Expression and Prognostic Value of a Novel B7-H3 (CD276) Antibody in Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Antibody Production
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Clinical Characterization of the AML Patients
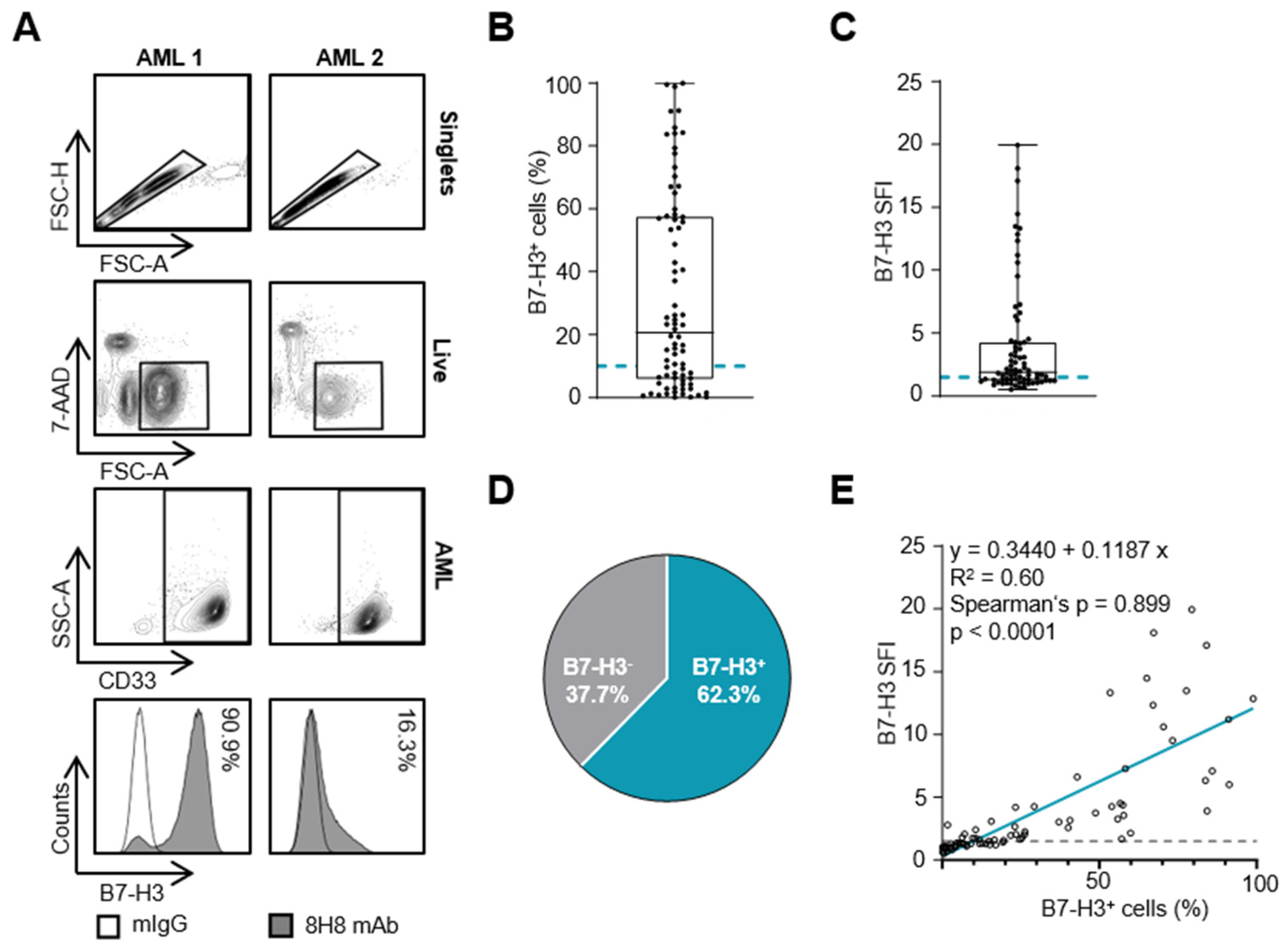
3.2. B7-H3 Expression on AML Blasts
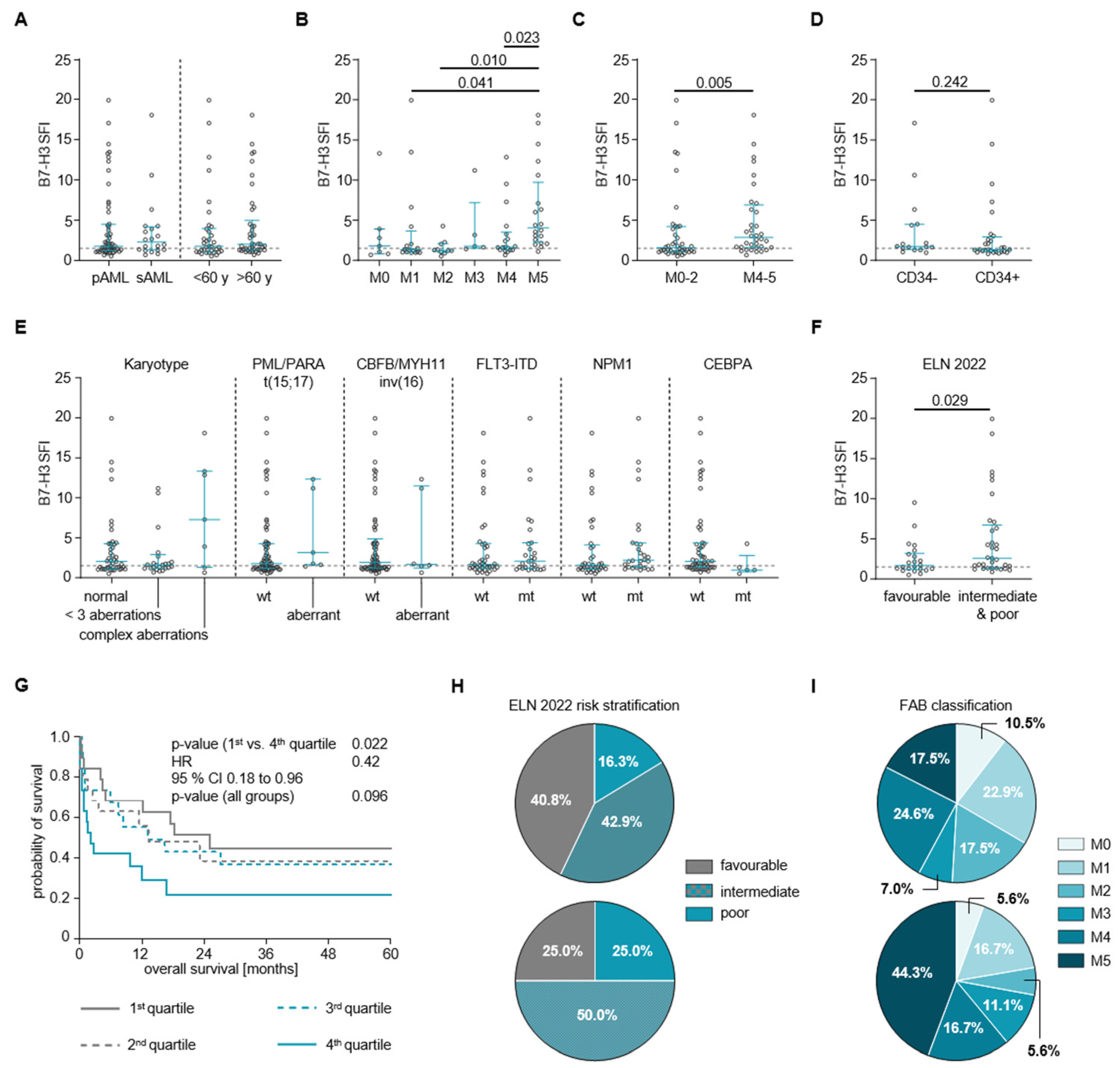
3.3. B7-H3 Expression and Clinical and Genetic Characteristics of AML Patients
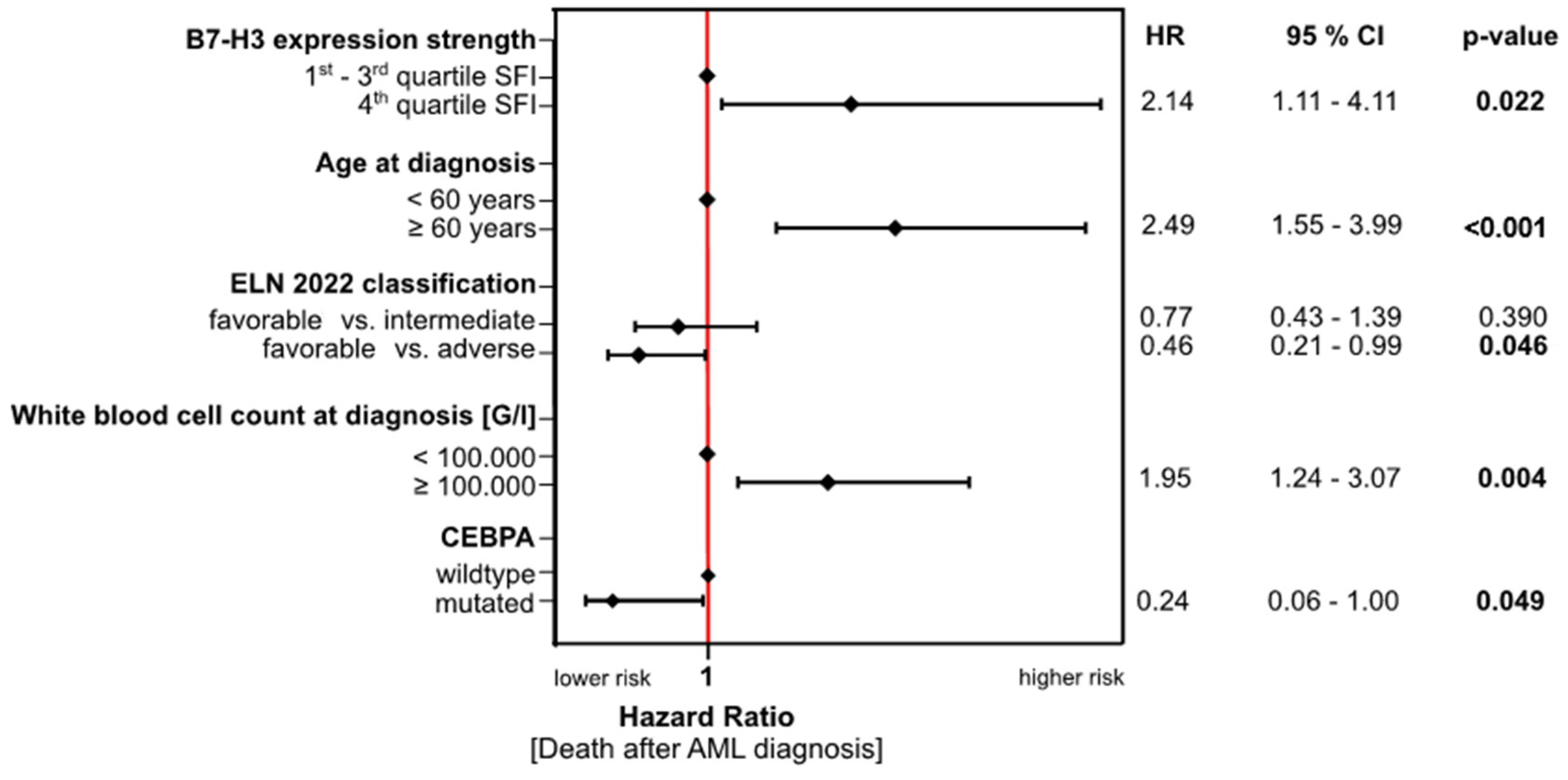
3.4. Association of B7-H3 Expression with Clinical Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Lubbert, M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef] [PubMed]
- Costa, A. Role of new immunophenotypic markers on prognostic and overall survival of acute myeloid leukemia: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 4138. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Fodstad, O.; Nunes-Xavier, C.E. B7-H3 Immune Checkpoint Protein in Human Cancer. Curr. Med. Chem. 2020, 27, 4062–4086. [Google Scholar] [CrossRef]
- Mortezaee, K. B7-H3 immunoregulatory roles in cancer. Biomed. Pharmacother. 2023, 163, 114890. [Google Scholar] [CrossRef]
- Michelakos, T.; Kontos, F.; Barakat, O.; Maggs, L.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3 targeted antibody-based immunotherapy of malignant diseases. Expert. Opin. Biol. Ther. 2021, 21, 587–602. [Google Scholar] [CrossRef]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Jin, W.L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune checkpoint of B7-H3 in cancer: From immunology to clinical immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zorko, N.; Elliott, A.; Lozada, J.A.; Radovich, M.; Heath, E.I.; Agarwal, N.; McKay, R.R.; Garje, R.; Bastos, B.R. Pan-cancer associations of B7-H3 (CD276) transcriptional expression across human malignancies. J. Clin. Oncol. 2023, 41, 2624. [Google Scholar] [CrossRef]
- Guery, T.; Roumier, C.; Berthon, C.; Renneville, A.; Preudhomme, C.; Quesnel, B. B7-H3 protein expression in acute myeloid leukemia. Cancer Med. 2015, 4, 1879–1883. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, X.; Wu, Y.; Xu, J.; Wang, L.; Chen, W.; Zhang, W.; Li, J.; Zhang, S.; Qiu, H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology 2015, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, T.; You, F.; Zhang, T.; Li, Y.; Ji, C.; Han, Z.; Sheng, B.; Zhai, X.; An, G.; et al. B7-H3 chimeric antigen receptor-modified T cell shows potential for targeted treatment of acute myeloid leukaemia. Eur. J. Med. Res. 2023, 28, 129. [Google Scholar] [CrossRef]
- Zekri, L.; Lutz, M.; Prakash, N.; Manz, T.; Klimovich, B.; Mueller, S.; Hoerner, S.; Hagelstein, I.; Engel, M.; Chashchina, A.; et al. An optimized IgG-based B7-H3xCD3 bispecific antibody for treatment of gastrointestinal cancers. Mol. Ther. 2023, 31, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Hagelstein, I.; Engel, M.; Hinterleitner, C.; Manz, T.; Marklin, M.; Jung, G.; Salih, H.R.; Zekri, L. B7-H3-targeting Fc-optimized antibody for induction of NK cell reactivity against sarcoma. Front. Immunol. 2022, 13, 1002898. [Google Scholar] [CrossRef]
- Lutz, M.S.; Zekri, L.; Wessling, L.; Berchtold, S.; Heitmann, J.S.; Lauer, U.M.; Jung, G.; Salih, H.R. IgG-based B7-H3xCD3 bispecific antibody for treatment of pancreatic, hepatic and gastric cancer. Front. Immunol. 2023, 14, 1163136. [Google Scholar] [CrossRef] [PubMed]
- Stefanczyk, S.A.; Hagelstein, I.; Lutz, M.S.; Muller, S.; Holzmayer, S.J.; Jarjour, G.; Zekri, L.; Heitmann, J.S.; Salih, H.R.; Marklin, M. Induction of NK cell reactivity against acute myeloid leukemia by Fc-optimized CD276 (B7-H3) antibody. Blood Cancer J. 2024, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M. Proposed revised criteria for the classification of acute myeloid leukemia: A report of the French-AmericanBritish Cooperative Group. Ann. Intern. Med. 1985, 103, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.G. Classification of acute myeloid leukemias–a comparison of FAB and immunophenotyping. Leukemia 1987, 1, 697–705. [Google Scholar] [PubMed]
- Zekri, L.; Vogt, F.; Osburg, L.; Muller, S.; Kauer, J.; Manz, T.; Pflugler, M.; Maurer, A.; Heitmann, J.S.; Hagelstein, I.; et al. An IgG-based bispecific antibody for improved dual targeting in PSMA-positive cancer. EMBO Mol. Med. 2021, 13, e11902. [Google Scholar] [CrossRef] [PubMed]
- Durben, M.; Schmiedel, D.; Hofmann, M.; Vogt, F.; Nubling, T.; Pyz, E.; Buhring, H.J.; Rammensee, H.G.; Salih, H.R.; Grosse-Hovest, L.; et al. Characterization of a bispecific FLT3 X CD3 antibody in an improved, recombinant format for the treatment of leukemia. Mol. Ther. 2015, 23, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Antohe, I.; Dascalescu, A.; Danaila, C.; Titieanu, A.; Zlei, M.; Ivanov, I.; Sireteanu, A.; Pavel, M.; Cianga, P. B7-Positive and B7-Negative Acute Myeloid Leukemias Display Distinct T Cell Maturation Profiles, Immune Checkpoint Receptor Expression, and European Leukemia Net Risk Profiles. Front. Oncol. 2020, 10, 264. [Google Scholar] [CrossRef]
- Golubovskaya, V. CAR-T Cells Targeting Immune Checkpoint Pathway Players. Front. Biosci. (Landmark Ed) 2022, 27, 121. [Google Scholar] [CrossRef]
- Lichtman, E.I.; Du, H.; Shou, P.; Song, F.; Suzuki, K.; Ahn, S.; Li, G.; Ferrone, S.; Su, L.; Savoldo, B.; et al. Preclinical Evaluation of B7-H3-specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 3141–3153. [Google Scholar] [CrossRef]
- Schorr, C.; Perna, F. Targets for chimeric antigen receptor T-cell therapy of acute myeloid leukemia. Front. Immunol. 2022, 13, 1085978. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Ly, S.; El-Dana, F.; Yuan, B.; Jaggupilli, A.; Grimm, S.; Bover, L.; Buhring, H.-J.; Battula, V.L. AML-327 Novel Anti–B7-H3 Blocking Antibody Enhances NK Cell–Mediated Cytotoxicity and Improves Outcomes in AML-Bearing Mice. Clin. Lymphoma Myeloma Leuk. 2022, 22, S236. [Google Scholar] [CrossRef]
- Tyagi, A.; Ly, S.; El-Dana, F.; Yuan, B.; Jaggupilli, A.; Grimm, S.; Konopleva, M.; Buhring, H.J.; Battula, V.L. Evidence supporting a role for the immune checkpoint protein B7-H3 in NK cell-mediated cytotoxicity against AML. Blood 2022, 139, 2782–2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Jin, Y.; Xia, P.H.; Lin, J.; Ma, J.C.; Li, T.; Liu, Z.Q.; Xiang, H.L.; Cheng, C.; Xu, Z.J.; et al. Integrated analysis reveals distinct molecular, clinical, and immunological features of B7-H3 in acute myeloid leukemia. Cancer Med. 2021, 10, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.; Qian, J.; Lin, J.; Chen, Q.; Yuan, Q.; Zhou, J.; Zhang, T.; Shi, J.; Zhou, H. Expression characteristic of 4Ig B7-H3 and 2Ig B7-H3 in acute myeloid leukemia. Bioengineered 2021, 12, 11987–12002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Gui, L.; Yan, X.; Zhou, X.; Ma, Y.; Yang, Z.; Fang, Y.; Zhang, H.; Shi, J. Expression and prognosis of the B7 family in acute myeloid leukemia. Ann. Transl. Med. 2021, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, C.; Liu, Z.; Yang, M.; Tang, X.; Wang, Y.; Zheng, M.; Huang, J.; Zhong, K.; Zhao, S.; et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol. Ther. Oncolytics 2020, 17, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Konstandin, N.P.; Pastore, F.; Herold, T.; Dufour, A.; Rothenberg-Thurley, M.; Hinrichsen, T.; Ksienzyk, B.; Tschuri, S.; Schneider, S.; Hoster, E.; et al. Genetic heterogeneity of cytogenetically normal AML with mutations of CEBPA. Blood Adv. 2018, 2, 2724–2731. [Google Scholar] [CrossRef]
- Van Doorn, S.B.V.W.; Erpelinck, C.; Meijer, J.; van Oosterhoud, S.; van Putten, W.L.; Valk, P.J.; Berna Beverloo, H.; Tenen, D.G.; Lowenberg, B.; Delwel, R. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol. J. 2003, 4, 31–40. [Google Scholar] [CrossRef]

| Mean | Range | |
|---|---|---|
| Age at diagnosis [years] | 60.9 | 26.0–89.0 |
| Sex | n = 77 | % of known |
| Female | 30 | 39.0 |
| Male | 47 | 61.0 |
| Primary/Secondary AML | ||
| Primary | 58 | 75.3 |
| Secondary | 19 | 24.7 |
| FAB classification | ||
| M0 | 7 | 9.3 |
| M1 | 16 | 21.3 |
| M2 | 11 | 14.7 |
| M3 | 6 | 8.0 |
| M4 | 17 | 22.7 |
| M5 | 18 | 24.0 |
| Unknown | 2 | - |
| WHO classification 2016 | ||
| Acute myeloid leukemia with myelodysplasia-related changes | 10 | 13.0 |
| Acute myeloid leukemia with recurrent genetic aberrations | 44 | 57.1 |
| Acute myeloid leukemia, not otherwise specified | 21 | 27.3 |
| Therapy-related myeloid neoplasms | 2 | 2.6 |
| ELN 2022 classification | ||
| Favorable | 25 | 38.5 |
| Intermediate | 28 | 43.0 |
| Poor | 12 | 18.5 |
| Unknown | 12 | - |
| Blood count at diagnosis | Mean | Range |
| WBC [G/L] | 110.9 | 4.6–448.3 |
| Hb [g/dL] | 8.6 | 3.8–12.9 |
| Plt [G/L] | 78.1 | 6.0–433.0 |
| Blasts [%] | Mean | Range |
| PB n = 63 | 80.4 | 23–100 |
| BM n = 36 | 74.7 | 12–98 |
| B7-H3 Low (SFI < 4.45) (n = 58) | B7-H3 High (SFI ≥ 4.45) (n = 19) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Range | n | Mean | Range | ||||||
| Age at diagnosis [years] | 58 | 62.7 | 26.0 | – | 89.0 | 19 | 67.0 | 37.0 | – | 85.0 | 0.239 ♦ |
| WBC [G/L] | 65.9 | 5.0 | – | 448.3 | 126.8 | 4.6 | – | 361.0 | 0.116 ♦ | ||
| Hb [g/dL] | 9.0 | 3.8 | – | 12.9 | 8.4 | 4.4 | – | 10.3 | 0.190 ♦ | ||
| Plt [G/L] | 43.5 | 6.0 | – | 433.0 | 41.0 | 6.0 | – | 124.0 | 0.324 ♦ | ||
| Sex | % within group | % within group | |||||||||
| Female | 23 | 39.7 | 7 | 36.8 | |||||||
| Male | 35 | 60.3 | 12 | 63.2 | 0.8274 ‡ | ||||||
| Primary/Secondary AML | |||||||||||
| Primary | 42 | 72.4 | 16 | 84.2 | |||||||
| Secondary | 16 | 27.6 | 3 | 15.8 | 0.301 ‡ | ||||||
| FAB classification | |||||||||||
| M0 | 6 | 10.5 | 1 | 5.6 | |||||||
| M1 | 13 | 22.8 | 3 | 16.7 | |||||||
| M2 | 10 | 17.5 | 1 | 5.6 | |||||||
| M3 | 4 | 7.0 | 2 | 11.1 | |||||||
| M4 | 14 | 24.6 | 3 | 16.7 | |||||||
| M5 | 10 | 17.5 | 8 | 44.4 | 0.241 ‡ | ||||||
| Unknown | 1 | - | 1 | - | |||||||
| WHO classification 2016 | |||||||||||
| Acute myeloid leukemia with myelodysplasia-related changes | 8 | 13.8 | 2 | 10.5 | |||||||
| Acute myeloid leukemia with recurrent genetic aberrations | 31 | 53.5 | 13 | 68.4 | |||||||
| Acute myeloid leukemia, not otherwise specified | 17 | 29.3 | 4 | 21.1 | |||||||
| Therapy-related myeloid neoplasms | 2 | 3.5 | 0 | 0.0 | 0.639 ‡ | ||||||
| ELN 2022 classification | |||||||||||
| Favorable | 21 | 42.9 | 4 | 25.0 | |||||||
| Intermediate | 20 | 40.8 | 8 | 50.0 | |||||||
| Poor | 8 | 16.3 | 4 | 25.0 | 0.422 ‡ | ||||||
| Unknown | 9 | - | 3 | - | |||||||
| 1st Induction | |||||||||||
| No | 14 | 24.1 | 8 | 42.1 | |||||||
| Yes | 44 | 75.9 | 11 | 57.9 | 0.132 ‡ | ||||||
| Anthracycline-based 1st induction | |||||||||||
| No | 17 | 29,8 | 11 | 64.7 | |||||||
| Yes | 40 | 70.2 | 6 | 35.3 | 0.007 ‡ | ||||||
| Unknown | 2 | - | 1 | - | |||||||
| Response to 1st induction | |||||||||||
| CR(i) | 25 | 67.6 | 7 | 87.5 | |||||||
| PR | 12 | 32.4 | 1 | 12.5 | 0.217 ‡ | ||||||
| Unknown | 21 | - | 11 | - | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefańczyk, S.A.; Hayn, C.; Heitmann, J.; Jung, S.; Zekri, L.; Märklin, M. Expression and Prognostic Value of a Novel B7-H3 (CD276) Antibody in Acute Myeloid Leukemia. Cancers 2024, 16, 2455. https://doi.org/10.3390/cancers16132455
Stefańczyk SA, Hayn C, Heitmann J, Jung S, Zekri L, Märklin M. Expression and Prognostic Value of a Novel B7-H3 (CD276) Antibody in Acute Myeloid Leukemia. Cancers. 2024; 16(13):2455. https://doi.org/10.3390/cancers16132455
Chicago/Turabian StyleStefańczyk, Sylwia A., Clara Hayn, Jonas Heitmann, Susanne Jung, Latifa Zekri, and Melanie Märklin. 2024. "Expression and Prognostic Value of a Novel B7-H3 (CD276) Antibody in Acute Myeloid Leukemia" Cancers 16, no. 13: 2455. https://doi.org/10.3390/cancers16132455
APA StyleStefańczyk, S. A., Hayn, C., Heitmann, J., Jung, S., Zekri, L., & Märklin, M. (2024). Expression and Prognostic Value of a Novel B7-H3 (CD276) Antibody in Acute Myeloid Leukemia. Cancers, 16(13), 2455. https://doi.org/10.3390/cancers16132455





