RAS-Beppu Classification: A New Recurrence Risk Classification System Incorporating the Beppu Score and RAS Status for Colorectal Liver Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Treatment Strategy for CRLM
2.3. Assessment of RAS Status
2.4. Beppu Classification
2.5. RAS-Beppu Classification
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Clinical Outcomes According to the Beppu and RAS-Beppu Classification Systems
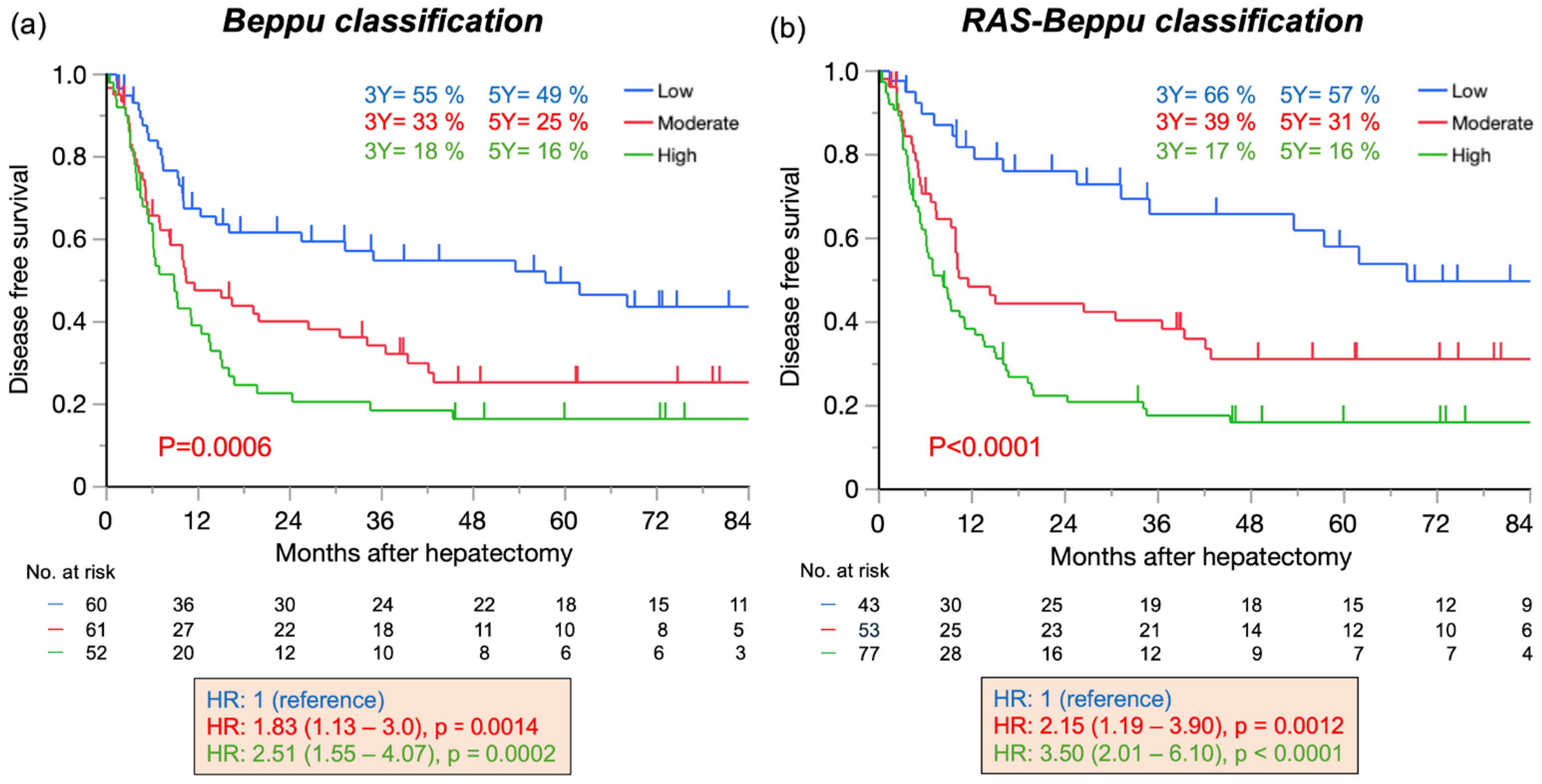
3.2. Disease-Free Survival According to the Beppu and RAS-Beppu Classifications
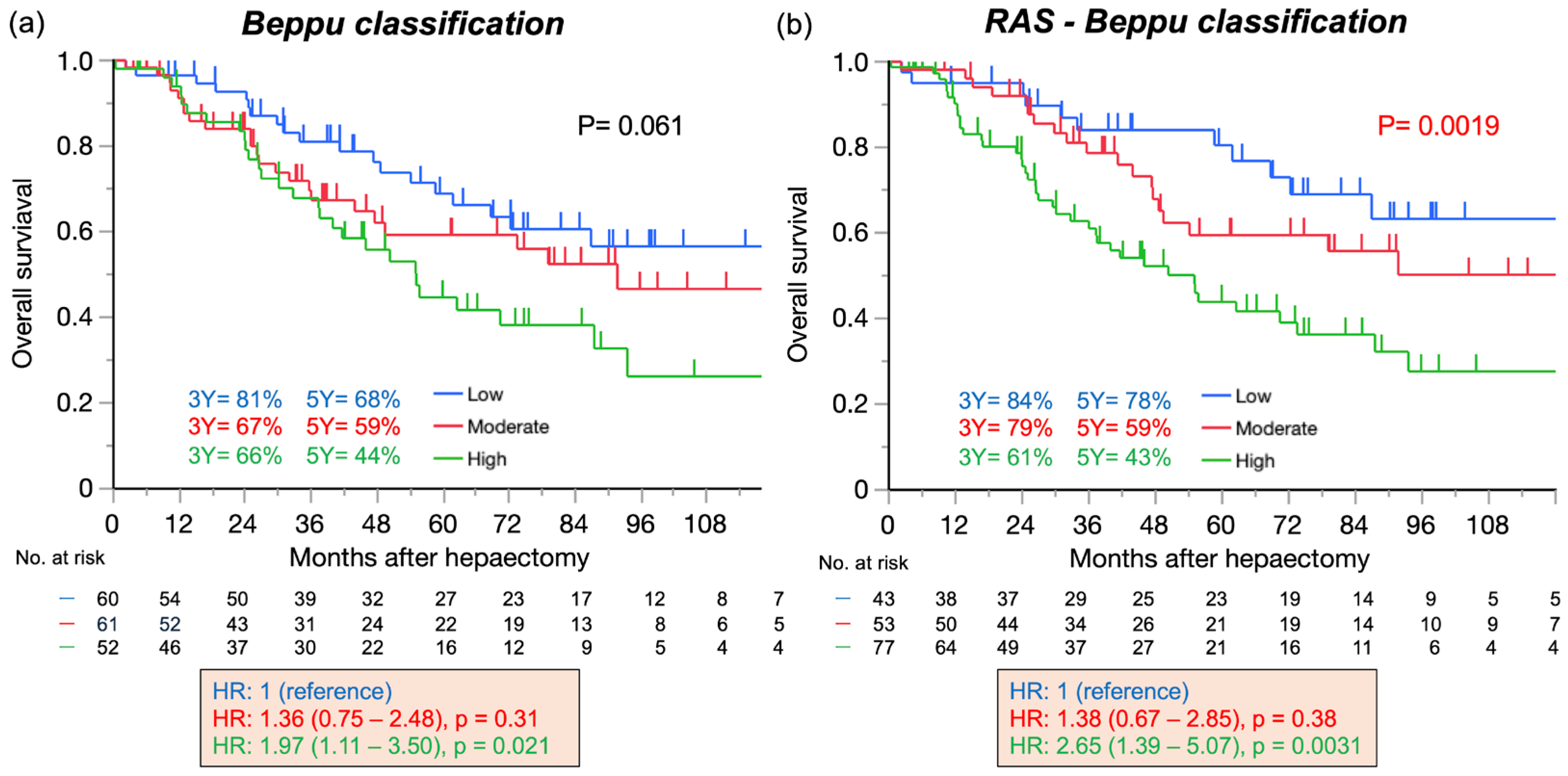
3.3. Overall Survival According to the Beppu and RAS-Beppu Classifications
3.4. Disease-Free Survival According to the Genetic and Morphological Evaluation (GAME) Score and the Modified Clinical Risk Score (CRS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsilimigras, D.I.; Hyer, J.M.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Poultsides, G.; Sasaki, K.; Aucejo, F.; Pawlik, T.M. Resection of Colorectal Liver Metastasis: Prognostic Impact of Tumor Burden vs KRAS Mutational Status. J. Am. Coll. Surg. 2021, 232, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Horino, T.; Tokunaga, R.; Miyamoto, Y.; Hiyoshi, Y.; Akiyama, T.; Daitoku, N.; Sakamoto, Y.; Yoshida, N.; Baba, H. The impact of the advanced lung cancer inflammation index on the outcomes of patients with metastatic colorectal cancer who receive chemotherapy. Int. J. Clin. Oncol. 2023, 28, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ignatavicius, P.; Oberkofler, C.E.; Chapman, W.C.; DeMatteo, R.P.; Clary, B.M.; D’Angelica, M.I.; Tanabe, K.K.; Hong, J.C.; Aloia, T.A.; Pawlik, T.M.; et al. Choices of Therapeutic Strategies for Colorectal Liver Metastases Among Expert Liver Surgeons: A Throw of the Dice? Ann. Surg. 2020, 272, 715–722. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D.; Association Française de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Nagashima, I.; Takada, T.; Adachi, M.; Nagawa, H.; Muto, T.; Okinaga, K. Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: Comparison of our scoring system to the positive number of risk factors. World J. Gastroenterol. 2006, 12, 6305–6309. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.; Donohue, J.H.; Que, F.G.; Farnell, M.B.; Schleck, C.D.; Ilstrup, D.M.; Nagorney, D.M. Hepatic resection for colorectal metastases: Value for risk scoring systems? Ann. Surg. 2007, 246, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Konopke, R.; Kersting, S.; Distler, M.; Dietrich, J.; Gastmeier, J.; Heller, A.; Kulisch, E.; Saeger, H. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009, 29, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Tez, M.; Tez, S. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann. Surg. 2008, 247, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Kato, T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J. Surg. 2008, 32, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Sakamoto, Y.; Hasegawa, K.; Honda, G.; Tanaka, K.; Kotera, Y.; Nitta, H.; Yoshidome, H.; Hatano, E.; Ueno, M.; et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: Multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2012, 19, 72–84. [Google Scholar] [CrossRef]
- Beppu, T.; Yamamura, K.; Sakamoto, K.; Honda, G.; Kobayashi, S.; Endo, I.; Hasegawa, K.; Kotake, K.; Itabashi, M.; Hashiguchi, Y.; et al. Validation study of the JSHBPS nomogram for patients with colorectal liver metastases who underwent hepatic resection in the recent era—A nationwide survey in Japan. J. Hepatobiliary Pancreat. Sci. 2023, 30, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Yamamura, K.; Imai, K.; Hayashi, H.; Miyamoto, Y. Recurrence-risk stratification using the Beppu score and selection of perioperative chemotherapy for colorectal liver metastases. J. Surg. Oncol. 2024, 129, 893–900. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Zimmitti, G.; Kopetz, S.E.; Shindoh, J.; Chen, S.S.; Andreou, A.; Curley, S.A.; Aloia, T.A.; Maru, D.M. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann. Surg. 2013, 258, 619–626; discussion 626–627. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Gholami, S.; Kim, Y.; Andreatos, N.; Rezaee, N.; Deshwar, A.; Buettner, S.; Allen, P.J.; Kingham, T.P.; et al. Genetic and Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br. J. Surg. 2018, 105, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef]
- Buisman, F.E.; Giardiello, D.; Kemeny, N.E.; Steyerberg, E.W.; Höppener, D.J.; Galjart, B.; Nierop, P.M.; Balachandran, V.P.; Cercek, A.; Drebin, J.A.; et al. Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur. J. Cancer 2022, 168, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Mehta, R.; Tsilimigras, D.I.; Sahara, K.; Paredes, A.Z.; Bagante, F.; Guglielmi, A.; Alexandrescu, S.; Poultsides, G.A.; Sasaki, K.; et al. Prognostic factors differ according to KRAS mutational status: A classification and regression tree model to define prognostic groups after hepatectomy for colorectal liver metastasis. Surgery 2020, 168, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Takematsu, T.; Mima, K.; Hayashi, H.; Kitano, Y.; Nakagawa, S.; Hiyoshi, Y.; Okabe, H.; Imai, K.; Miyamoto, Y.; Baba, H. RAS mutation status in combination with the JSHBPS nomogram may be useful for preoperative identification of colorectal liver metastases with high risk of recurrence and mortality after hepatectomy. J. Hepatobiliary Pancreat. Sci. 2023, 31, 69–79. [Google Scholar] [PubMed]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789–3799. [Google Scholar]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Serviddio, G.; Vendemiale, G.; Muscatiello, N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J. Gastroenterol. 2016, 22, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Hasegawa, K.; Matsuyama, Y.; Shindoh, J.; Mise, Y.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Discrepancy between recurrence-free survival and overall survival in patients with resectable colorectal liver metastases: A potential surrogate endpoint for time to surgical failure. Ann. Surg. Oncol. 2014, 21, 1817–1824. [Google Scholar] [CrossRef]
- Adams, R.B.; Aloia, T.A.; Loyer, E.; Pawlik, T.M.; Taouli, B.; Vauthey, J.N. Selection for hepatic resection of colorectal liver metastases: Expert consensus statement. HPB 2013, 15, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Kitano, Y.; Hayashi, H.; Matsumoto, T.; Kinoshita, S.; Sato, H.; Shiraishi, Y.; Nakao, Y.; Kaida, T.; Imai, K.; Yamashita, Y.-I.; et al. Borderline resectable for colorectal liver metastases: Present status and future perspective. World J. Gastrointest. Surg. 2021, 13, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, T.; Nara, S.; Ban, D.; Mizui, T.; Murase, Y.; Esaki, M.; Shimada, K.; Hashimoto, T.; Makuuchi, M. Objective Definition and Optimized Strategy for “Biologically Borderline Resectable” Colorectal Liver Metastases. World J. Surg. 2023, 47, 2834–2845. [Google Scholar] [CrossRef]
- Maki, H.; Jain, A.J.; Haddad, A.; Lendoire, M.; Chun, Y.S.; Vauthey, J.N. Locoregional treatment for colorectal liver metastases aiming for precision medicine. Ann. Gastroenterol. Surg. 2023, 7, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.K.; Wu, H.-T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients with Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Newhook, T.E.; Overman, M.J.; Chun, Y.S.; Dasari, A.; Tzeng, C.D.; Cao, H.S.T.; Raymond, V.; Parseghian, C.; Johnson, B.; Nishioka, Y.; et al. Prospective Study of Perioperative Circulating Tumor DNA Dynamics in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann. Surg. 2023, 277, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Øgaard, N.; Reinert, T.; Henriksen, T.V.; Frydendahl, A.; Aagaard, E.; Ørntoft, M.W.; Larsen, M.Ø.; Knudsen, A.R.; Mortensen, F.V.; Andersen, C.L. Tumour-agnostic circulating tumour DNA analysis for improved recurrence surveillance after resection of colorectal liver metastases: A prospective cohort study. Eur. J. Cancer 2022, 163, 163–176. [Google Scholar] [CrossRef] [PubMed]

| N = 173 | |
|---|---|
| Median age (range) | 66 (25–94) |
| Gender: M/F | 60/113 |
| Median BMI (range) | 22.7 (14.9–32.5) |
| Primary tumor sight: Right/Left | 40/133 |
| Primary tumor: T 0–2/3–4 | 19/151 |
| Distribution of liver metastasis: Unilobar/Bilobar | 118/54 |
| Median CEA at diagnosis (mg/dL) (range) | 9.5 (0.5–3377) |
| RAS mutations (%) | 64 (37) |
| Median Beppu score (range) | 7 (0–21) |
| Chemotherapy (%) | 115 (66) |
| Preoperative | 72 (42) |
| Adjuvant | 75 (44) |
| Operative procedures: Non-AR/AR | 109/64 |
| Laparoscopic surgery (%) | 55 (32) |
| TSH or preoperative PVE (%) | 5 (3) |
| Radiofrequency ablation (%) | 22 (12.7) |
| Median operation time (min) (range) | 428 (90–1222) |
| Median intraoperative blood loss (mL) (range) | 342 (0–4057) |
| Transfusion of RBC (%) | 12 (7) |
| Clavien-Dindo class ≥ III (%) | 29 (17) |
| Resection status: R0/R1 | 121/26 |
| DFS | Beppu Classification | RAS-Beppu Classification | ||||
|---|---|---|---|---|---|---|
| Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value | |
| RAS: mutant/wild | 2.25 (1.54–3.29) | <0.0001 | 2.35 (1.54–3.59) | <0.0001 | ||
| Preoperative chemotherapy | 1.63 (1.12–2.38) | 0.010 | 1.08 (0.67–1.72) | 0.76 | 1.02 (0.66–1.60) | 0.92 |
| Resection margin: R1/R0 | 1.76 (1.05–2.96) | 0.033 | 1.66 (0.97–2.85) | 0.067 | 1.43 (0.83–2.45) | 0.20 |
| Beppu classification | 0.0009 | 0.017 | ||||
| Moderate/Low | 1.83 | 0.014 | 1.41 | 0.21 | ||
| (1.13–3.0) | (0.82–2.46) | |||||
| High/Low | 2.51 | 0.0002 | 2.28 | 0.005 | ||
| (1.55–4.07) | (1.28–4.05) | |||||
| High/Moderate | 1.37 | 0.155 | 1.61 | 0.064 | ||
| (0.89–2.11) | (0.97–2.67) | |||||
| RAS-Beppu classification | <0.0001 | 0.0002 | ||||
| Moderate/Low | 2.15 | 0.012 | 2.22 | 0.015 | ||
| (1.19–3.90) | (1.17–4.22) | |||||
| High/Low | 3.50 | <0.0001 | 3.78 | <0.0001 | ||
| (2.00–6.10) | (2.00–7.12) | |||||
| High/Moderate | 1.62 | 0.025 | 1.70 | 0.030 | ||
| (1.06–2.48) | (1.05–2.74) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajiri, T.; Mima, K.; Beppu, T.; Hayashi, H.; Horino, T.; Adachi, Y.; Imai, K.; Masuda, T.; Miyamoto, Y.; Iwatsuki, M. RAS-Beppu Classification: A New Recurrence Risk Classification System Incorporating the Beppu Score and RAS Status for Colorectal Liver Metastases. Cancers 2025, 17, 640. https://doi.org/10.3390/cancers17040640
Tajiri T, Mima K, Beppu T, Hayashi H, Horino T, Adachi Y, Imai K, Masuda T, Miyamoto Y, Iwatsuki M. RAS-Beppu Classification: A New Recurrence Risk Classification System Incorporating the Beppu Score and RAS Status for Colorectal Liver Metastases. Cancers. 2025; 17(4):640. https://doi.org/10.3390/cancers17040640
Chicago/Turabian StyleTajiri, Takuya, Kosuke Mima, Toru Beppu, Hiromitsu Hayashi, Taichi Horino, Yuki Adachi, Katsunori Imai, Toshiro Masuda, Yuji Miyamoto, and Masaaki Iwatsuki. 2025. "RAS-Beppu Classification: A New Recurrence Risk Classification System Incorporating the Beppu Score and RAS Status for Colorectal Liver Metastases" Cancers 17, no. 4: 640. https://doi.org/10.3390/cancers17040640
APA StyleTajiri, T., Mima, K., Beppu, T., Hayashi, H., Horino, T., Adachi, Y., Imai, K., Masuda, T., Miyamoto, Y., & Iwatsuki, M. (2025). RAS-Beppu Classification: A New Recurrence Risk Classification System Incorporating the Beppu Score and RAS Status for Colorectal Liver Metastases. Cancers, 17(4), 640. https://doi.org/10.3390/cancers17040640







