ns | 71.8 ± 63.7 | ns |
| Total protein (g/L) | 74.6 ± 5.0 | ns | 74.5 ± 6.7 | ns | 73.7 ± 7.9 | ns |
| Albumin (g/L) | 41.5 ± 4.2 | <0.001 | 35.9 ± 4.8 | 0.005 | 39.3 ± 4.8 | 0.05 |
| Gamma-globulin (g/L) | 14.6 ± 4.6 | <0.001 | 20.7 ± 6.1 | <0.005 | 15.2 ± 4.8 | ns |
| Alpha-fetoprotein (μg/L) | 8.2 ± 17.6 | <0.05 | 22.4 ± 50.6 | ns | 12.5 ± 19.9 | ns |
| Cases Without Events | p-Level0 vs. 1 | Cases with HE | p-Level1 vs. 2 | Cases with EHE | p-Level0 vs. 2 | |
|---|---|---|---|---|---|---|
| Liver NITs | Group 0 | Group 1 | Group 2 | |||
| N° cases (%) * | 309 (90%) | 60 (79%) | 40 (77%) | |||
| LSM ** at BT *** | 11.7 ± 7.7 | <0.001 | 32.6 ± 12.0 | <0.01 | 15.2 ± 9.1 | ns |
| LSM at last FU | 7.5 ± 4.1 | <0.001 | 28.3 ± 14.4 | <0.005 | 10.1 ± 7.4 | ns |
| APRI at BT | 1.0 ± 0.9 | <0.001 | 2.5 ± 1.8 | <0.001 | 1.4 ± 1.2 | <0.05 |
| APRI at last FU | 0.4 ± 0.2 | <0.001 | 1.6 ± 1.2 | <0.01 | 0.6 ± 0.5 | ns |
| FORNS at BT | 8.1 ± 1.5 | <0.001 | 11.5 ± 1.6 | <0.01 | 9.2 ± 1.8 | <0.01 |
| FORNS at last FU | 7.3 ± 1.2 | <0.001 | 10.9 ± 1.7 | <0.001 | 8.2 ± 1.7 | <0.01 |
| FIB-4 at BT | 2.5 ± 1.4 | <0.001 | 8.5 ± 4.3 | <0.001 | 4.4 ± 2.7 | <0.005 |
| FIB-4 at last FU | 1.7 ± 0.7 | <0.001 | 6.9 ± 4.4 | <0.001 | 3.0 ± 2.1 | <0.05 |
| LSPS at BT | 1.0 ± 0.8 | <0.001 | 8.1 ± 5.0 | <0.001 | 1.8 ± 1.6 | <0.05 |
| LSPS at last FU | 0.6 ± 0.5 | <0.001 | 6.9 ± 5.4 | <0.001 | 1.0 ± 0.9 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalletto, L.; Bertoli, E.; Mescoli, C.; Aliberti, C.; Quaranta, M.G.; Kondili, L.; Chemello, L. Long-Term Risk of Hepatic and Extrahepatic-Related Events After Direct Antiviral Therapy for Chronic Hepatitis C: A Prospective Long-Term Study Cohort. Cancers 2025, 17, 1528. https://doi.org/10.3390/cancers17091528
Cavalletto L, Bertoli E, Mescoli C, Aliberti C, Quaranta MG, Kondili L, Chemello L. Long-Term Risk of Hepatic and Extrahepatic-Related Events After Direct Antiviral Therapy for Chronic Hepatitis C: A Prospective Long-Term Study Cohort. Cancers. 2025; 17(9):1528. https://doi.org/10.3390/cancers17091528
Chicago/Turabian StyleCavalletto, Luisa, Eleonora Bertoli, Claudia Mescoli, Camillo Aliberti, Maria Giovanna Quaranta, Loreta Kondili, and Liliana Chemello. 2025. "Long-Term Risk of Hepatic and Extrahepatic-Related Events After Direct Antiviral Therapy for Chronic Hepatitis C: A Prospective Long-Term Study Cohort" Cancers 17, no. 9: 1528. https://doi.org/10.3390/cancers17091528
APA StyleCavalletto, L., Bertoli, E., Mescoli, C., Aliberti, C., Quaranta, M. G., Kondili, L., & Chemello, L. (2025). Long-Term Risk of Hepatic and Extrahepatic-Related Events After Direct Antiviral Therapy for Chronic Hepatitis C: A Prospective Long-Term Study Cohort. Cancers, 17(9), 1528. https://doi.org/10.3390/cancers17091528









