Histotripsy of Liver Tumors: Patient Selection, Ethical Discussions, and How We Do It
Simple Summary
Abstract
1. Introduction
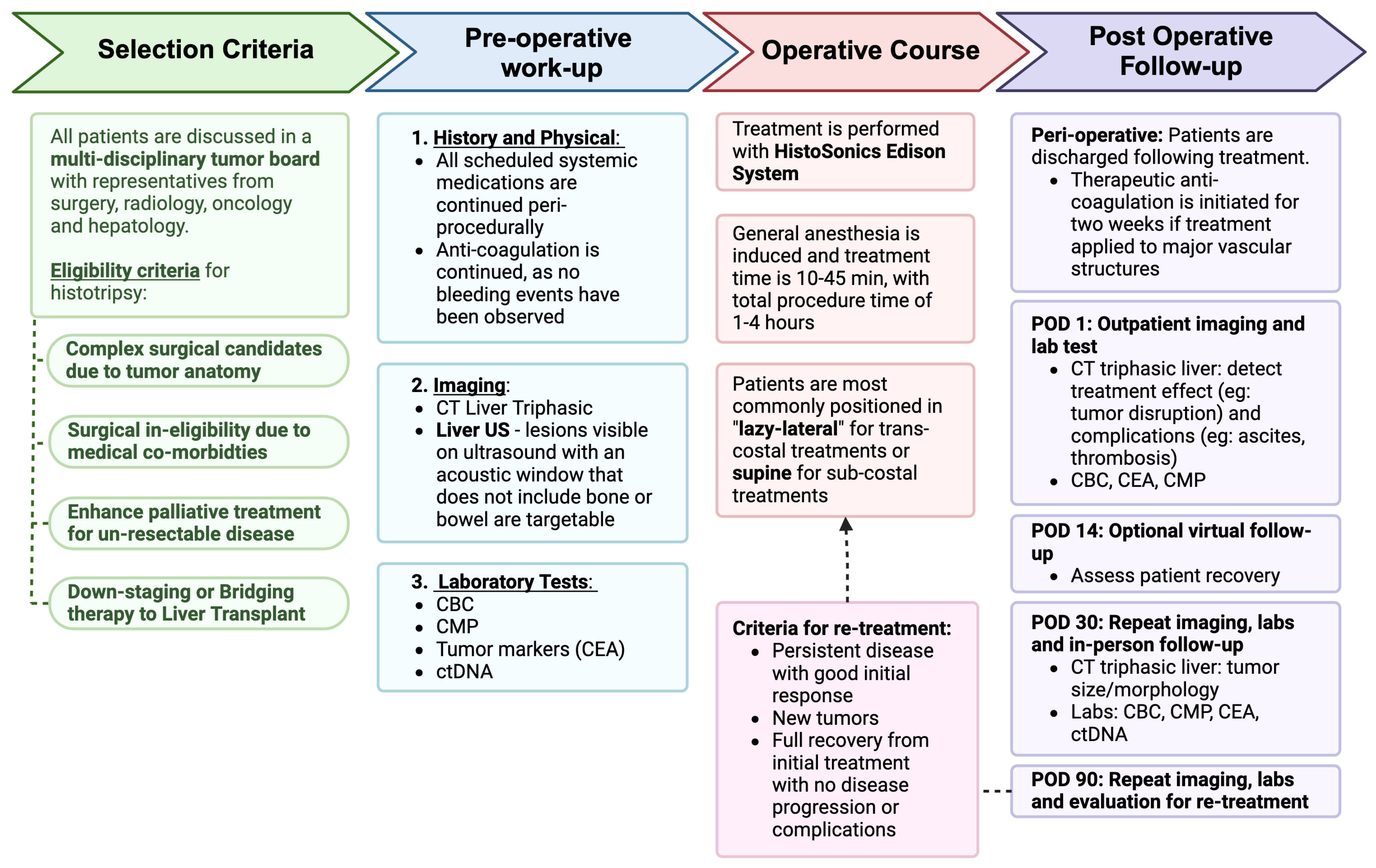
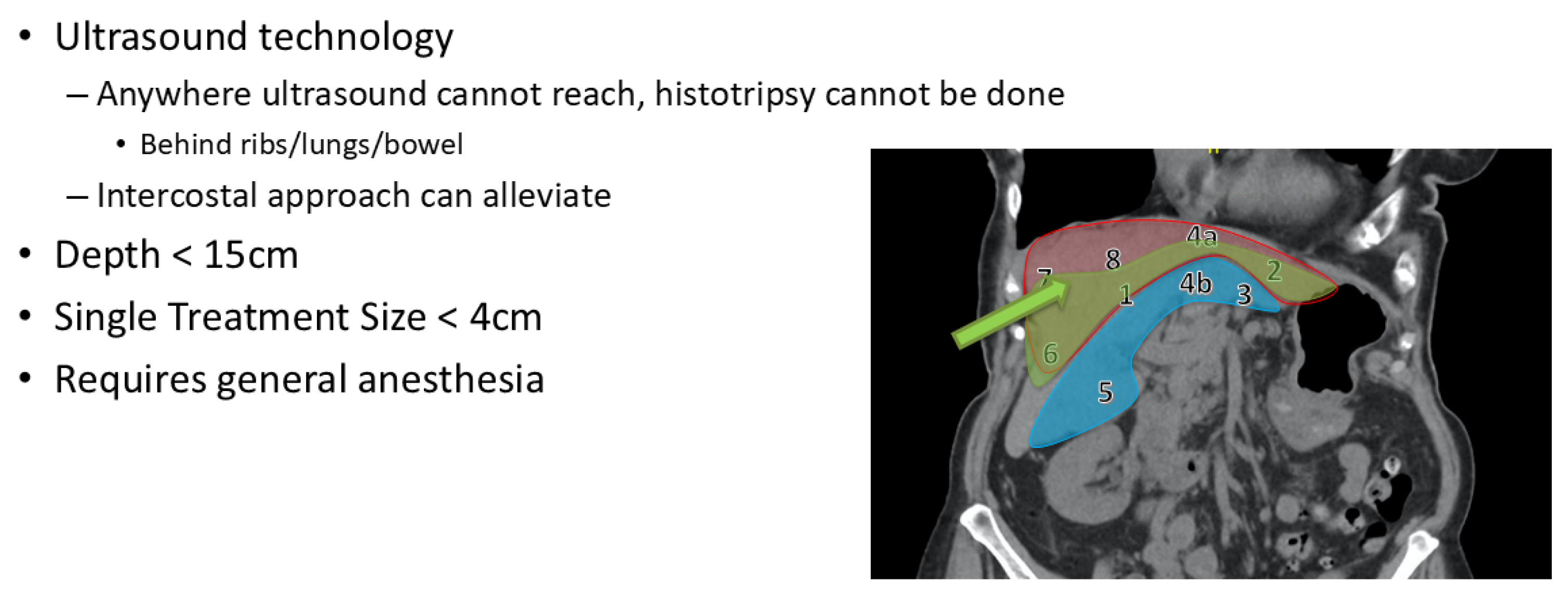
2. Patient Selection
3. How We Do It
3.1. Pre-Treatment Protocol
3.1.1. Laboratory Tests
3.1.2. Circulating Tumor DNA (ctDNA)
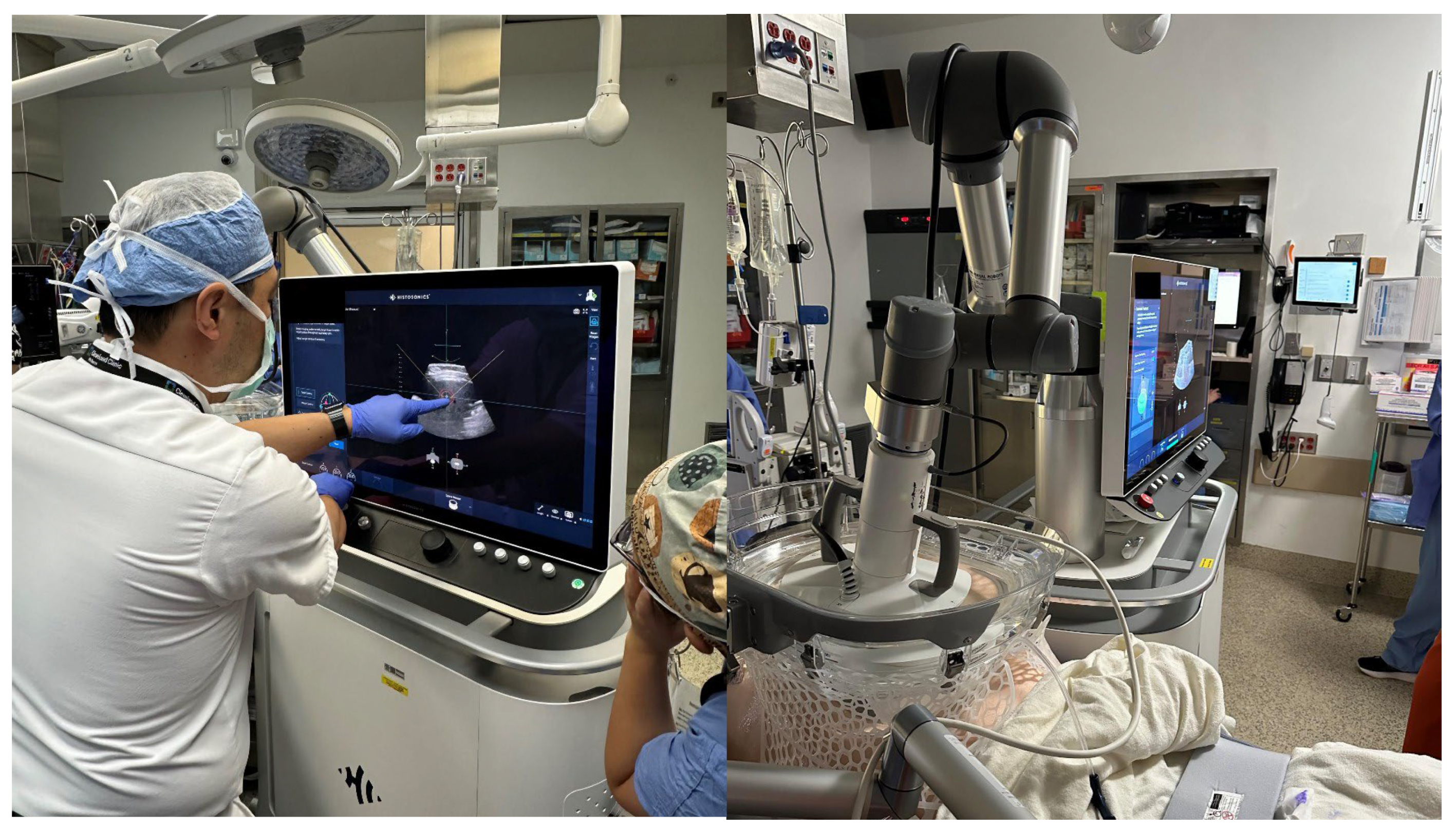
3.2. Procedure
3.3. Post-Treatment Follow-Up
3.3.1. POD 1
3.3.2. POD 14
3.3.3. POD 30
3.3.4. POD 90
3.4. Management of Concomitant Therapies
4. Discussion and Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Canc Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Angeli-Pahim, I.; Chambers, A.; Duarte, S.; Zarrinpar, A. Current Trends in Surgical Management of Hepatocellular Carcinoma. Cancers 2023, 15, 5378. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, Z.-Y.; Yan, X.-L.; Yang, W.; Yan, K.; Xing, B.-C. Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: A propensity score analysis. World J. Surg. Oncol. 2018, 16, 207. [Google Scholar] [CrossRef]
- Solheim, J.M.; Dueland, S.; Line, P.D.; Hagness, M. Transplantation for Nonresectable Colorectal Liver Metastases: Long-Term Follow-Up of the First Prospective Pilot Study. Ann. Surg. 2023, 278, 239–245. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Anders, R.; Are, C.; Brown, D.; Chang, D.T.; et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J. Natl. Compr. Canc Netw. 2019, 17, 302–310. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Canc Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef]
- Crocetti, L.; Scalise, P.; Bozzi, E.; Candita, G.; Cioni, R. Thermal ablation of hepatocellular carcinoma. J. Med. Imaging Radiat. Oncol. 2023, 67, 817–831. [Google Scholar] [CrossRef]
- Song, J.; Cao, L.; Ma, K.; Li, J.; Wang, X.; Chen, J.; Zheng, S. Laparoscopic liver resection versus radiofrequency ablation for small hepatocellular carcinoma: Randomized clinical trial. Br. J. Surg. 2024, 111, znae099. [Google Scholar] [CrossRef]
- Tinguely, P.; Ruiter, S.J.S.; Engstrand, J.; de Haas, R.J.; Nilsson, H.; Candinas, D.; de Jong, K.P.; Freedman, J. A prospective multicentre trial on survival after Microwave Ablation VErsus Resection for Resectable Colorectal liver metastases (MAVERRIC). Eur. J. Cancer 2023, 187, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc. Interv. Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Solbiati, L.; Meloni, M.F.; Gazelle, G.S.; Halpern, E.F.; Goldberg, S.N. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003, 226, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, Y.; Yu, X.; Dong, B. Malignant liver tumors: Treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009, 251, 933–940. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.C.; Maybody, M.; Brody, L.A.; et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef]
- Sapisochin, G.; Barry, A.; Doherty, M.; Fischer, S.; Goldaracena, N.; Rosales, R.; Russo, M.; Beecroft, R.; Ghanekar, A.; Bhat, M.; et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol. 2017, 67, 92–99. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef]
- Liang, P.; Yu, J.; Yu, X.L.; Wang, X.H.; Wei, Q.; Yu, S.Y.; Li, H.X.; Sun, H.T.; Zhang, Z.X.; Liu, H.C.; et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: A multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut 2012, 61, 1100–1101. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.H.; Choi, B.I.; de Baère, T.; Dodd, G.D., 3rd; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—A 10-year update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Worlikar, T.; Zhang, M.; Ganguly, A.; Hall, T.L.; Shi, J.; Zhao, L.; Lee, F.T.; Mendiratta-Lala, M.; Cho, C.S.; Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers 2022, 14, 1612. [Google Scholar] [CrossRef]
- Worlikar, T.; Hall, T.; Zhang, M.; Mendiratta-Lala, M.; Green, M.; Cho, C.S.; Xu, Z. Insights from in vivo preclinical cancer studies with histotripsy. Int. J. Hyperth. 2024, 41, 2297650. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-man histotripsy of hepatic tumors: The THERESA trial, a feasibility study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Lei, S.v.d.; Dijkstra, M.; Versteeg, K.S.; Buffart, T.E.; Lissenberg-Witte, B.I.; Swijnenburg, R.-J.; Tol, M.P.v.d.; Puijk, R.S.; Group, C.T.C. Surgery versus thermal ablation for small-size colorectal liver metastases (COLLISION): An international, multicenter, phase III randomized controlled trial. J. Clin. Oncol. 2024, 42, LBA3501. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Burns, K.; Ong, E.; Couillard, A.; Parikh, N.D.; Caoili, E.; Kim, J.; Aucejo, F.; Schlegel, A.; Knott, E.; et al. The First International Experience with Histotripsy: A Safety Analysis of 230 Cases. J. Gastrointest. Surg. 2025, 29, 102000. [Google Scholar] [CrossRef]
- Uysal, M.; Wehrle, C.J.; Coppa, C.; Kamath, S.; Krishnamurthi, S.; Martin, C.; Hag, M.E.; Khalil, M.; Fujiki, M.; Schlegel, A.; et al. Bridging therapy with histotripsy prior to liver transplantation for hepatocellular carcinoma: A first case report. Exp. Hematol. Oncol. 2025, 14, 20. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Kim, Y.; Owens, G.; Roberts, W.; Cain, C.; Xu, Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys. Med. Biol. 2014, 59, 253–270. [Google Scholar] [CrossRef]
- Pepple, A.L.; Guy, J.L.; McGinnis, R.; Felsted, A.E.; Song, B.; Hubbard, R.; Worlikar, T.; Garavaglia, H.; Dib, J.; Chao, H.; et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front. Immunol. 2023, 14, 1012799. [Google Scholar] [CrossRef]
- Hong, H.; Wehrle, C.J.; Zhang, M.; Fares, S.; Stitzel, H.; Garib, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Ma, W.W.; et al. Circulating Tumor DNA Profiling in Liver Transplant for Hepatocellular Carcinoma, Cholangiocarcinoma, and Colorectal Liver Metastases: A Programmatic Proof of Concept. Cancers 2024, 16, 927. [Google Scholar] [CrossRef]
- Raj, R.; Wehrle, C.J.; Aykun, N.; Stitzel, H.; Ma, W.W.; Krishnamurthi, S.; Estfan, B.; Kamath, S.; Kwon, D.C.H.; Aucejo, F. Immunotherapy Plus Locoregional Therapy Leading to Curative-Intent Hepatectomy in HCC: Proof of Concept Producing Durable Survival Benefits Detectable with Liquid Biopsy. Cancers 2023, 15, 5220. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Kwon, D.C.H.; Miller, C.; Walsh, R.M.; Aucejo, F. Tumor Mutational Burden from Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma After Resection: An Emerging Biomarker for Surveillance. Ann. Surg. 2024, 280, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Fujiki, M.; Hashimoto, K.; Quintini, C.; et al. Liquid Biopsy by ctDNA in Liver Transplantation for Colorectal Cancer Liver Metastasis. J. Gastrointest. Surg. 2023, 27, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Stackhouse, K.; Chang, J.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Walsh, R.M.; et al. Circulating Tumor DNA in Colorectal Cancer Liver Metastasis: Analysis of Patients Receiving Liver Resection and Transplant. JCO Clin. Cancer Inform. 2023, 7, e2300111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Fang, C.; Cortis, K.; Yusuf, G.T.; Gregory, S.; Lewis, D.; Kane, P.; Peddu, P. Complications from percutaneous microwave ablation of liver tumours: A pictorial review. Br. J. Radiol. 2019, 92, 20180864. [Google Scholar] [CrossRef]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef]
- Zheng, R.Q.; Kudo, M.; Inui, K.; Suetomi, Y.; Minami, Y.; Chung, H.; Kawasaki, T. Transient portal vein thrombosis caused by radiofrequency ablation for hepatocellular carcinoma. J. Gastroenterol. 2003, 38, 101–103. [Google Scholar] [CrossRef]
- Vogl, T.J.; Freichel, J.; Gruber-Rouh, T.; Nour-Eldin, N.A.; Bechstein, W.O.; Zeuzem, S.; Naguib, N.N.N.; Stefenelli, U.; Adwan, H. Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years. Cancers 2024, 16, 1756. [Google Scholar] [CrossRef]
- Puls, R.; Langner, S.; Rosenberg, C.; Hegenscheid, K.; Kuehn, J.P.; Noeckler, K.; Hosten, N. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J. Vasc. Interv. Radiol. 2009, 20, 225–234. [Google Scholar] [CrossRef]
- Lee, H.; Heo, J.S.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; Choi, S.H.; Choi, D.W. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: A propensity score analysis. World J. Gastroenterol. 2015, 21, 3300–3307. [Google Scholar] [CrossRef]
- Hong, K.; McBride, J.D.; Georgiades, C.S.; Reyes, D.K.; Herman, J.M.; Kamel, I.R.; Geschwind, J.F. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: Comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2009, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Groeschl, R.T.; Pilgrim, C.H.; Hanna, E.M.; Simo, K.A.; Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A.; Bloomston, M.; Schmidt, C.; et al. Microwave ablation for hepatic malignancies: A multiinstitutional analysis. Ann. Surg. 2014, 259, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Kiefer, M.V.; Sun, W.; Haller, D.; Fraker, D.L.; Tuite, C.M.; Stavropoulos, S.W.; Mondschein, J.I.; Soulen, M.C. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2011, 117, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Hong, H.; Gross, A.; Liu, Q.; Ali, K.; Cazzaniga, B.; Miyazaki, Y.; Tuul, M.; Modaresi Esfeh, J.; Khalil, M.; et al. The impact of normothermic machine perfusion and acuity circles on waitlist time, mortality, and cost in liver transplantation: A multicenter experience. Liver Transpl. 2025, 31, 438–449. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Zhang, M.; Khalil, M.; Pita, A.; Modaresi Esfeh, J.; Diago-Uso, T.; Kim, J.; Aucejo, F.; Kwon, D.C.H.; Ali, K.; et al. Impact of Back-to-Base Normothermic Machine Perfusion on Complications and Costs: A Multicenter, Real-World Risk-Matched Analysis. Ann. Surg. 2024, 280, 300–310. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Kusakabe, J.; Akabane, M.; Maspero, M.; Zervos, B.; Modaresi Esfeh, J.; Whitsett Linganna, M.; Imaoka, Y.; Khalil, M.; Pita, A.; et al. Expanding Selection Criteria in Deceased Donor Liver Transplantation for Hepatocellular Carcinoma: Long-term Follow-up of a National Registry and 2 Transplant Centers. Transplantation 2024, 108, 2386–2395. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Raj, R.; Maspero, M.; Satish, S.; Eghtesad, B.; Pita, A.; Kim, J.; Khalil, M.; Calderon, E.; Orabi, D.; et al. Risk assessment in liver transplantation for hepatocellular carcinoma: Long-term follow-up of a two-centre experience. Int. J. Surg. 2024, 110, 2818–2831. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2024, 22, e240029. [Google Scholar] [CrossRef]
- Dopazo, C.; Søreide, K.; Rangelova, E.; Mieog, S.; Carrion-Alvarez, L.; Diaz-Nieto, R.; Primavesi, F.; Stättner, S. Hepatocellular carcinoma. Eur. J. Surg. Oncol. 2024, 50, 107313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uysal, M.; Wehrle, C.J.; Satish, S.; Knott, E.; Hong, H.; Allkushi, E.; Schlegel, A.; Berber, E.; Aucejo, F.; Kim, J.; et al. Histotripsy of Liver Tumors: Patient Selection, Ethical Discussions, and How We Do It. Cancers 2025, 17, 1100. https://doi.org/10.3390/cancers17071100
Uysal M, Wehrle CJ, Satish S, Knott E, Hong H, Allkushi E, Schlegel A, Berber E, Aucejo F, Kim J, et al. Histotripsy of Liver Tumors: Patient Selection, Ethical Discussions, and How We Do It. Cancers. 2025; 17(7):1100. https://doi.org/10.3390/cancers17071100
Chicago/Turabian StyleUysal, Melis, Chase J. Wehrle, Sangeeta Satish, Emily Knott, Hanna Hong, Erlind Allkushi, Andrea Schlegel, Eren Berber, Federico Aucejo, JaeKeun Kim, and et al. 2025. "Histotripsy of Liver Tumors: Patient Selection, Ethical Discussions, and How We Do It" Cancers 17, no. 7: 1100. https://doi.org/10.3390/cancers17071100
APA StyleUysal, M., Wehrle, C. J., Satish, S., Knott, E., Hong, H., Allkushi, E., Schlegel, A., Berber, E., Aucejo, F., Kim, J., & Kwon, D. C. H. (2025). Histotripsy of Liver Tumors: Patient Selection, Ethical Discussions, and How We Do It. Cancers, 17(7), 1100. https://doi.org/10.3390/cancers17071100





