Intrafractional Motion in Online-Adaptive Magnetic Resonance-Guided Radiotherapy of Adrenal Metastases Leads to Reduced Target Volume Coverage and Elevated Organ-at-Risk Doses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
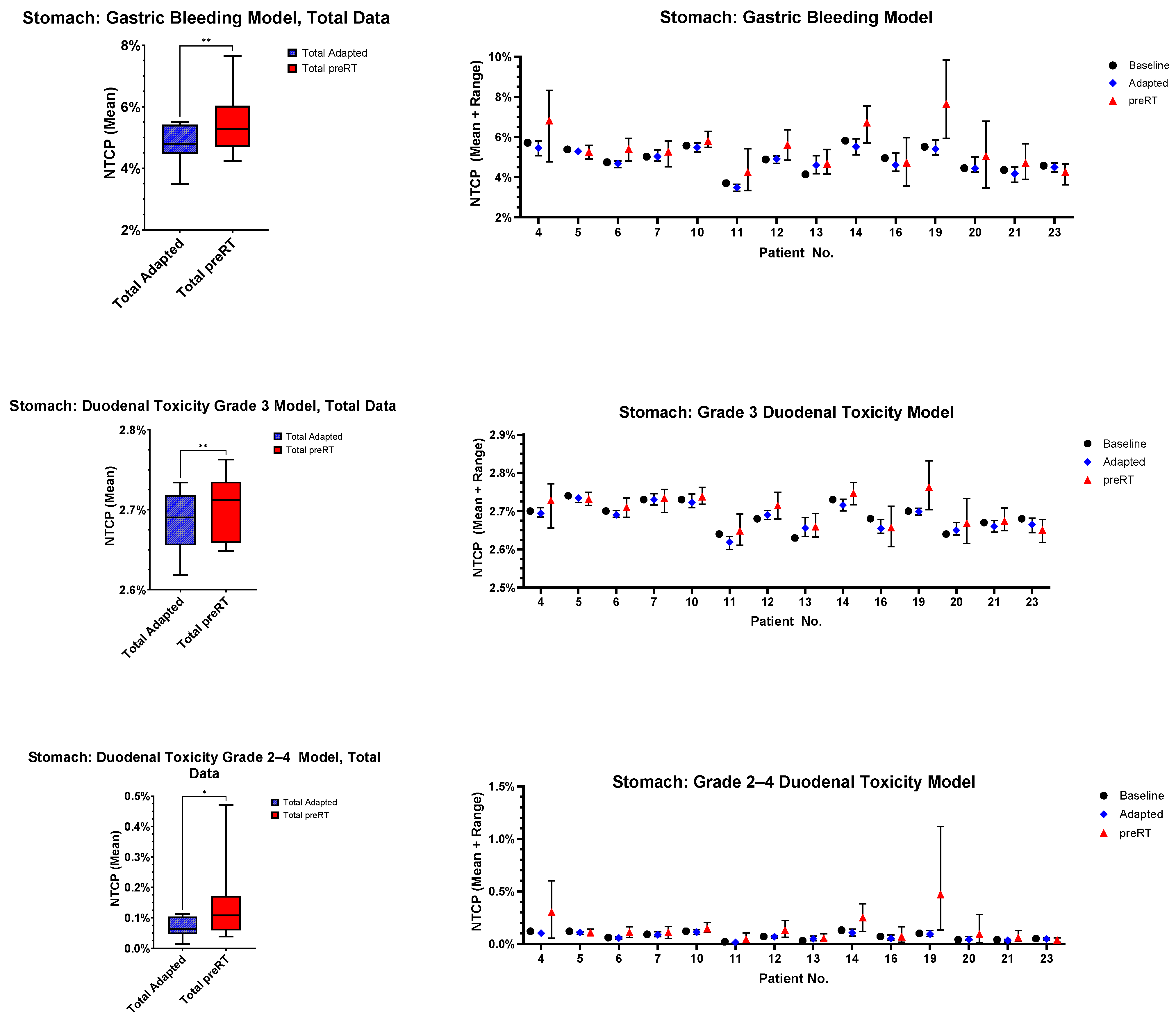
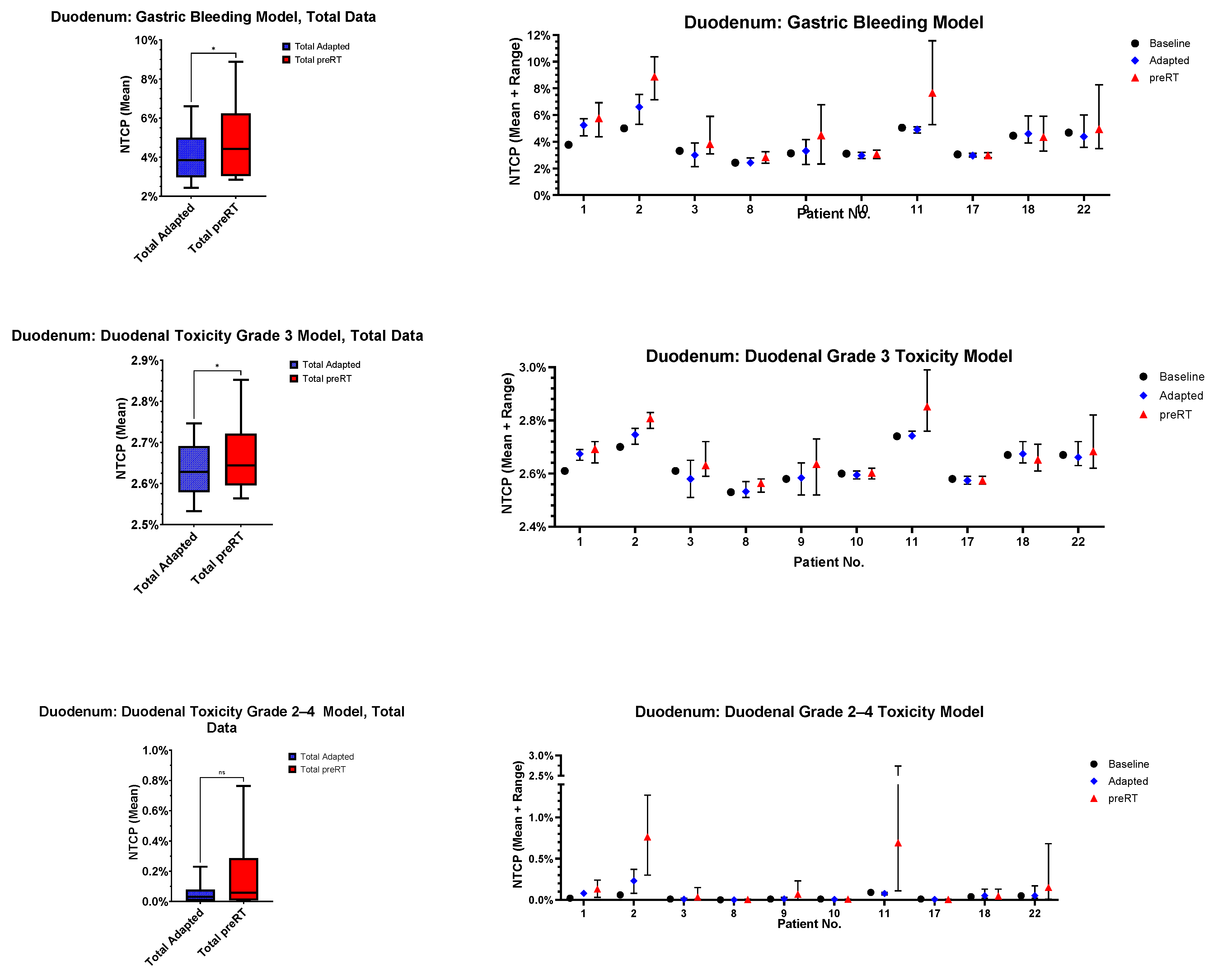
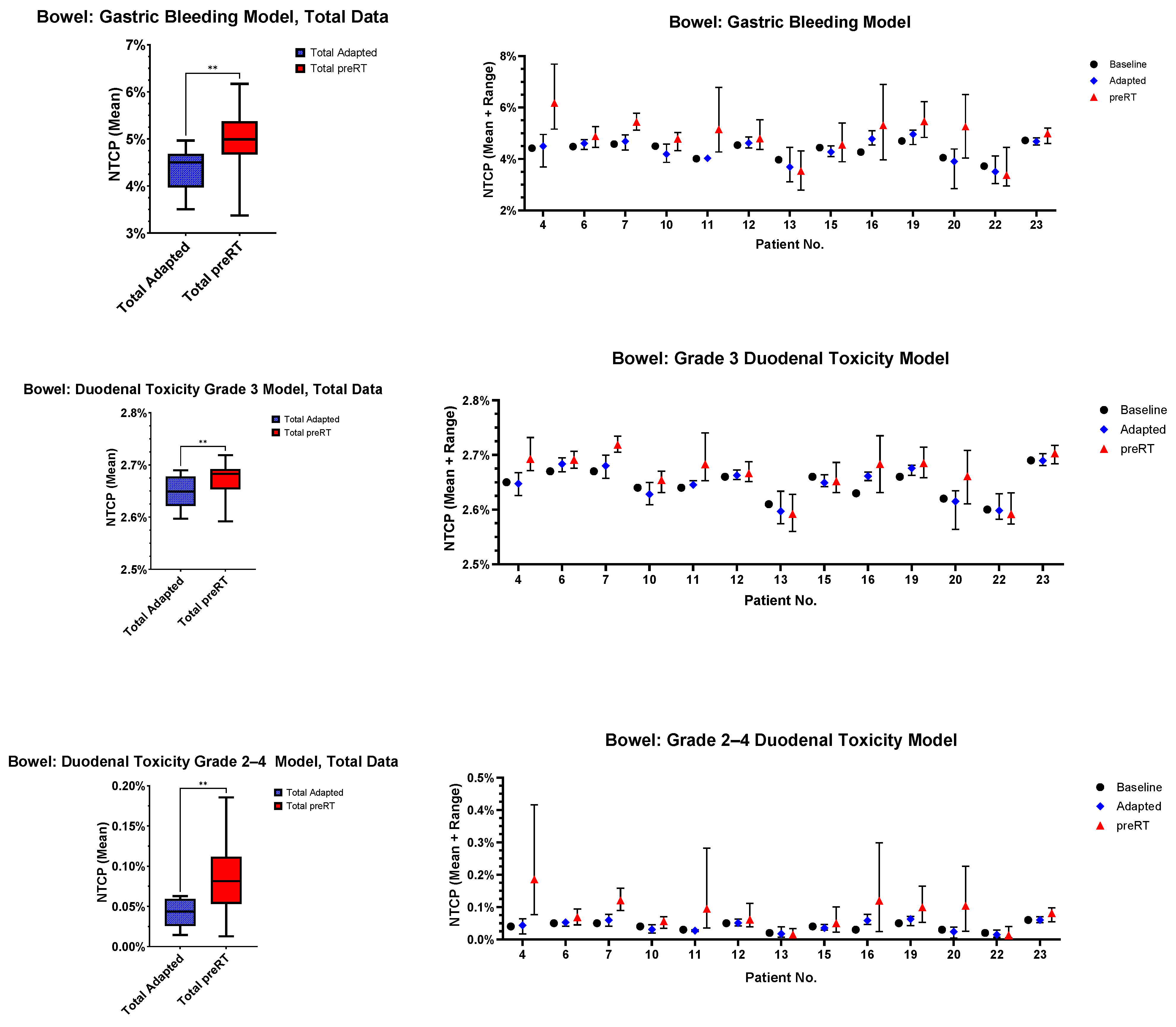
- Gastric bleeding model (TD50 = 180, n = 0.12, m = 0.49, D ref = 2.0 Gy, α/β = 2.5 Gy) [32];
- Duodenal toxicity ≥ grade 3 model (TD50 = 299.1, n = 0.193, m = 0.51, D ref = 2.0 Gy, α/β = 4.0 Gy) [33];
- Duodenal toxicity grades 2–4 model (TD50 = 24.6, n = 0.12, m = 0.23, D ref = 25.0 Gy, α/β = 4.0 Gy) [34].
3. Results
3.1. Patient and Treatment Characteristics
3.2. Position Corrections and Time Intervals
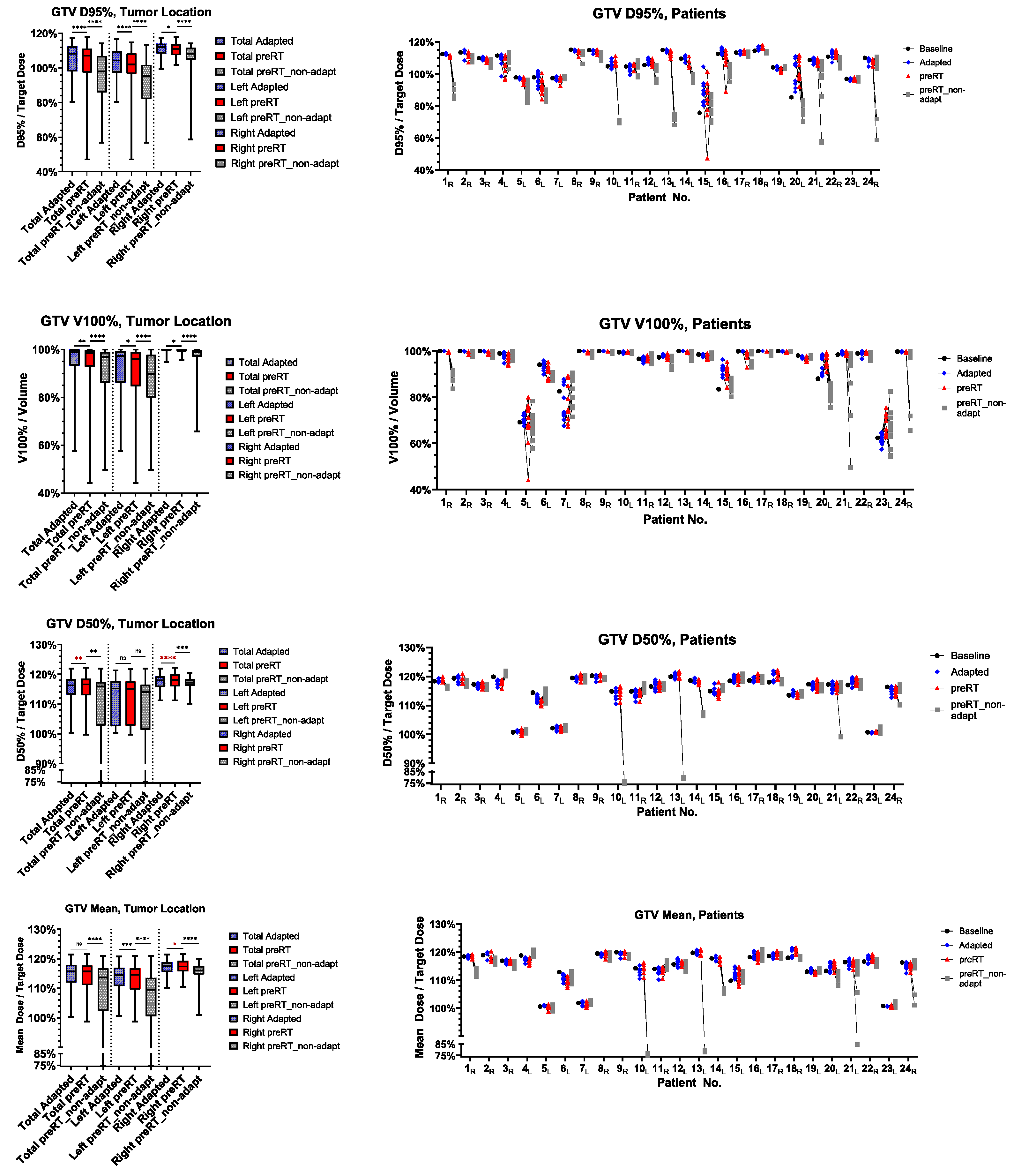
3.3. Target Volume Coverage
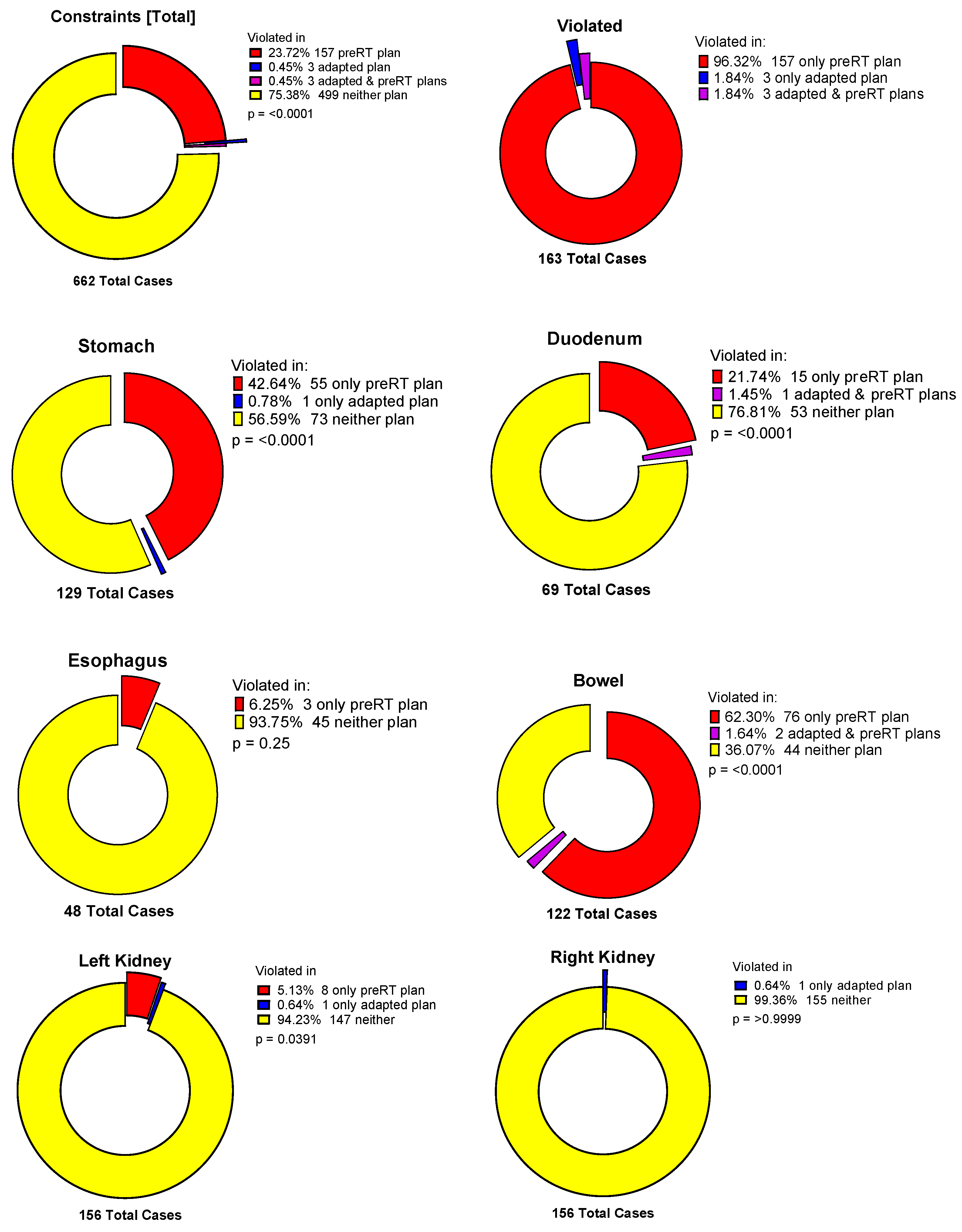
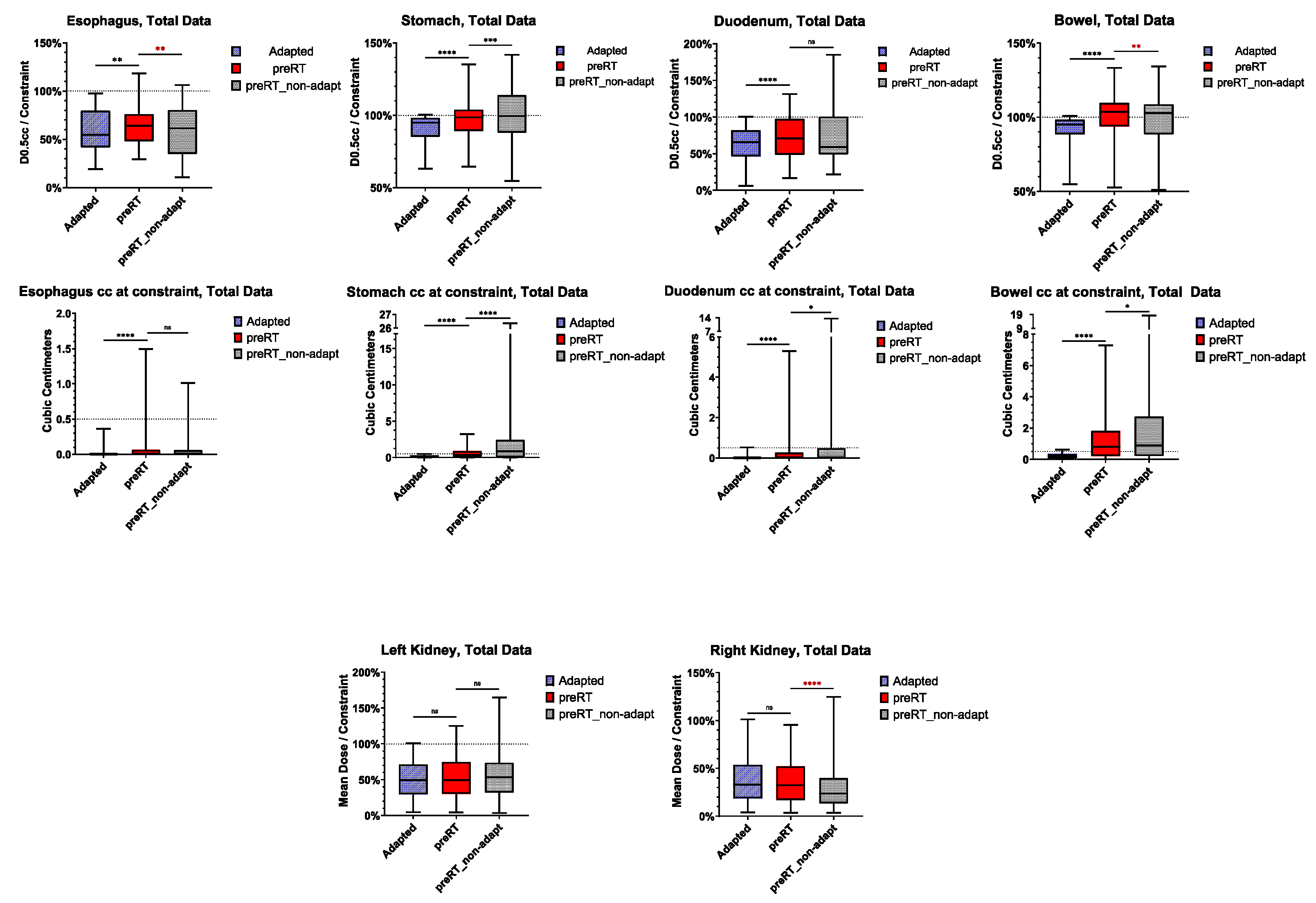
3.4. Violation of OAR Constraints
3.5. Normal Tissue Complication Probability (NTCP)
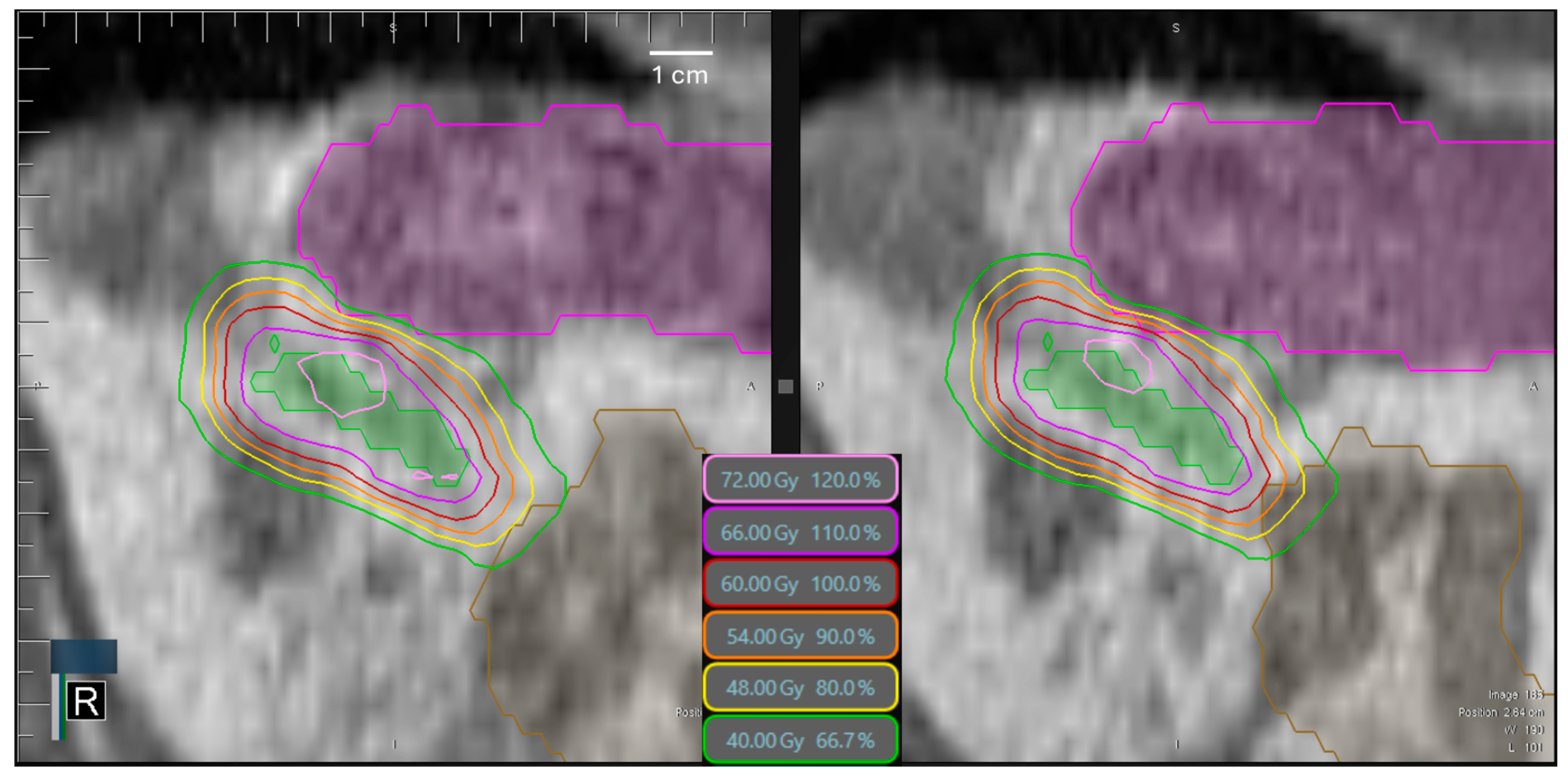
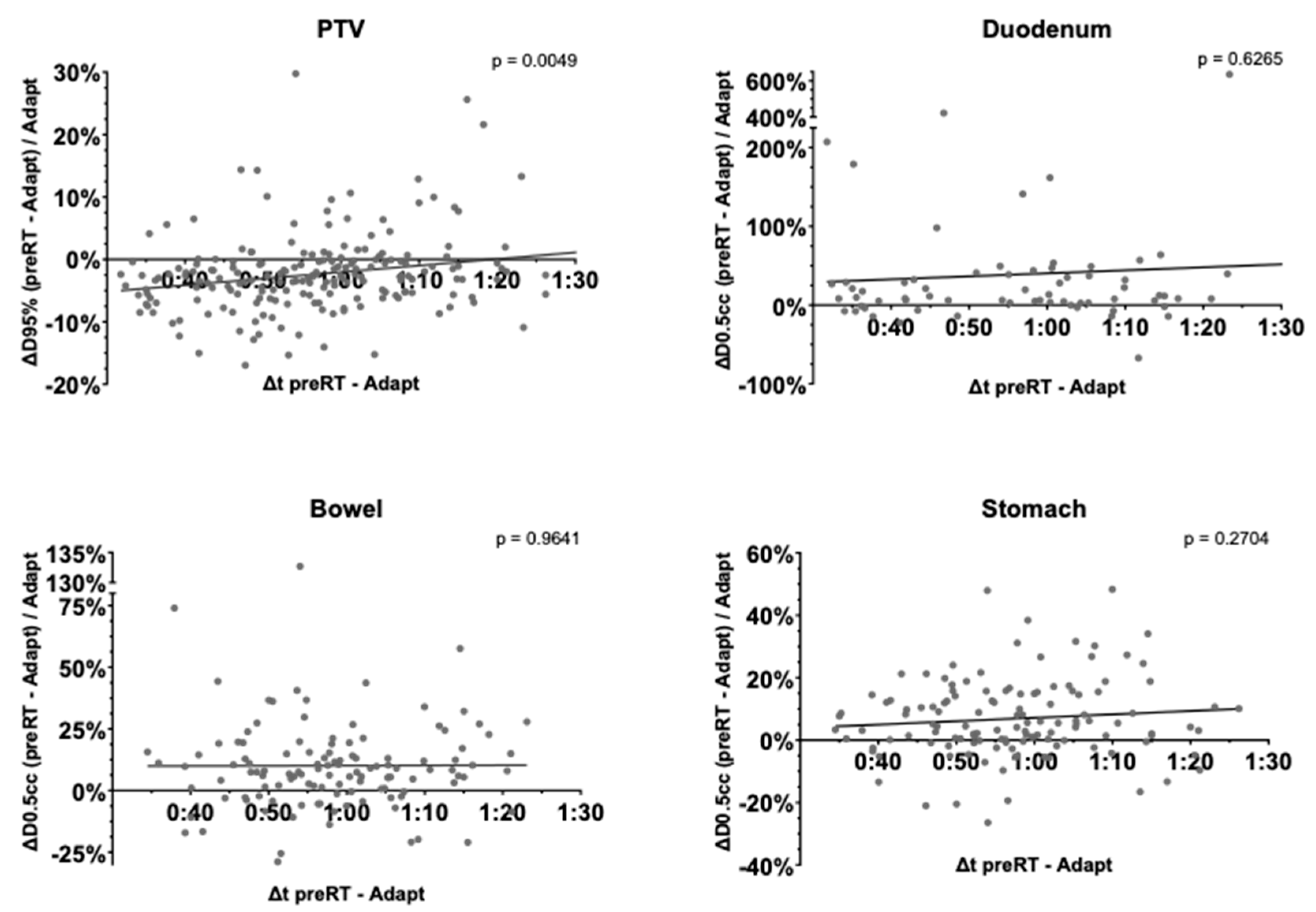
3.6. Time-Dependence of PTV Coverage and OAR Overdosage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.C.; Baal, J.D.; Baal, U.; Pai, J.; Gottschalk, A.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Stereotactic Body Radiation Therapy of Adrenal Metastases: A Pooled Meta-Analysis and Systematic Review of 39 Studies with 1006 Patients. Int. J. Radiat. Oncol. 2020, 107, 48–61. [Google Scholar] [CrossRef]
- Chalkidou, A.; Macmillan, T.; Grzeda, M.T.; Peacock, J.; Summers, J.; Eddy, S.; Coker, B.; Patrick, H.; Powell, H.; Berry, L.; et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: A prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021, 22, 98–106. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, P.G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non–Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; Preeshagul, I.; et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): An open-label, randomised, controlled, phase 2 study. Lancet 2023, 403, 171–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Kashani, R.; Lawrence, T.S.; Balter, J.M. Real-time prediction of stomach motions based upon gastric contraction and breathing models. Phys. Med. Biol. 2022, 68, 015001. [Google Scholar] [CrossRef]
- Fast, M.F.; Cao, M.; Parikh, P.; Sonke, J.-J. Intrafraction Motion Management With MR-Guided Radiation Therapy. Semin. Radiat. Oncol. 2023, 34, 92–106. [Google Scholar] [CrossRef]
- Palacios, M.A.; Bohoudi, O.; Bruynzeel, A.M.; Koste, J.R.v.S.d.; Cobussen, P.; Slotman, B.J.; Lagerwaard, F.J.; Senan, S. Role of Daily Plan Adaptation in MR-Guided Stereotactic Ablative Radiation Therapy for Adrenal Metastases. Int. J. Radiat. Oncol. 2018, 102, 426–433. [Google Scholar] [CrossRef]
- Chen, H.; Schneiders, F.L.; Bruynzeel, A.M.E.; Lagerwaard, F.J.; Koste, J.R.v.S.d.; Cobussen, P.; Bohoudi, O.; Slotman, B.J.; Louie, A.V.; Senan, S. Impact of daily plan adaptation on organ-at-risk normal tissue complication probability for adrenal lesions undergoing stereotactic ablative radiation therapy. Radiother. Oncol. 2021, 163, 14–20. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Kotecha, R.; Tom, M.C.; Chuong, M.D.; Contreras, J.A.; Romaguera, T.; Alvarez, D.; McCulloch, J.; Herrera, R.; Hernandez, R.J.; et al. CT-guided versus MR-guided radiotherapy: Impact on gastrointestinal sparing in adrenal stereotactic body radiotherapy. Radiother. Oncol. 2021, 166, 101–109. [Google Scholar] [CrossRef]
- Hoegen, P.; Katsigiannopulos, E.; Buchele, C.; Regnery, S.; Weykamp, F.; Sandrini, E.; Ristau, J.; Liermann, J.; Meixner, E.; Forster, T.; et al. Stereotactic magnetic resonance-guided online adaptive radiotherapy of adrenal metastases combines high ablative doses with optimized sparing of organs at risk. Clin. Transl. Radiat. Oncol. 2022, 39, 100567. [Google Scholar] [CrossRef] [PubMed]
- Crijns, S.P.M.; Kok, J.G.M.; Lagendijk, J.J.W.; Raaymakers, B.W. Towards MRI-guided linear accelerator control: Gating on an MRI accelerator. Phys. Med. Biol. 2011, 56, 4815–4825. [Google Scholar] [CrossRef]
- Ehrbar, S.; Käser, S.B.; Chamberlain, M.; Krayenbühl, J.; Wilke, L.; Mayinger, M.; Schüler, H.G.; Guckenberger, M.; Andratschke, N.; Tanadini-Lang, S. MR-guided beam gating: Residual motion, gating efficiency and dose reconstruction for stereotactic treatments of the liver and lung. Radiother. Oncol. 2022, 174, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, J.; Chen, F.; Hill, G.; Spiegel, J.; Mehta, V. Duty Cycle Selection of Gating in Lung SBRT With Flatting Filter–Free Beams. Int. J. Radiat. Oncol. 2014, 90, S900. [Google Scholar] [CrossRef]
- Klüter, S.; Katayama, S.; Spindeldreier, C.K.; Koerber, S.A.; Major, G.; Alber, M.; Akbaba, S.; Debus, J.; Hörner-Rieber, J. First prospective clinical evaluation of feasibility and patient acceptance of magnetic resonance-guided radiotherapy in Germany. Strahlenther. Und Onkol. 2020, 196, 691–698. [Google Scholar] [CrossRef]
- Placidi, L.; Cusumano, D.; Boldrini, L.; Votta, C.; Pollutri, V.; Antonelli, M.V.; Chiloiro, G.; Romano, A.; De Luca, V.; Catucci, F.; et al. Quantitative analysis of MRI-guided radiotherapy treatment process time for tumor real-time gating efficiency. J. Appl. Clin. Med. Phys. 2020, 21, 70–79. [Google Scholar] [CrossRef]
- Tyagi, N.; Liang, J.; Burleson, S.; Subashi, E.; Scripes, P.G.; Tringale, K.R.; Romesser, P.B.; Reyngold, M.; Crane, C.H. Feasibility of ablative stereotactic body radiation therapy of pancreas cancer patients on a 1.5 Tesla magnetic resonance-linac system using abdominal compression. Phys. Imaging Radiat. Oncol. 2021, 19, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.N.; Cunningham, J.M.; Arch, J.V.; Chetty, I.J.; Parikh, P.J.; Dolan, J.L. Impact of intrafraction motion in pancreatic cancer treatments with MR-guided adaptive radiation therapy. Front. Oncol. 2023, 13, 1298099. [Google Scholar] [CrossRef]
- Uchinami, Y.; Kanehira, T.; Fujita, Y.; Miyamoto, N.; Yokokawa, K.; Koizumi, F.; Shido, M.; Takahashi, S.; Otsuka, M.; Yasuda, K.; et al. Evaluation of short-term gastrointestinal motion and its impact on dosimetric parameters in stereotactic body radiation therapy for pancreatic cancer. Clin. Transl. Radiat. Oncol. 2023, 39, 100576. [Google Scholar] [CrossRef]
- Teoh, S.; George, B.; Owens, R.; Bungay, H.; Maughan, T.; Mukherjee, S. Dosimetric Impact of Gastrointestinal Tract Variability during Online Adaptive MR-Guided Hypofractionated Stereotactic Ablative Radiotherapy (SABR) to the Pancreas. Int. J. Radiat. Oncol. 2022, 114, e599. [Google Scholar] [CrossRef]
- Zhang, Y.; Balter, J.; Dow, J.; Cao, Y.; Lawrence, T.S.; Kashani, R. Development of an abdominal dose accumulation tool and assessments of accumulated dose in gastrointestinal organs. Phys. Med. Biol. 2023, 68, 075004. [Google Scholar] [CrossRef]
- Bernchou, U.; Schytte, T.; Bertelsen, A.; Lorenzen, E.L.; Brink, C.; Mahmood, F. Impact of abdominal compression on intra-fractional motion and delivered dose in magnetic resonance image-guided adaptive radiation ablation of adrenal gland metastases. Phys. Medica 2023, 114, 102682. [Google Scholar] [CrossRef]
- Buchele, C.; Renkamp, C.K.; Regnery, S.; Behnisch, R.; Rippke, C.; Schlüter, F.; Hoegen-Saßmannshausen, P.; Debus, J.; Hörner-Rieber, J.; Alber, M.; et al. Intrafraction organ movement in adaptive MR-guided radiotherapy of abdominal lesions—Dosimetric impact and how to detect its extent in advance. Radiat. Oncol. 2024, 19, 80. [Google Scholar] [CrossRef]
- Diez, P.; Hanna, G.; Aitken, K.; van As, N.; Carver, A.; Colaco, R.; Conibear, J.; Dunne, E.; Eaton, D.; Franks, K.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Bohoudi, O.; Bruynzeel, A.; Senan, S.; Cuijpers, J.; Slotman, B.; Lagerwaard, F.; Palacios, M. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother. Oncol. 2017, 125, 439–444. [Google Scholar] [CrossRef]
- Lyman, J.T.; Wolbarst, A.B. Optimization of radiation therapy, III: A method of assessing complication probabilities from dose-volume histograms. Int. J. Radiat. Oncol. 1987, 13, 103–109. [Google Scholar] [CrossRef]
- Kutcher, G.J.; Burman, C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method gerald. Int. J. Radiat. Oncol. 1989, 16, 1623–1630. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- Nenoff, L.; Sudhyadhom, A.; Lau, J.; Sharp, G.C.; Paganetti, H.; Pursley, J. Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes. Cancers 2023, 15, 4592. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Medica 2007, 23, 115–125. [Google Scholar] [CrossRef]
- Pan, C.; Dawson, L.; McGinn, C.; Lawrence, T.; Haken, R.T. Analysis of radiation-induced gastric and duodenal bleeds using the Lyman-Kutcher-Burman model. Int. J. Radiat. Oncol. 2003, 57, S217–S218. [Google Scholar] [CrossRef]
- Holyoake, D.L.; Aznar, M.; Mukherjee, S.; Partridge, M.; Hawkins, M.A. Modelling duodenum radiotherapy toxicity using cohort dose-volume-histogram data. Radiother. Oncol. 2017, 123, 431–437. [Google Scholar] [CrossRef]
- Murphy, J.D.; Christman-Skieller, C.; Kim, J.; Dieterich, S.; Chang, D.T.; Koong, A.C. A Dosimetric Model of Duodenal Toxicity After Stereotactic Body Radiotherapy for Pancreatic Cancer. Int. J. Radiat. Oncol. 2010, 78, 1420–1426. [Google Scholar] [CrossRef]
- Buergy, D.; Würschmidt, F.; Gkika, E.; Hörner-Rieber, J.; Knippen, S.; Gerum, S.; Balermpas, P.; Henkenberens, C.; Voglhuber, T.; Kornhuber, C.; et al. Stereotactic body radiotherapy of adrenal metastases—A dose-finding study. Int. J. Cancer 2022, 151, 412–421. [Google Scholar] [CrossRef]
- Ugurluer, G.; Schneiders, F.L.; Corradini, S.; Boldrini, L.; Kotecha, R.; Kelly, P.; Portelance, L.; Camilleri, P.; Ben-David, M.A.; Poiset, S.; et al. Factors influencing local control after MR-guided stereotactic body radiotherapy (MRgSBRT) for adrenal metastases. Clin. Transl. Radiat. Oncol. 2024, 46, 100756. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.; Kotecha, R.; Herrera, R.; Kutuk, T.; Fahey, M.; Wuthrick, E.; Grass, G.D.; Hoffe, S.; Frakes, J.; Chuong, M.D.; et al. Multi-institutional experience of MR-guided stereotactic body radiation therapy for adrenal gland metastases. Clin. Transl. Radiat. Oncol. 2024, 45, 100719. [Google Scholar] [CrossRef]
- Michalet, M.; Bettaïeb, O.; Khalfi, S.; Ghorbel, A.; Valdenaire, S.; Debuire, P.; Aillères, N.; Draghici, R.; De Méric De Bellefon, M.; Charissoux, M.; et al. Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results. J. Clin. Med. 2022, 12, 291. [Google Scholar] [CrossRef]
- Hoegen-Saßmannshausen, P.; Jessen, I.; Buchele, C.; Schlüter, F.; Rippke, C.; Renkamp, C.K.; Weykamp, F.; Regnery, S.; Liermann, J.; Meixner, E.; et al. Clinical Outcomes of Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiotherapy of Adrenal Metastases from a Single Institution. Cancers 2024, 16, 2273. [Google Scholar] [CrossRef]
- Schneiders, F.L.; van Vliet, C.; Giraud, N.; Bruynzeel, A.M.; Slotman, B.J.; Palacios, M.A.; Senan, S. Clinical outcomes of MR-guided adrenal stereotactic ablative radiotherapy with preferential sparing of organs at risk. Clin. Transl. Radiat. Oncol. 2023, 43, 100680. [Google Scholar] [CrossRef]
- Purdie, T.G.; Bissonnette, J.-P.; Franks, K.; Bezjak, A.; Payne, D.; Sie, F.; Sharpe, M.B.; Jaffray, D.A. Cone-Beam Computed Tomography for On-Line Image Guidance of Lung Stereotactic Radiotherapy: Localization, Verification, and Intrafraction Tumor Position. Int. J. Radiat. Oncol. 2007, 68, 243–252. [Google Scholar] [CrossRef]
- Keizer, D.d.M.; Kerkmeijer, L.; Willigenburg, T.; van Lier, A.; Hartogh, D.; Zyp, J.v.d.V.v.; Breugel, E.d.G.-V.; Raaymakers, B.; Lagendijk, J.; de Boer, J. Prostate intrafraction motion during the preparation and delivery of MR-guided radiotherapy sessions on a 1.5T MR-Linac. Radiother. Oncol. 2020, 151, 88–94. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Tawk, B.; Knoll, M.; Hoegen-Saßmannshausen, P.; Liermann, J.; Huber, P.E.; Lifferth, M.; Lang, C.; Häring, P.; Gnirs, R.; et al. Clinical Workflow of Cone Beam Computer Tomography-Based Daily Online Adaptive Radiotherapy with Offline Magnetic Resonance Guidance: The Modular Adaptive Radiotherapy System (MARS). Cancers 2024, 16, 1210. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, S.; Weiss, Y.; Chuong, M.D.; Bassiri, N.; Gutierrez, A.N.; Kotecha, R.; Mehta, M.P.; Mittauer, K.E. Case report: Intrafraction dose-guided tracking for gastrointestinal organ-at-risk isotoxicity delivery on an MR-guided radiotherapy system. Front. Oncol. 2024, 14, 1357916. [Google Scholar] [CrossRef]
- Kontaxis, C.; Bol, G.H.; Lagendijk, J.J.W.; Raaymakers, B.W. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys. Med. Biol. 2015, 60, 7485–7497. [Google Scholar] [CrossRef]
- Kontaxis, C.; Bol, G.H.; Stemkens, B.; Glitzner, M.; Prins, F.M.; Kerkmeijer, L.G.W.; Lagendijk, J.J.W.; Raaymakers, B.W. Towards fast online intrafraction replanning for free-breathing stereotactic body radiation therapy with the MR-linac. Phys. Med. Biol. 2017, 62, 7233–7248. [Google Scholar] [CrossRef]

| Age [y], Median (Range) | 63.1 (38.0–81.2) |
|---|---|
| Sex | |
| Female | 9 |
| Male | 14 |
| Primary tumor | |
| NSCLC | 12 |
| SCLC | 3 |
| Melanoma | 4 |
| Renal cell carcinoma | 2 |
| Esophageal cancer | 1 |
| Liposarcoma | 1 |
| Treatment situation | |
| Oligometastasis | 8 |
| Oligoprogression | 15 |
| Laterality | |
| Left | 13 |
| Right | 9 |
| Bilateral | 1 |
| Fractionation/prescription style | |
| 5 × 10.0 Gy/80% isodose | 5 |
| 6 × 7.5 Gy/80% isodose | 2 |
| 8 × 7.5 Gy/80% isodose | 3 |
| 8 × 5.0 Gy/80% isodose | 2 |
| 10 × 5.0 Gy/80% isodose | 9 |
| 12 × 4.0 Gy/homogeneous | 3 |
| Frequency of adaptation | |
| Total fractions prescribed | 203 |
| Total fractions treated | 202 |
| Adapted | 198 |
| Non-adapted | 4 |
| Not deemed necessary | 2 |
| MR-linac maintenance | 2 |
| GTV size [cc], Median (Range) | 37.2 (9.9–292.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoegen-Saßmannshausen, P.; Hartschuh, T.P.; Renkamp, C.K.; Buchele, C.; Schlüter, F.; Sandrini, E.; Weykamp, F.; Regnery, S.; Meixner, E.; König, L.; et al. Intrafractional Motion in Online-Adaptive Magnetic Resonance-Guided Radiotherapy of Adrenal Metastases Leads to Reduced Target Volume Coverage and Elevated Organ-at-Risk Doses. Cancers 2025, 17, 1533. https://doi.org/10.3390/cancers17091533
Hoegen-Saßmannshausen P, Hartschuh TP, Renkamp CK, Buchele C, Schlüter F, Sandrini E, Weykamp F, Regnery S, Meixner E, König L, et al. Intrafractional Motion in Online-Adaptive Magnetic Resonance-Guided Radiotherapy of Adrenal Metastases Leads to Reduced Target Volume Coverage and Elevated Organ-at-Risk Doses. Cancers. 2025; 17(9):1533. https://doi.org/10.3390/cancers17091533
Chicago/Turabian StyleHoegen-Saßmannshausen, Philipp, Tobias P. Hartschuh, Claudia Katharina Renkamp, Carolin Buchele, Fabian Schlüter, Elisabetta Sandrini, Fabian Weykamp, Sebastian Regnery, Eva Meixner, Laila König, and et al. 2025. "Intrafractional Motion in Online-Adaptive Magnetic Resonance-Guided Radiotherapy of Adrenal Metastases Leads to Reduced Target Volume Coverage and Elevated Organ-at-Risk Doses" Cancers 17, no. 9: 1533. https://doi.org/10.3390/cancers17091533
APA StyleHoegen-Saßmannshausen, P., Hartschuh, T. P., Renkamp, C. K., Buchele, C., Schlüter, F., Sandrini, E., Weykamp, F., Regnery, S., Meixner, E., König, L., Debus, J., Klüter, S., & Hörner-Rieber, J. (2025). Intrafractional Motion in Online-Adaptive Magnetic Resonance-Guided Radiotherapy of Adrenal Metastases Leads to Reduced Target Volume Coverage and Elevated Organ-at-Risk Doses. Cancers, 17(9), 1533. https://doi.org/10.3390/cancers17091533









