Preclinical Assessment of Dactinomycin in KMT2A-Rearranged Infant Acute Lymphoblastic Leukemia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Drug and Sensitivity Testing
2.3. Assessment of In Vivo Efficacy
2.4. Statistical Analyses
3. Results
3.1. Anti-Neoplastic Drug Screen Identifies Activity of Dactinomycin in Infant ALL
3.2. In Vitro Assessment of Dactinomycin in Infant ALL
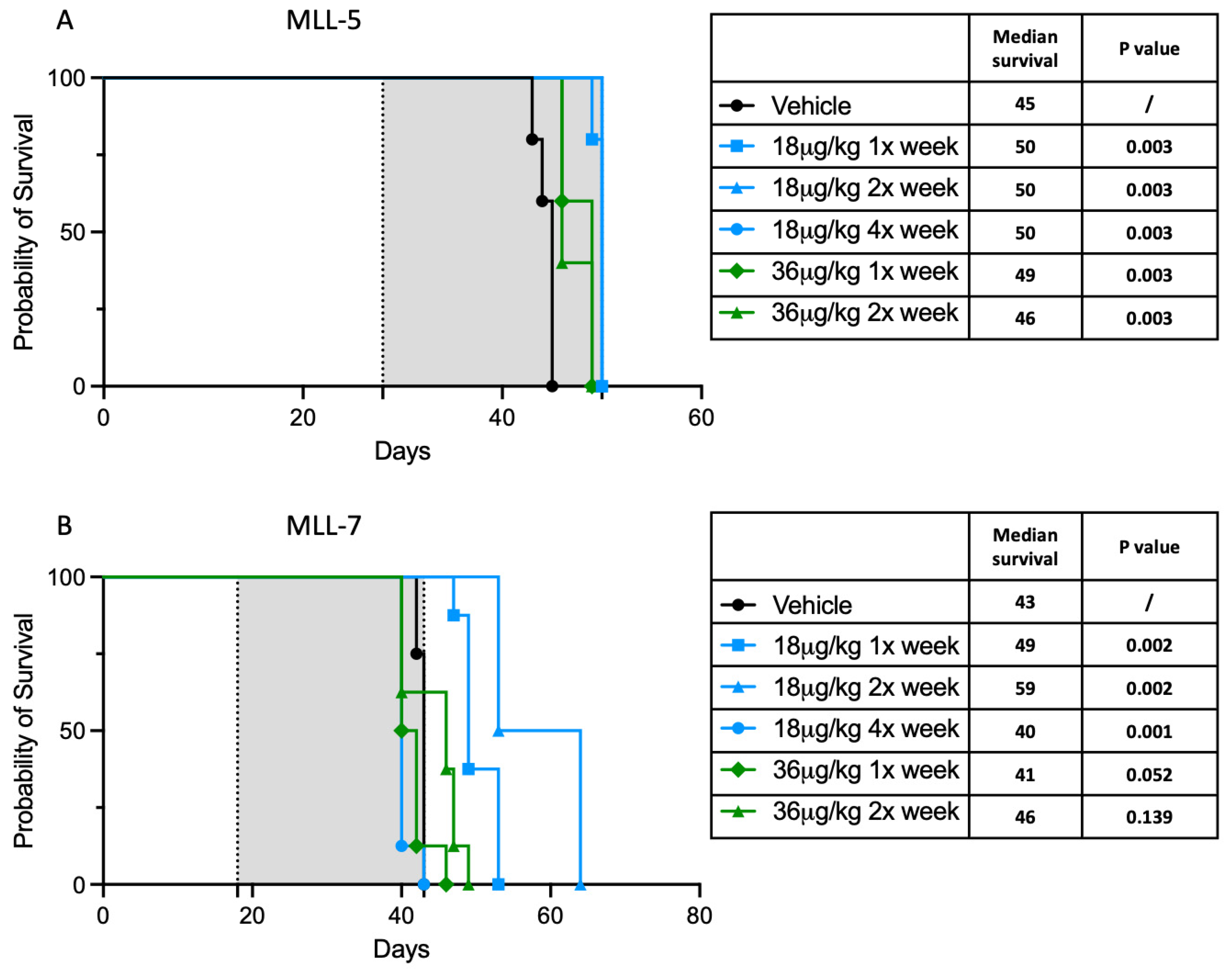
3.3. Dactinomycin Has a Minimal In Vivo Survival Benefit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| EFS | Event-free survival |
| FCS | Fetal calf serum |
| FDA | Food and Drug Administration |
| iALL | Infant acute lymphoblastic leukemia |
| IC50 | Half-maximal inhibitory concentration |
| JAK | Janus kinase |
| KMT2A | Lysine methyltransferase 2A |
| MTD | Maximum tolerated dose |
| NOD/SCID | Nonobese diabetic/severe combined immunodeficiency |
| NPM1 | Nucleophosmin1 |
| PBS | Phosphate buffered saline |
| PDX | Patient-derived xenograft |
References
- National Cancer Institute. NCCR*Explorer: An Interactive Website for NCCR Cancer Statistics. Available online: https://nccrexplorer.ccdi.cancer.gov (accessed on 10 September 2024).
- Hunger, S.P.; Mullighan, C.G. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef]
- Kotecha, R.S. Updates in infant acute lymphoblastic leukemia and the potential for targeted therapy. Hematology 2022, 2022, 611–617. [Google Scholar] [CrossRef]
- Kotecha, R.S.; Pieters, R.; Stutterheim, J. KMT2A-rearranged acute lymphoblastic leukaemia. EJC Paediatr. Oncol. 2024, 4, 100204. [Google Scholar] [CrossRef]
- Hilden, J.M.; Dinndorf, P.A.; Meerbaum, S.O.; Sather, H.; Villaluna, D.; Heerema, N.A.; McGlennen, R.; Smith, F.O.; Woods, W.G.; Salzer, W.L.; et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: Report on CCG 1953 from the Children’s Oncology Group. Blood 2006, 108, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, Z.E.; Hilden, J.M.; Jones, T.L.; Devidas, M.; Winick, N.J.; Willman, C.L.; Harvey, R.C.; Chen, I.M.; Behm, F.G.; Pullen, J.; et al. Intensified chemotherapy without SCT in infant ALL: Results from COG P9407 (Cohort 3). Pediatr. Blood Cancer 2015, 62, 419–426. [Google Scholar] [CrossRef]
- Brown, P.A.; Kairalla, J.A.; Hilden, J.M.; Dreyer, Z.E.; Carroll, A.J.; Heerema, N.A.; Wang, C.; Devidas, M.; Gore, L.; Salzer, W.L.; et al. FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children’s Oncology Group trial AALL0631. Leukemia 2021, 35, 1279–1290. [Google Scholar] [CrossRef]
- Guest, E.M.; Kairalla, J.A.; Devidas, M.; Hibbitts, E.; Carroll, A.J.; Heerema, N.A.; Kubaney, H.R.; August, M.A.; Ramesh, S.; Yoo, B.; et al. Azacitidine as epigenetic priming for chemotherapy is safe and well-tolerated in infants with newly diagnosed KMT2A-rearranged acute lymphoblastic leukemia: Children’s Oncology Group trial AALL15P1. Haematologica 2024, 109, 3918–3927. [Google Scholar] [CrossRef]
- Pieters, R.; Schrappe, M.; De Lorenzo, P.; Hann, I.; De Rossi, G.; Felice, M.; Hovi, L.; LeBlanc, T.; Szczepanski, T.; Ferster, A.; et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 2007, 370, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; De Lorenzo, P.; Ancliffe, P.; Aversa, L.A.; Brethon, B.; Biondi, A.; Campbell, M.; Escherich, G.; Ferster, A.; Gardner, R.A.; et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international phase III randomized study. J. Clin. Oncol. 2019, 37, 2246–2256. [Google Scholar] [CrossRef]
- Tomizawa, D.; Koh, K.; Sato, T.; Kinukawa, N.; Morimoto, A.; Isoyama, K.; Kosaka, Y.; Oda, T.; Oda, M.; Hayashi, Y.; et al. Outcome of risk-based therapy for infant acute lymphoblastic leukemia with or without an MLL gene rearrangement, with emphasis on late effects: A final report of two consecutive studies, MLL96 and MLL98, of the Japan Infant Leukemia Study Group. Leukemia 2007, 21, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Tomizawa, D.; Moriya Saito, A.; Watanabe, T.; Miyamura, T.; Hirayama, M.; Takahashi, Y.; Ogawa, A.; Kato, K.; Sugita, K.; et al. Early use of allogeneic hematopoietic stem cell transplantation for infants with MLL gene-rearrangement-positive acute lymphoblastic leukemia. Leukemia 2015, 29, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.A.; Huang, M.; Jeha, S.; Deyell, R.J.; Lewis, V.A.; Chang, B.H.; Lowe, E.J.; Frediani, J.; Vezina, C.; Michon, B.; et al. Outcome of infants treated on Total Therapy for Infants with Acute Lymphoblastic Leukemia I: Results from a non-randomized multi-center study. Blood 2023, 142, 823. [Google Scholar] [CrossRef]
- Fechina, L.; Popov, A.; Tsaur, G.; Henze, G.; Shorikov, E.; Makarova, O.; Khlebnikova, O.; Zhukova, Y.; Arakaev, O.; Streneva, O.; et al. Combination of chemotherapy and all-trans retinoic acid for the treatment KMT2A-rearranged infant acute lymphoblastic leukemia. Results of the MLL-Baby trial. Leukemia 2023, 37, 2276–2281. [Google Scholar] [CrossRef]
- Tomizawa, D.; Miyamura, T.; Imamura, T.; Watanabe, T.; Moriya Saito, A.; Ogawa, A.; Takahashi, Y.; Hirayama, M.; Taki, T.; Deguchi, T.; et al. A risk-stratified therapy for infants with acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial. Blood 2020, 136, 1813–1823. [Google Scholar] [CrossRef]
- Mironova, D.; Saraswati, C.M.; Downie, P.; Lai, C.Y.; Cook, E.; Carruthers, V.; Moukhaiber, P.; Molloy, F.; Serov, J.; McKinnon, E.; et al. Late effects in survivors of infant acute lymphoblastic leukaemia—A study of the Australian and New Zealand Children’s Haematology/Oncology Group. Blood Cancer J. 2023, 13, 150. [Google Scholar] [CrossRef]
- Van der Sluis, I.M.; de Lorenzo, P.; Kotecha, R.S.; Attarbaschi, A.; Escherich, G.; Nysom, K.; Stary, J.; Ferster, A.; Brethon, B.; Locatelli, F.; et al. Blinatumomab added to chemotherapy in infant lymphoblastic leukemia. N. Engl. J. Med. 2023, 388, 1572–1581. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Deloitte. Unleash AI’s potential. In Measuring the Return from Pharmaceutical Innovation, 14th ed.; Deloitte: London, UK, 2024; Available online: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-rd-roi-14th-edition.pdf (accessed on 10 September 2024).
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, U. Actinomycin. Chemistry and mechanism of action. Chem. Rev. 1974, 74, 625–652. [Google Scholar] [CrossRef]
- Green, D.M.; Sallan, S.E.; Krishan, A. Actinomycin D in childhood acute lymphocytic leukemia. Cancer Treat. Rep. 1978, 62, 829–831. [Google Scholar]
- Gionfriddo, I.; Brunetti, L.; Mezzasoma, F.; Milano, F.; Cardinali, V.; Ranieri, R.; Venanzi, A.; Pierangeli, S.; Vetro, C.; Spinozzi, G.; et al. Dactinomycin induces complete remission associated with nucleolar stress response in relapsed/refractory NPM1-mutated AML. Leukemia 2021, 35, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, M.N.; Ford, J.; Cheung, L.C.; Heng, J.; Singh, S.; Wells, J.; Failes, T.W.; Arndt, G.M.; Smithers, N.; Prinjha, R.K.; et al. Systematic chemical and molecular profiling of MLL-rearranged infant acute lymphoblastic leukemia reveals efficacy of romidepsin. Leukemia 2017, 31, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.C.; de Kraa, R.; Oommen, J.; Chua, G.A.; Singh, S.; Hughes, A.M.; Ferrari, E.; Ford, J.; Chiu, S.K.; Stam, R.W.; et al. Preclinical evaluation of carfilzomib for infant KMT2A-rearranged acute lymphoblastic leukemia. Front. Oncol. 2021, 11, 631594. [Google Scholar] [CrossRef] [PubMed]
- Bliss, C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.; Carol, H.; Evans, K.; High, L.; Mendomo, A.; Robbins, A.; Meyer, C.; Venn, N.C.; Marschalek, R.; Henderson, M.; et al. Effective targeting of the P53–MDM2 axis in preclinical models of infant MLL-rearranged acute lymphoblastic leukemia. Clin. Cancer Res. 2015, 21, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Sobell, H.M. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 1985, 82, 5328–5331. [Google Scholar] [CrossRef]
- Kamens, J.L.; Nance, S.; Koss, C.; Xu, B.; Cotton, A.; Lam, J.W.; Garfinkle, E.A.R.; Nallagatla, P.; Smith, A.M.R.; Mitchell, S.; et al. Proteasome inhibition targets the KMT2A transcriptional complex in acute lymphoblastic leukemia. Nat. Commun. 2023, 14, 809. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef]
- Schmidt, C.; Schubert, N.A.; Brabetz, S.; Mack, N.; Schwalm, B.; Chan, J.A.; Selt, F.; Herold-Mende, C.; Witt, O.; Milde, T.; et al. Preclinical drug screen reveals topotecan, actinomycin D, and volasertib as potential new therapeutic candidates for ETMR brain tumor patients. Neuro Oncol. 2017, 19, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Merkel, O.; Wacht, N.; Sifft, E.; Melchardt, T.; Hamacher, F.; Kocher, T.; Denk, U.; Hofbauer, J.P.; Egle, A.; Scheideler, M.; et al. Actinomycin D induces p53-independent cell death and prolongs survival in high-risk chronic lymphocytic leukemia. Leukemia 2012, 26, 2508–2516. [Google Scholar] [CrossRef]
- Wu, H.C.; Rérolle, D.; Berthier, C.; Hleihel, R.; Sakamoto, T.; Quentin, S.; Benhenda, S.; Morganti, C.; Wu, C.; Conte, L.; et al. Actinomycin D targets NPM1c-primed mitochondria to restore PML-driven senescence in AML therapy. Cancer Discov. 2021, 11, 3198–3213. [Google Scholar] [CrossRef]


| Cell Lines | Vincristine | Daunorubicin | Dexamethasone | Cytarabine | Methotrexate | Mercaptopurine | L-Asparaginase | Thioguanine | 4-Hydroxy-Cyclophosphamide |
|---|---|---|---|---|---|---|---|---|---|
| PER-490 | −1.94 | −2.77 | −0.08 | 1.59 | −1.43 | −2.13 | −1.17 | −2.91 | −0.49 |
| PER-494 | 0.34 | −3.29 | 0.71 | 3.42 | −6.01 | −6.68 | −0.86 | −7.21 | −3 |
| PER-785 | 0.83 | 0.92 | −1.19 | 0.51 | 0.07 | 0.75 | 1.61 | 1.08 | 0.77 |
| PER-826 | −4.68 | −4.48 | −2.5 | −0.8 | −6.27 | −3.17 | −1.76 | −1.9 | −2.87 |
| PER-910 | 0.68 | −0.44 | 0.41 | 1.77 | 0.23 | −1.85 | −3.46 | −1.55 | 0.51 |
| PER-784 | −6.65 | −4.47 | −6.04 | 0.62 | −9.9 | −2.7 | −6.74 | −5.61 | −3.67 |
| Colour key: | |||||||||
| <−10 | Antagonistic | ||||||||
| −10 to 10 | Additive | ||||||||
| >10 | Synergistic | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.K.; Ferrari, E.; Oommen, J.; Malinge, S.; Cheung, L.C.; Kotecha, R.S. Preclinical Assessment of Dactinomycin in KMT2A-Rearranged Infant Acute Lymphoblastic Leukemia. Cancers 2025, 17, 527. https://doi.org/10.3390/cancers17030527
Chiu SK, Ferrari E, Oommen J, Malinge S, Cheung LC, Kotecha RS. Preclinical Assessment of Dactinomycin in KMT2A-Rearranged Infant Acute Lymphoblastic Leukemia. Cancers. 2025; 17(3):527. https://doi.org/10.3390/cancers17030527
Chicago/Turabian StyleChiu, Sung K., Emanuela Ferrari, Joyce Oommen, Sebastien Malinge, Laurence C. Cheung, and Rishi S. Kotecha. 2025. "Preclinical Assessment of Dactinomycin in KMT2A-Rearranged Infant Acute Lymphoblastic Leukemia" Cancers 17, no. 3: 527. https://doi.org/10.3390/cancers17030527
APA StyleChiu, S. K., Ferrari, E., Oommen, J., Malinge, S., Cheung, L. C., & Kotecha, R. S. (2025). Preclinical Assessment of Dactinomycin in KMT2A-Rearranged Infant Acute Lymphoblastic Leukemia. Cancers, 17(3), 527. https://doi.org/10.3390/cancers17030527






