Trends in Outcome of Hematopoietic Stem Cell Transplantation: 5000 Transplantations and 30 Years of Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Clinical Definitions and Statistical Analysis
3. Results
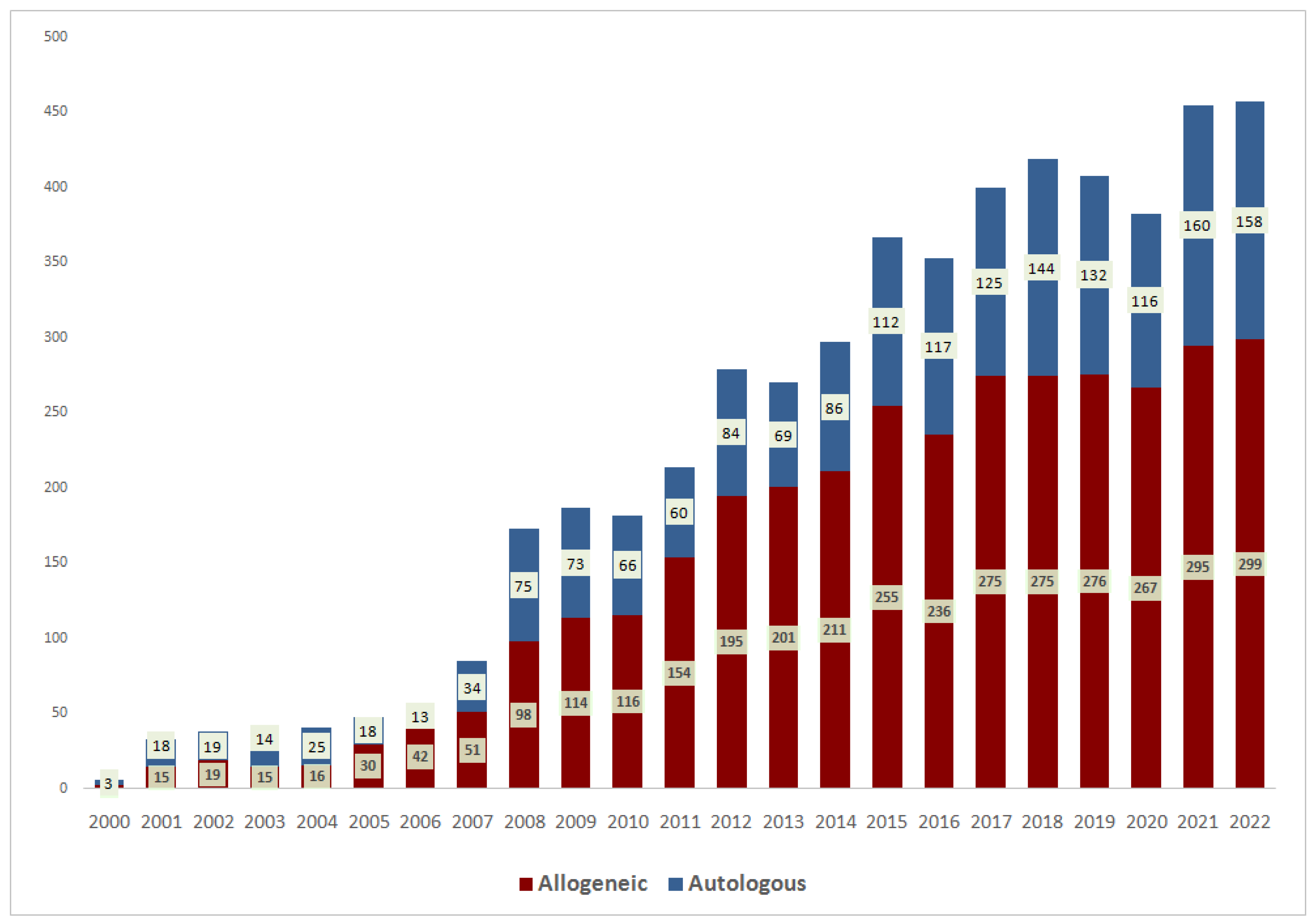
3.1. General Trends
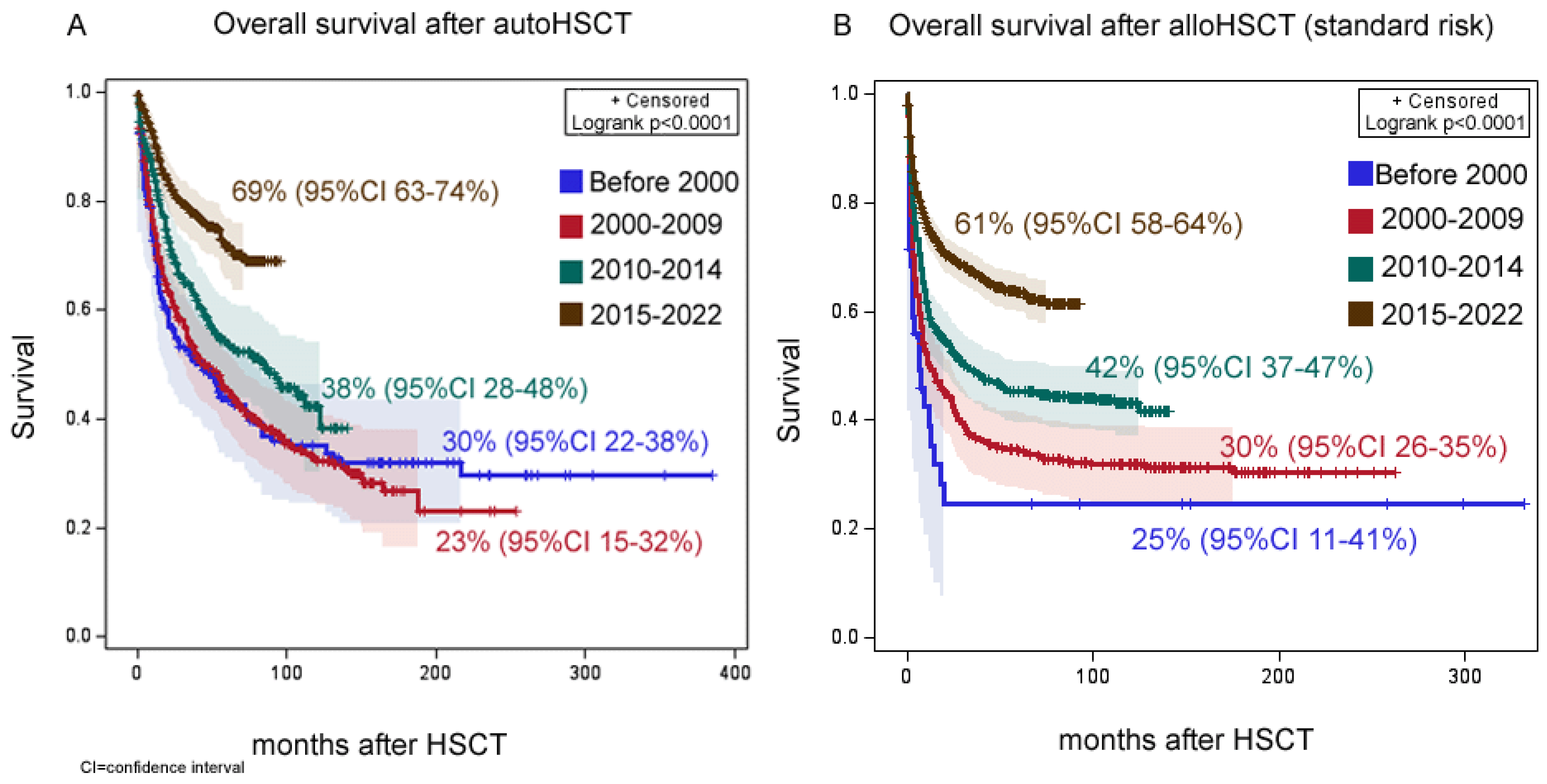
3.2. Survival Outcomes
3.2.1. Autologous HCT
3.2.2. Allogeneic HCT
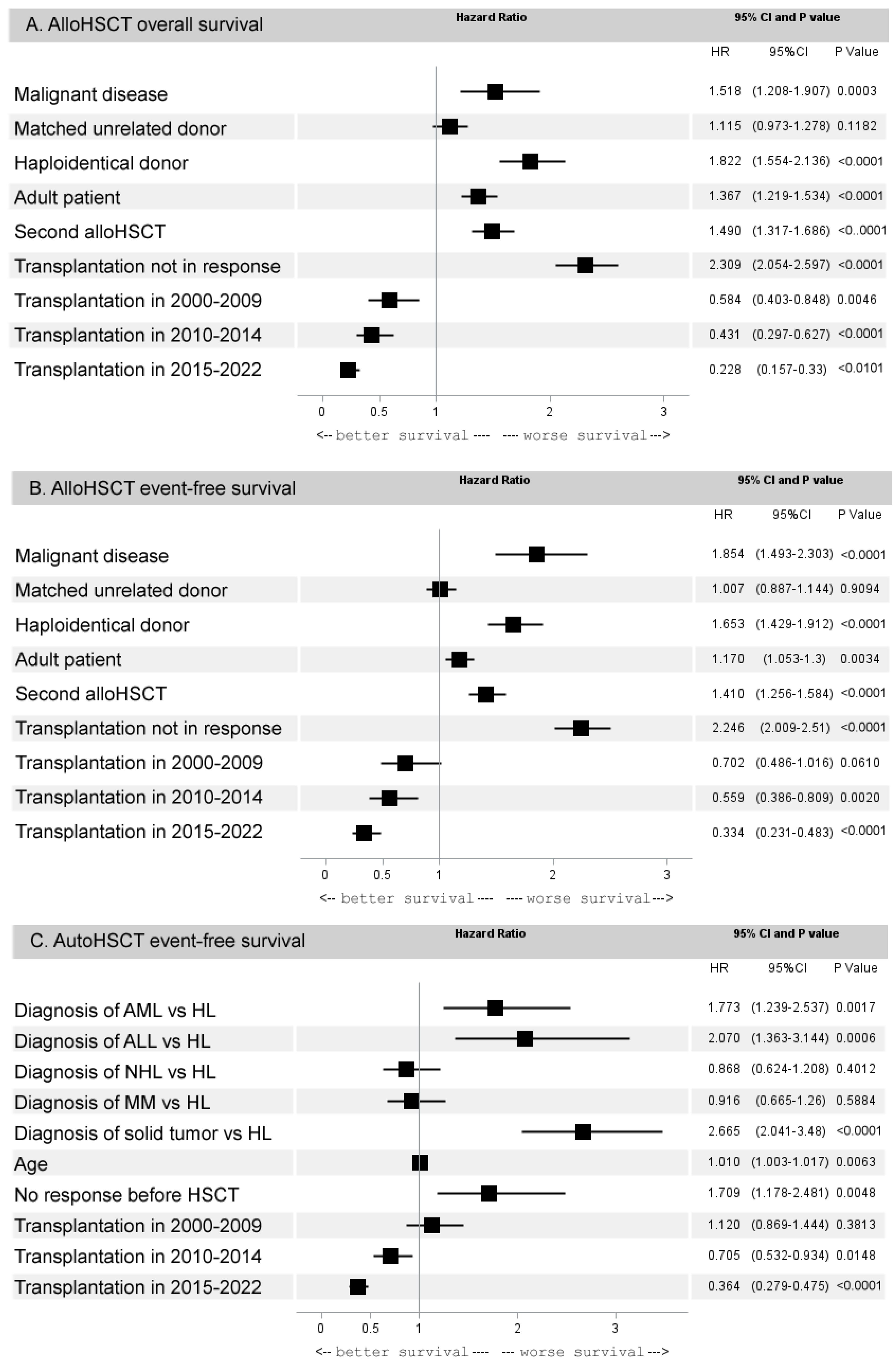
3.2.3. Multivariate Analysis of 5-Year Outcomes of HCT
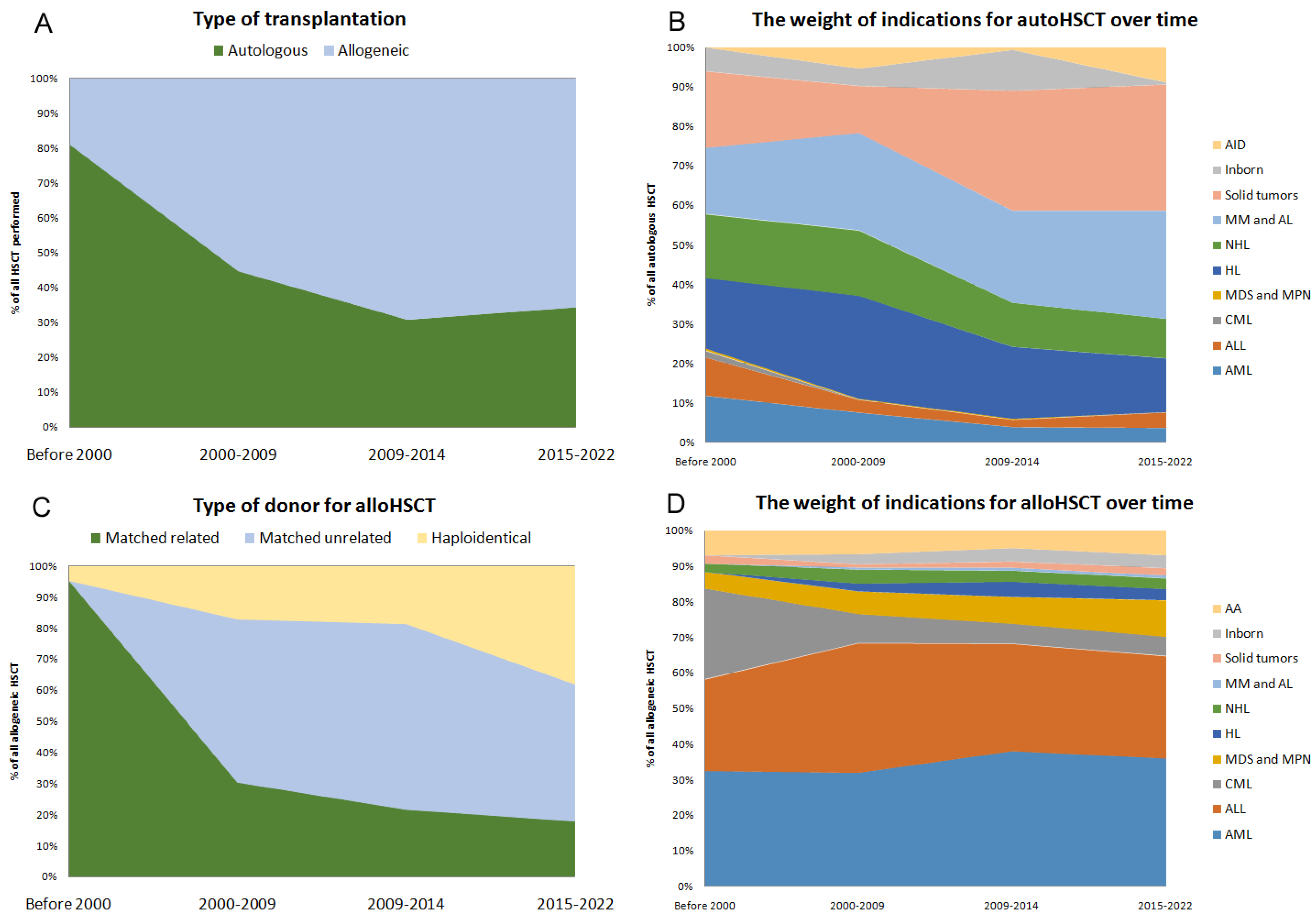
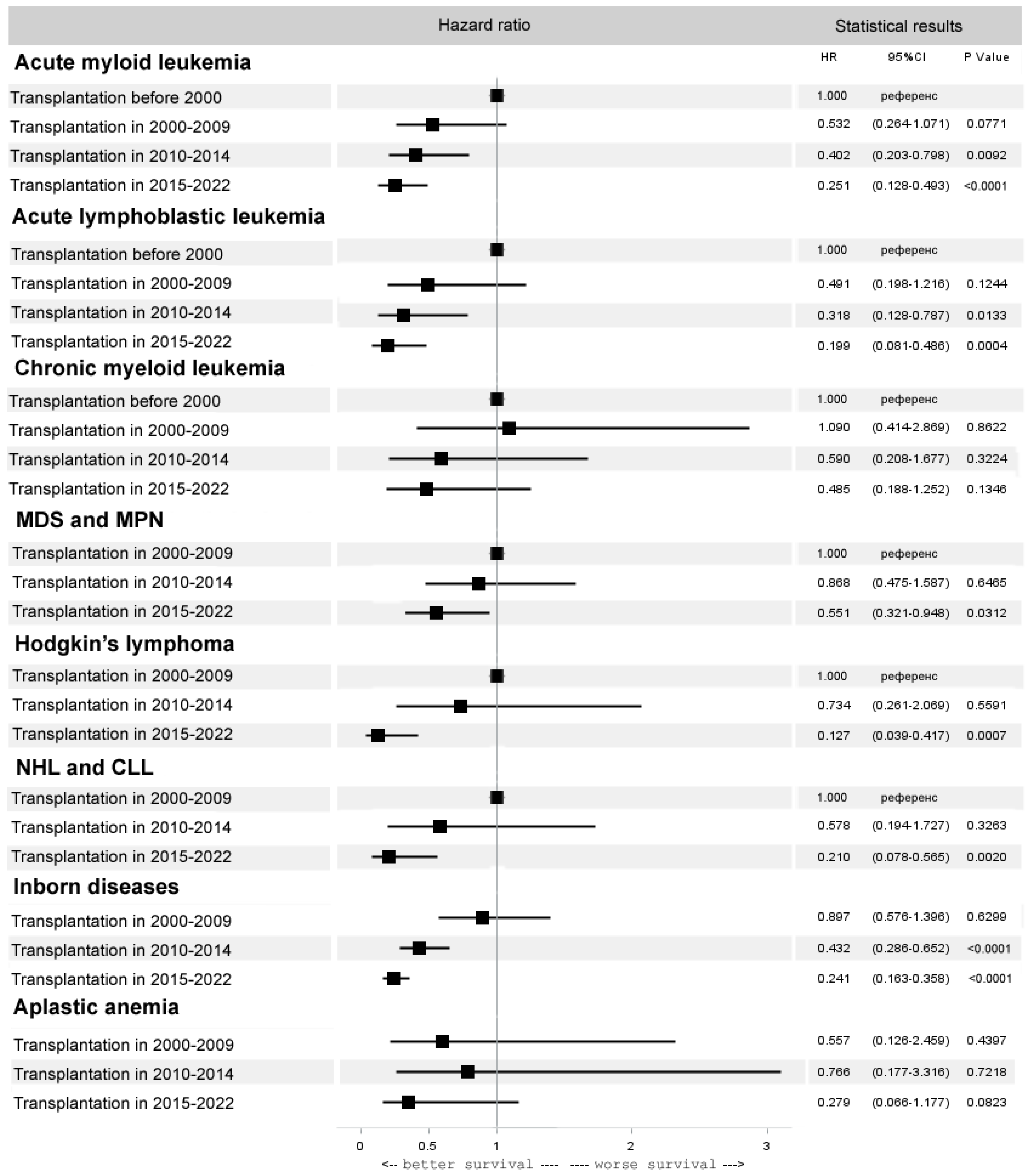
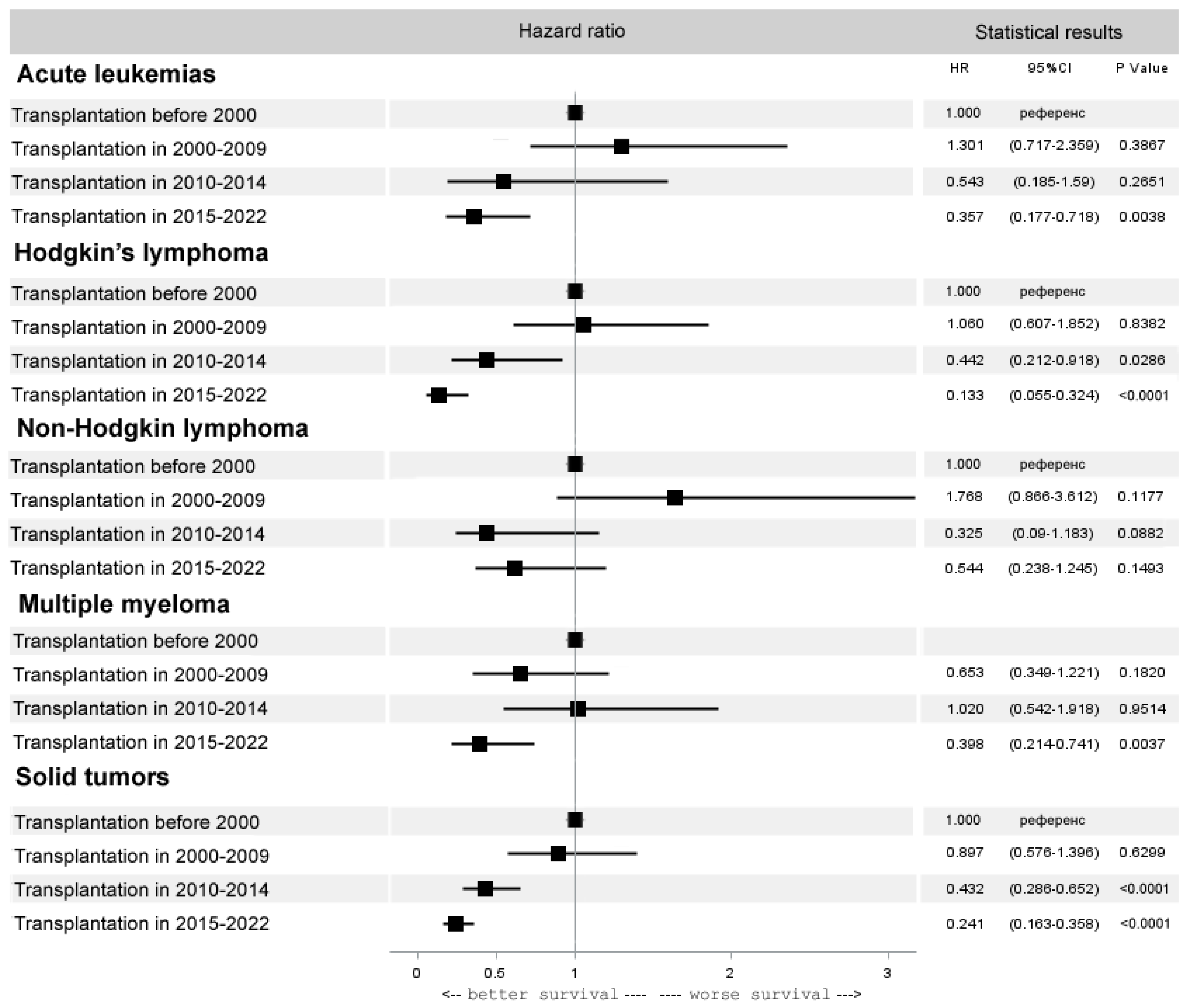
3.3. Trends in Specific Diseases
3.3.1. Allogeneic HCT
3.3.2. Autologous HCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penack, O.; Peczynski, C.; Mohty, M.; Yakoub-Agha, I.; Styczynski, J.; Montoto, S.; Duarte, R.F.; Kröger, N.; Schoemans, H.; Koenecke, C.; et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020, 4, 6283–6290. [Google Scholar] [CrossRef] [PubMed]
- Kanate, A.S.; Majhail, N.S.; Savani, B.N.; Bredeson, C.; Champlin, R.E.; Crawford, S.; Giralt, S.A.; LeMaistre, C.F.; Marks, D.I.; Omel, J.L.; et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2020, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Khvedelidze, I.; Romejko-Jarosinska, J.; Tanase, A.; Akhtar, S.; Ben Othman, T.; Ma’koseh, M.; Afanasyev, B.; et al. The outcome of patients with Hodgkin lymphoma and early relapse after autologous stem cell transplant has improved in recent years. Leukemia 2022, 36, 1646–1653, Erratum in: Leukemia 2022, 36, 2150. [Google Scholar] [CrossRef] [PubMed]
- Hegerova, L.; Cao, Q.; Lazaryan, A.; McClune, B.L.; Weisdorf, D.J.; Brunstein, C.G.; Bachanova, V. Improving outcomes after allogeneic hematopoietic cell transplantation for Hodgkin lymphoma in the brentuximab vedotin era. Bone Marrow Transplant. 2017, 52, 697–703. [Google Scholar] [CrossRef]
- Snowden, J.A.; Saccardi, R.; Orchard, K.; Ljungman, P.; Duarte, R.F.; Labopin, M.; McGrath, E.; Brook, N.; de Elvira, C.R.; Gordon, D.; et al. Benchmarking of survival outcomes following haematopoietic stem cell transplantation: A review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 2020, 55, 681–694, Erratum in Bone Marrow Transplant. 2020, 55, 838–839. [Google Scholar] [CrossRef]
- O’Meara, A.; Holbro, A.; Meyer, S.; Martinez, M.; Medinger, M.; Buser, A.; Halter, J.; Heim, D.; Gerull, S.; Bucher, C.; et al. Forty years of haematopoietic stem cell transplantation: A review of the Basel experience. Swiss Med. Wkly. 2014, 144, w13928. [Google Scholar] [CrossRef]
- Cooper, J.P.; Storer, B.E.; Granot, N.; Gyurkocza, B.; Sorror, M.L.; Chauncey, T.R.; Shizuru, J.; Franke, G.N.; Maris, M.B.; Boyer, M.; et al. Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: Improved outcomes over two decades. Haematologica 2021, 106, 1599–1607. [Google Scholar] [CrossRef]
- Gratwohl, A.; Sureda, A.; Baldomero, H.; Gratwohl, M.; Dreger, P.; Kröger, N.; Ljungman, P.; McGrath, E.; Mohty, M.; Nagler, A.; et al. Economics and Outcome After Hematopoietic Stem Cell Transplantation: A Retrospective Cohort Study. EBioMedicine 2015, 2, 2101–2109. [Google Scholar] [CrossRef]
- Bagnenko, S.F.; Zander, A.R.; Wagemaker, G.; Hehlmann, R.; Fehse, B.; Kulagin, A.D.; Zubarovskaya, L.S.; Moiseev, I.S.; Markova, I.V.; Chukhlovin, A.B. In memory of Professor Boris Afanasyev (28 August 1947–16 March 2020). Cell. Ther. Transplant. 2020, 9, 4–7. [Google Scholar] [CrossRef]
- Afanasiev, B.V.; Zubarovskaya, L.S.; Moiseev, I.S. Allogeneic Hematopoietic Stem Cell Transplantation in Children: Now, Problems and Prospects. Russ. J. Pediatr. Hematol. Oncol. 2015, 2, 28–42. [Google Scholar] [CrossRef]
- McNicholl, F. A History of Haematology. from Herodotus to HIV (Oxford Medical Histories). Ulster. Med. J. 2017, 86, 50. [Google Scholar]
- Isaev, A.A.; Deev, R.V.; Kuliev, A.; Plaxa, I.L.; Stancheva, N.V.; Borovkova, A.S.; Potapov, I.V.; Pomerantseva, E.A.; Chogovadze, A.G.; Boyarsky, K.Y.; et al. First experience of hematopoietic stem cell transplantation treatment of Shwachman-Diamond syndrome using unaffected HLA-matched sibling donor produced through preimplantation HLA typing. Bone Marrow Transplant. 2017, 52, 1249–1252. [Google Scholar] [CrossRef]
- Moiseev, I.S.; Pirogova, O.V.; Alyanski, A.L.; Babenko, E.V.; Gindina, T.L.; Darskaya, E.I.; Slesarchuk, O.A.; Bondarenko, S.N.; Afanasyev, B.V. Graft-versus-Host Disease Prophylaxis in Unrelated Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil. Biol. Blood Marrow Transplant. 2016, 22, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J.; Ebmt, F.T.I.D.W.P.; Tridello, G.; Koster, L.; Iacobelli, S.; van Biezen, A.; van der Werf, S.; Mikulska, M.; Gil, L.; Cordonnier, C.; et al. Infectious Diseases Working Party EBMT. Death after hematopoietic stem cell transplantation: Changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020, 55, 126–136. [Google Scholar] [PubMed]
- Hildegard, T.G.; Eikema, D.-J.; Koster, L.; Penack, O.; Yakoub-Agha, I.; Montoto, S.; Chabannon, C.; Styczynski, J.; Nagler, A.; Robin, M.; et al. Incidence of Acute Graft-Versus-Host Disease and Survival after Allogeneic Hematopoietic Cell Transplantation over Time: A Study from the Transplant Complications and Chronic Malignancies Working Party of the EBMT. Blood 2018, 132 (Suppl. S1), 2120. [Google Scholar]
- Moiseev, I.S.; Pirogova, O.V.; Babenko, E.V.; Gindina, T.L.; Darskaya, E.I.; Morozova, E.V.; Bondarenko, S.N.; Afanasyev, B.V. Single-agent post-transplantation cyclophosphamide versus calcineurin-based graft-versus-host disease prophylaxis in matched related bone marrow transplantation. Cell. Ther. Transplant. 2017, 6, 52–59. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Battiwalla, M.; Hashmi, S.; Majhail, N.; Pavletic, S.; Savani, B.N.; Shelburne, N. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: Developing Recommendations to Improve Survivorship and Long-Term Outcomes. Biol. Blood Marrow Transplant. 2017, 23, 6–9. [Google Scholar] [CrossRef]
- Afanasyev, B.; Moiseev, I.; Volkov, N.; Lepik, K.; Mikhailova, N.; Bondarenko, S.; Zubarovskaya, L.; Morozova, E.; Paina, O.; Kozhokar, P.; et al. Achievements and Challenges of Evidence-Based Medicine in Hematopoietic Stem Cell Transplantation: An Analysis of Single-Center and Multicenter Trials. Clin. Oncohematol. 2020, 13, 260–272. [Google Scholar] [CrossRef]
- Marinelli, T.; Wee, L.Y.A.; Rowe, E.; Chhetri, R.; Friel, O.; Higgins, G.; Bardy, P.; Singhal, D.; Pradhan, A.; Crawford, L.; et al. Respiratory Viruses Cause Late Morbidity in Recipients of Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Corbacioglu, S.; de la Cámara, R.; Dolstra, H.; Glass, B.; Greco, R.; Mohty, M.; Neven, B.; et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: A report from the EBMT activity survey. Bone Marrow Transplant. 2022, 57, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Gevorgian, A.G.; Kozlov, A.V.; Tolkunova, P.S.; Kazantzev, I.V.; Yukhta, T.V.; Morozova, E.V.; Kulagin, A.D.; Punanov, Y.A.; Zheludkova, O.G.; Zubarovskaya, L.S. Tandem autologous hematopoietic stem cell transplantation for embryonal brain tumors in infants and very young children. Bone Marrow Transplant. 2022, 57, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Picardi, A. Is There Still a Role for Autologous Stem Cell Transplantation for the Treatment of Acute Myeloid Leukemia? Cancers 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; de Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef]
- Ayuk, F.; Beelen, D.W.; Bornhäuser, M.; Stelljes, M.; Zabelina, T.; Finke, J.; Kobbe, G.; Wolff, D.; Wagner, E.M.; Christopeit, M.; et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 2558–2567. [Google Scholar] [CrossRef]
- Saber, W.; Opie, S.; Rizzo, J.D.; Zhang, M.J.; Horowitz, M.M.; Schriber, J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 2012, 119, 3908–3916. [Google Scholar] [CrossRef]
- Brissot, E.; Labopin, M.; Ehninger, G.; Stelljes, M.; Brecht, A.; Ganser, A.; Tischer, J.; Kröger, N.; Afanasyev, B.; Finke, J.; et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: A report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica 2019, 104, 524–532. [Google Scholar] [CrossRef]
- Sanz, J.; Galimard, J.E.; Labopin, M.; Afanasyev, B.; Angelucci, E.; Ciceri, F.; Blaise, D.; Cornelissen, J.J.; Meijer, E.; Diez-Martin, J.L.; et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: A comparative study of the ALWP EBMT. J. Hematol. Oncol. 2020, 13, 46. [Google Scholar] [CrossRef]
- Barkhordar, M.; Kasaeian, A.; Tavakoli, S.; Vaezi, M.; Kamranzadeh Foumani, H.; Bahri, T.; Babakhani, D.; Mirzakhani, L.; Mousavi, A.; Mousavi, S.A.; et al. Selection of Suitable Alternative Donor in the Absence of Matched Sibling Donor: A Retrospective Single-Center Study to Compare between Haploidentical, 10/10 and 9/10 Unrelated Donor Transplantation. Int. J. Hematol. Oncol. Stem. Cell Res. 2021, 15, 51–60. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Hill, B.T.; Lamanna, N.; Barr, P.M.; Ujjani, C.S.; Brander, D.M.; Howlett, C.; Skarbnik, A.P.; Cheson, B.D.; Zent, C.S.; et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: Results from a multicenter study of 683 patients. Ann. Oncol. 2017, 28, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Shah, G.; Schöder, H.; Ganesan, N.; Drill, E.; Hancock, H.; Davey, T.; Perez, L.; Ryu, S.; Sohail, S.; et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J. Clin. Oncol. 2021, 39, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Bair, S.M.; Strelec, L.E.; Feldman, T.A.; Ahmed, G.; Armand, P.; Shah, N.N.; Singavi, A.N.; Reddy, N.; Khan, N.; Andreadis, C.; et al. Outcomes and Toxicities of Programmed Death-1 (PD-1) Inhibitors in Hodgkin Lymphoma Patients in the United States: A Real-World, Multicenter Retrospective Analysis. Oncologist 2019, 24, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, P.A.; Kang, H.J.; Yoo, K.H.; Zecca, M.; Cho, B.; Lucchini, G.; Nemecek, E.R.; Schultz, K.R.; Stepensky, P.; Chaudhury, S.; et al. Ibrutinib Treatment of Pediatric Chronic Graft-versus-Host Disease: Primary Results from the Phase 1/2 iMAGINE Study. Transplant. Cell. Ther. 2022, 28, 771.e1–771.e10. [Google Scholar] [CrossRef]
- Niederwieser, C.; Morozova, E.; Zubarovskaya, L.; Zabelina, T.; Klyuchnikov, E.; Janson, D.; Wolschke, C.; Christopeit, M.; Ayuk, F.; Moiseev, I.; et al. Risk factors for outcome after allogeneic stem cell transplantation in patients with advanced phase CML. Bone Marrow Transplant. 2021, 56, 2834–2841. [Google Scholar] [CrossRef]
- Devillier, R.; Eikema, D.J.; Dufour, C.; Aljurf, M.; Wu, D.; Maschan, A.; Kulagin, A.; Halkes, C.J.M.; Collin, M.; Snowden, J.; et al. Graft-versus-host disease and relapse/rejection-free survival after allogeneic transplantation for idiopathic severe aplastic anemia: A comprehensive analysis from the SAAWP of the EBMT. Haematologica 2023, 108, 2305–2315. [Google Scholar] [CrossRef]
| Autologous, n (%) | Allogeneic, n (%) | |
|---|---|---|
| Number | 1948 (38) | 3237 (62) |
| Age | ||
| Median (range), years | 30 (0–71) | 23 (0–77) |
| Children | 636 (32.7) | 1190 (36.8) |
| Adults | 1312 (67.3) | 2047 (63.2) |
| Diagnosis | ||
| Acute myeloid leukemia | 105 (5.4) | 1159 (35.8) |
| Acute lymphoblastic leukemia | 77 (4.0) | 977 (30.2) |
| Chronic myeloid leukemia | 4 (0.2) | 201 (6.2) |
| Myeloproliferative diseases and myelodysplastic syndrome | 3 (0.2) * | 302 (9.3) |
| Hodgkin’s lymphoma | 341 (17.5) | 107 (3.3) |
| Non-Hodgkin’s lymphomas Chronic lymphocytic leukemia | 238 (12.2) 6 (0.3) | 63 (1.9) 40 (1.2) |
| Multiple myeloma | 488 (25.0) | 17 (0.5) |
| Solid tumors | 568 (29) | 65 (2.0) |
| Aplastic anemia | 1 (0.1) ** | 210 (6.5) |
| Congenital diseases | 1 (0.1) *** | 96 (3.0) |
| Autoimmune diseases | 116 (6.0) | 0 (0) |
| Number of transplantations for single patient | ||
| 1st HCT | 1738 (89.2) | 2655 (82.0) |
| 2nd HCT | 209 (10.7) | 499 (15.4) |
| 3rd HCT | 1 (0.1) | 77 (2.4) |
| 4th HCT | 0 (0) | 6 (0.2) |
| Donor | ||
| Matched related | NA | 707 (21.8) |
| Matched unrelated | NA | 1568 (48.4) |
| Mismatched related | NA | 962 (29.7) |
| Indications for transplantation | ||
| Standard-risk HCT | 1886 (96.8) | 2701 (83.4) |
| Salvage HCT **** | 62 (3.2) | 536 (16.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubarovskaya, L.S.; Moiseev, I.S.; Vladovskaya, M.D.; Mikhailova, N.B.; Morozova, E.V.; Bykova, T.A.; Vlasova, Y.Y.; Paina, O.V.; Kazantsev, I.V.; Slesarchuk, O.A.; et al. Trends in Outcome of Hematopoietic Stem Cell Transplantation: 5000 Transplantations and 30 Years of Single-Center Experience. Cancers 2023, 15, 4758. https://doi.org/10.3390/cancers15194758
Zubarovskaya LS, Moiseev IS, Vladovskaya MD, Mikhailova NB, Morozova EV, Bykova TA, Vlasova YY, Paina OV, Kazantsev IV, Slesarchuk OA, et al. Trends in Outcome of Hematopoietic Stem Cell Transplantation: 5000 Transplantations and 30 Years of Single-Center Experience. Cancers. 2023; 15(19):4758. https://doi.org/10.3390/cancers15194758
Chicago/Turabian StyleZubarovskaya, Ludmila Stepanovna, Ivan Sergeevich Moiseev, Maria Dmidrievna Vladovskaya, Natalia Borisovna Mikhailova, Elena Vladislavovna Morozova, Tatyana Alexandrovna Bykova, Yulia Yurievna Vlasova, Olesya Vladimirovna Paina, Ilya Viktorovich Kazantsev, Olga Alexandrovna Slesarchuk, and et al. 2023. "Trends in Outcome of Hematopoietic Stem Cell Transplantation: 5000 Transplantations and 30 Years of Single-Center Experience" Cancers 15, no. 19: 4758. https://doi.org/10.3390/cancers15194758
APA StyleZubarovskaya, L. S., Moiseev, I. S., Vladovskaya, M. D., Mikhailova, N. B., Morozova, E. V., Bykova, T. A., Vlasova, Y. Y., Paina, O. V., Kazantsev, I. V., Slesarchuk, O. A., Smirnova, A. G., Osipova, A. A., Stelmakh, L. V., Polushin, A. Y., Goloshchapov, O. V., Bogomolny, M. P., Estrina, M. A., Popova, M. O., Kucher, M. A., ... Afanasyev, B. V. (2023). Trends in Outcome of Hematopoietic Stem Cell Transplantation: 5000 Transplantations and 30 Years of Single-Center Experience. Cancers, 15(19), 4758. https://doi.org/10.3390/cancers15194758







