Allogeneic Stem Cell Transplantation in Multiple Myeloma: Risk Factors and Outcomes in the Era of New Therapeutic Options—A Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient, Disease, and Transplant Characteristics
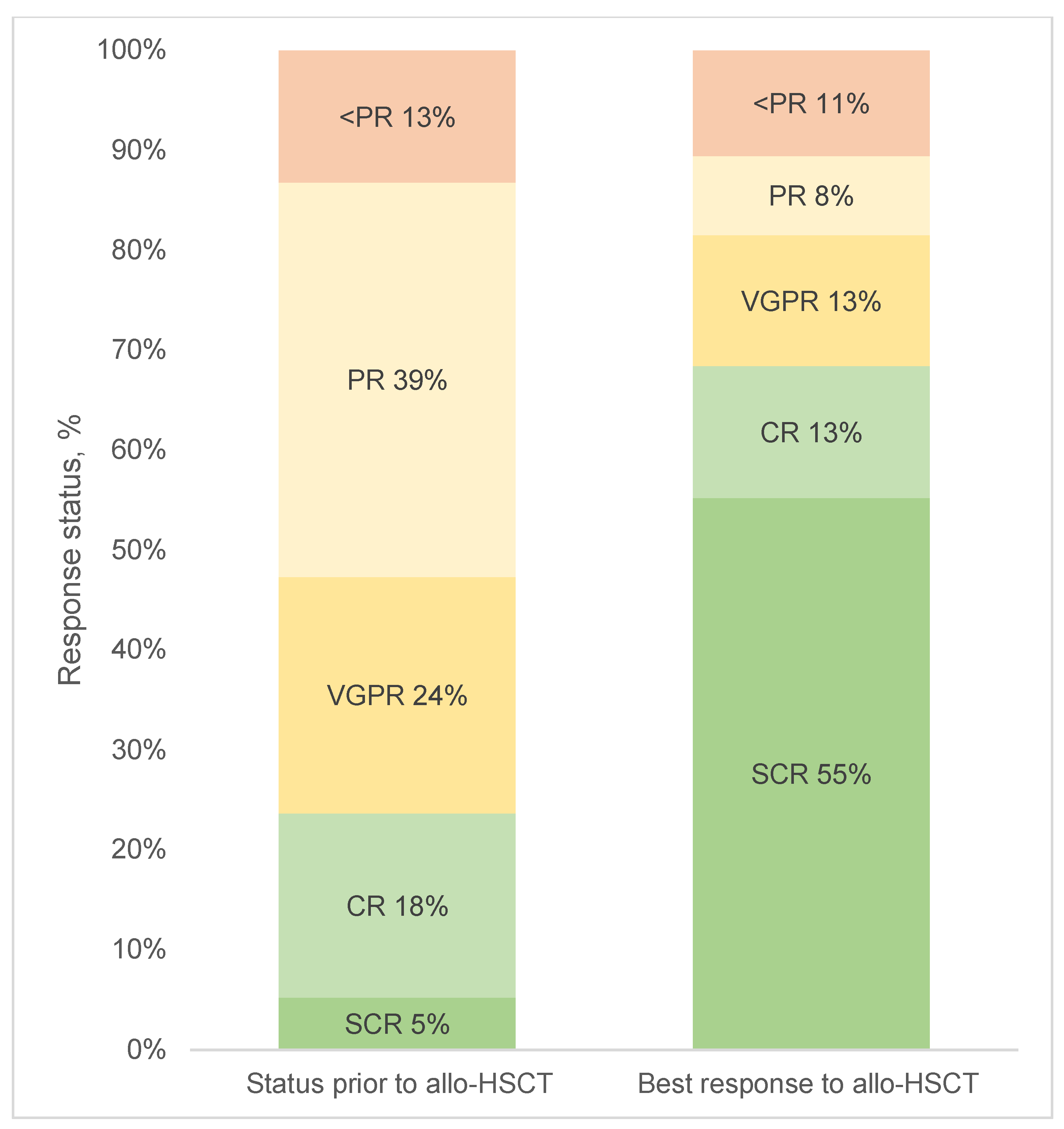
3.2. Response to Allo-HSCT and Maintenance
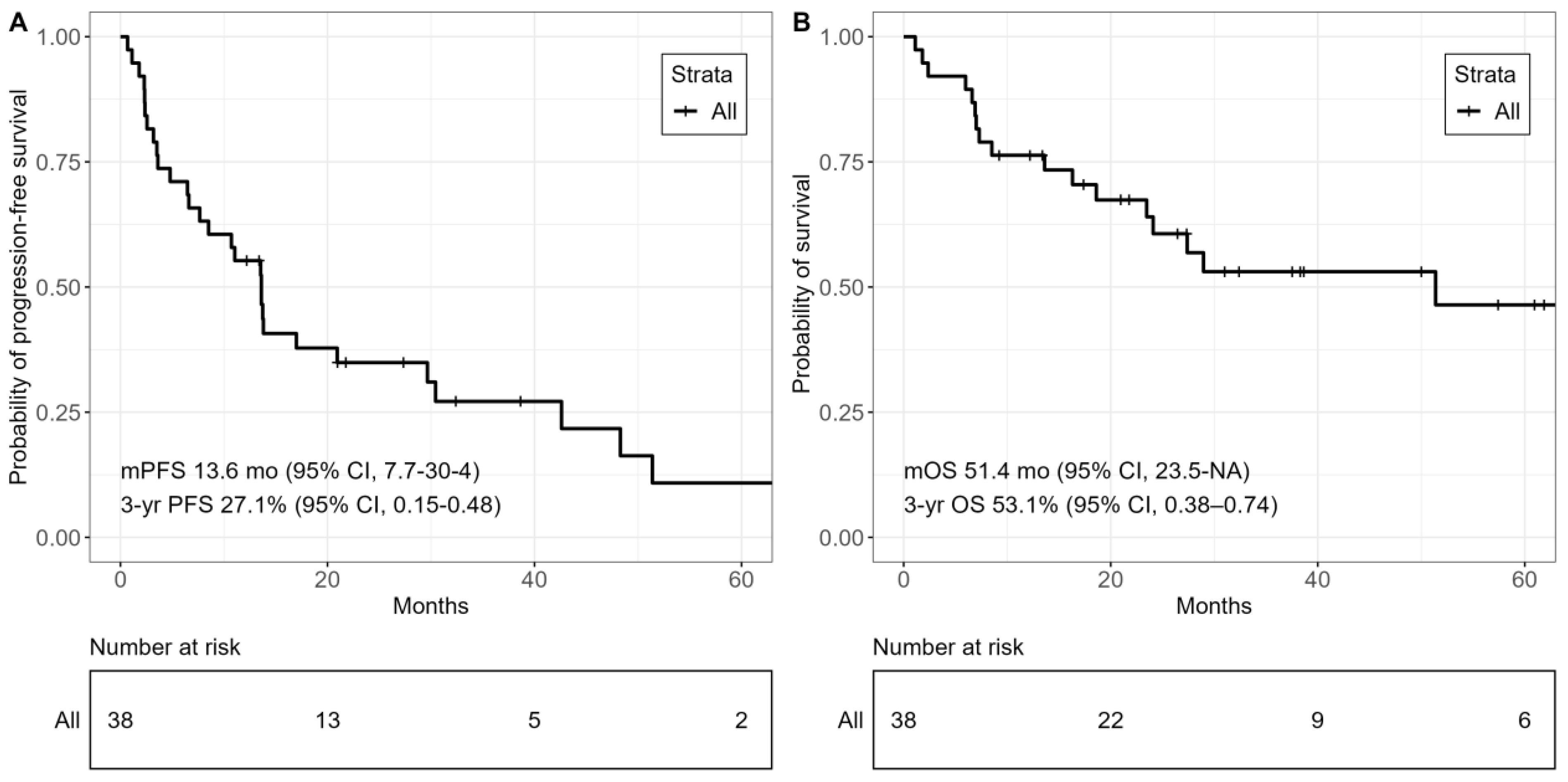
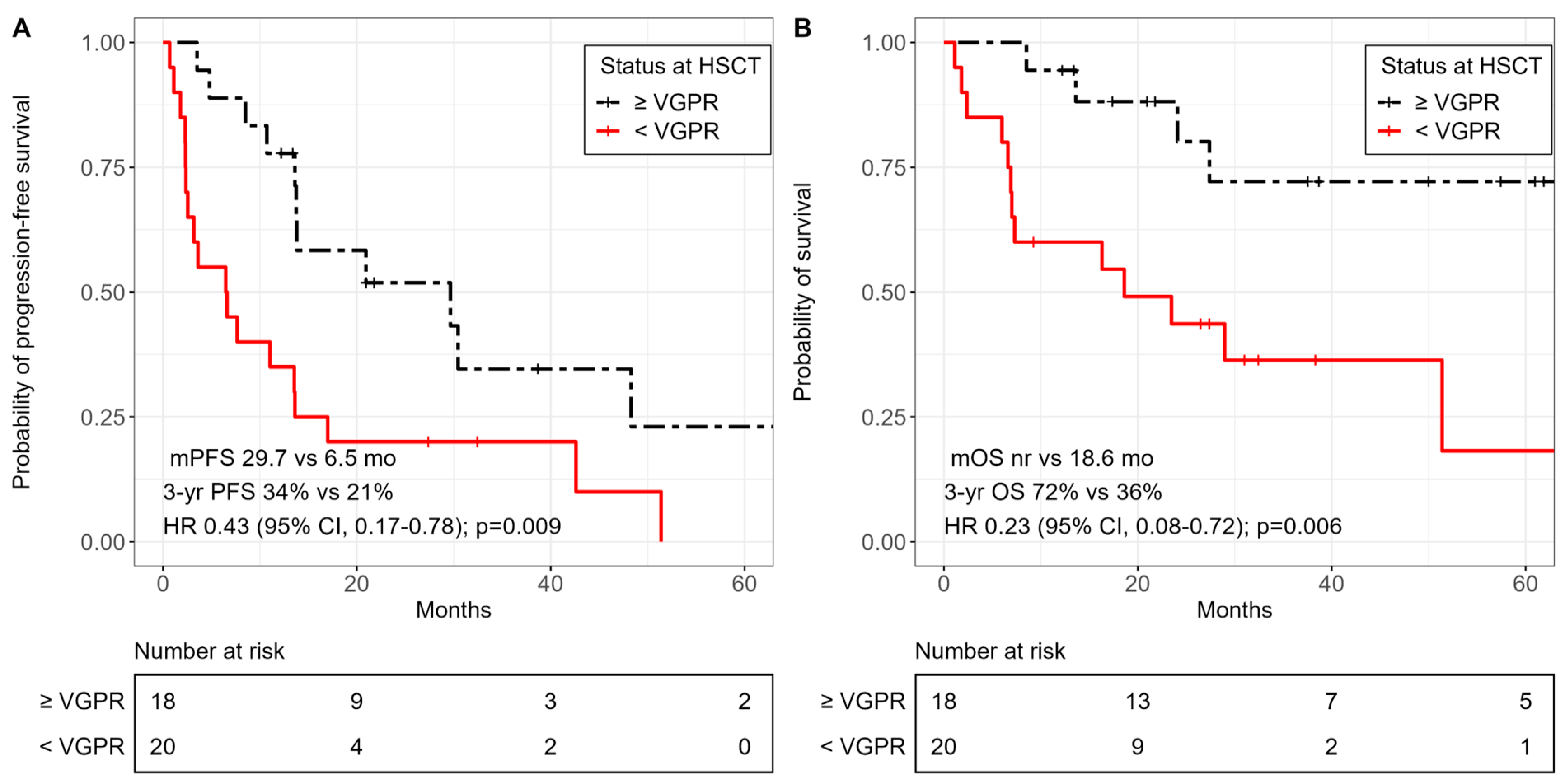
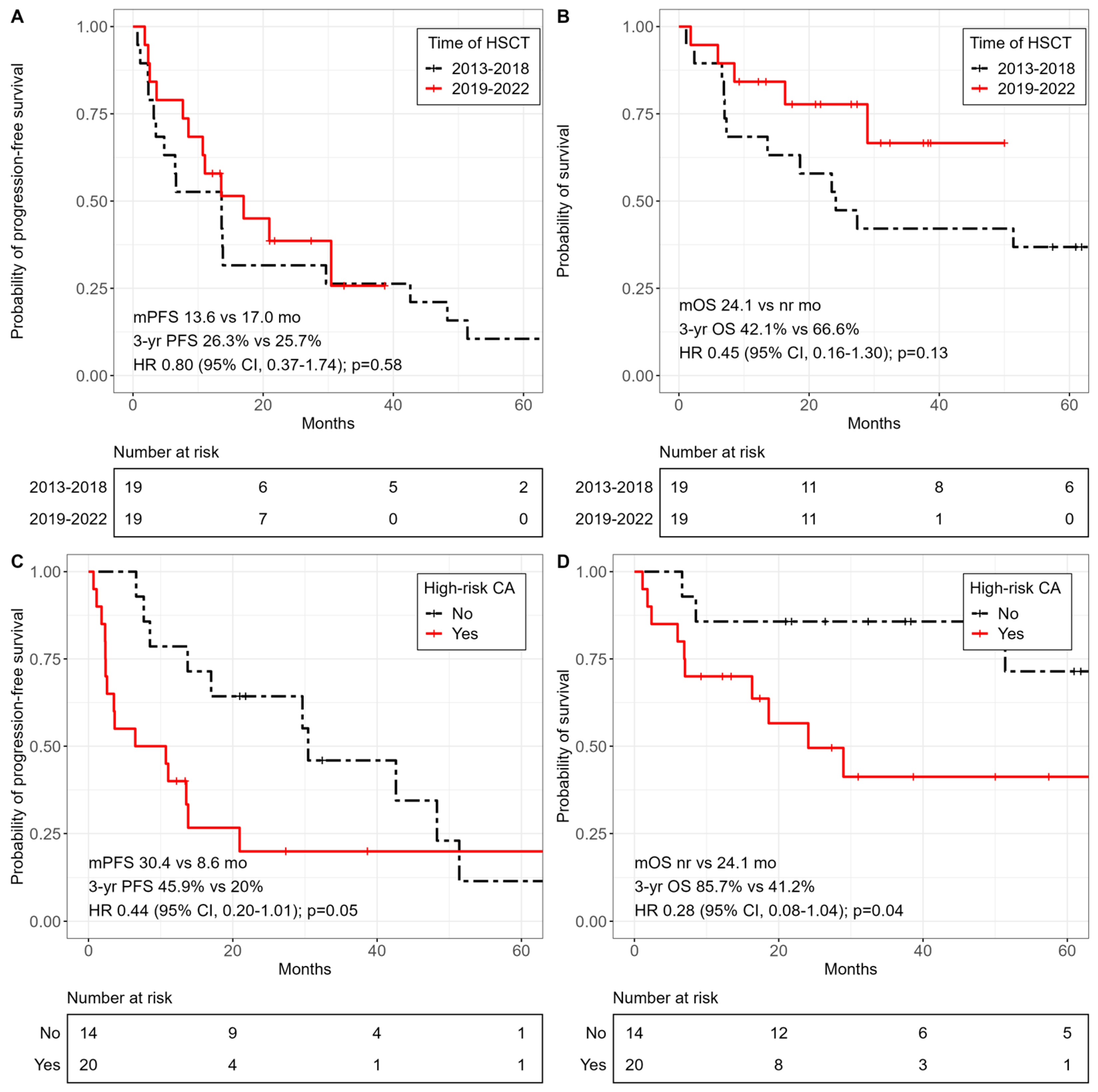
3.3. Survival Outcomes
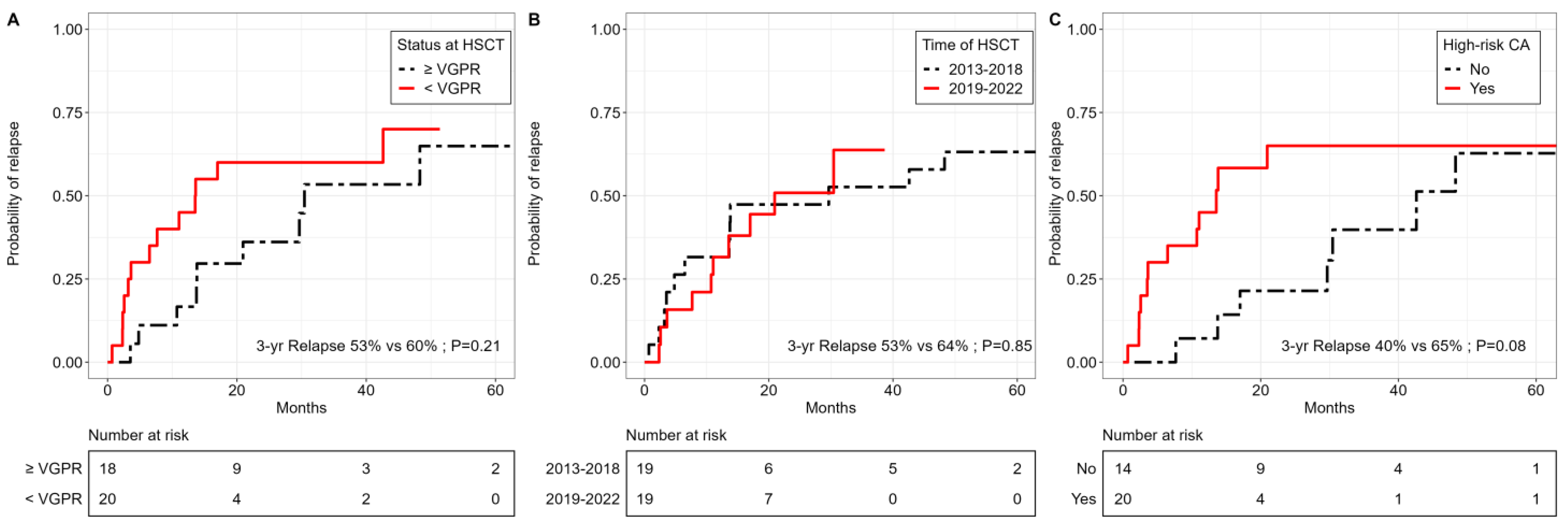
3.4. Relapse Incidence, GHVD and Non-Relapse Mortality
3.5. Subsequent Therapies and Outcomes after Relapse Post-Allo-HSCT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Berenson, J.R. Predicting Outcomes and Monitoring Disease in Patients with Multiple Myeloma. Clin. Adv. Hematol. Oncol. 2023, 21, 484–493. [Google Scholar]
- Ramasamy, K.; Gay, F.; Weisel, K.; Zweegman, S.; Mateos, M.V.; Richardson, P. Improving outcomes for patients with relapsed multiple myeloma: Challenges and considerations of current and emerging treatment options. Blood Rev. 2021, 49, 100808. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Weisel, K.; De Stefano, V.; Goldschmidt, H.; Delforge, M.; Mohty, M.; Cavo, M.; Vij, R.; Lindsey-Hill, J.; Dytfeld, D.; et al. LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 2022, 36, 1371–1376. [Google Scholar] [CrossRef]
- Charalampous, C.; Goel, U.; Kapoor, P.; Binder, M.; Buadi, F.K.; Cook, J.; Dingli, D.; Dispenzieri, A.; Fonder, A.L.; Gertz, M.A.; et al. Outcomes of patients with primary refractory multiple myeloma in the era of triplet and quadruplet induction therapy. Blood Adv. 2023, 7, 4371–4380. [Google Scholar] [CrossRef]
- Giralt, S.; Garderet, L.; Durie, B.; Cook, G.; Gahrton, G.; Bruno, B.; Hari, P.; Lokhorst, H.; McCarthy, P.; Krishnan, A.; et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol. Blood Marrow Transplant. 2015, 21, 2039–2051. [Google Scholar] [CrossRef]
- Greil, C.; Engelhardt, M.; Finke, J.; Wäsch, R. Allogeneic Stem Cell Transplantation in Multiple Myeloma. Cancers 2021, 14, 55. [Google Scholar] [CrossRef]
- Sobh, M.; Michallet, M.; Gahrton, G.; Iacobelli, S.; van Biezen, A.; Schönland, S.; Petersen, E.; Schaap, N.; Bonifazi, F.; Volin, L.; et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: Trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia 2016, 30, 2047–2054. [Google Scholar] [CrossRef]
- Crawley, C.; Iacobelli, S.; Björkstrand, B.; Apperley, J.F.; Niederwieser, D.; Gahrton, G. Reduced-intensity conditioning for myeloma: Lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood 2007, 109, 3588–3594. [Google Scholar] [CrossRef]
- Costa, L.J.; Iacobelli, S.; Pasquini, M.C.; Modi, R.; Giaccone, L.; Blade, J.; Schonland, S.; Evangelista, A.; Perez-Simon, J.A.; Hari, P.; et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. 2020, 55, 1810–1816. [Google Scholar] [CrossRef]
- Gahrton, G.; Iacobelli, S.; Garderet, L.; Yakoub-Agha, I.; Schönland, S. Allogeneic Transplantation in Multiple Myeloma—Does It Still Have a Place? J. Clin. Med. 2020, 9, 2180. [Google Scholar] [CrossRef]
- López-Corral, L.; Caballero-Velázquez, T.; López-Godino, O.; Rosiñol, L.; Pérez-Vicente, S.; Fernandez-Avilés, F.; Krsnik, I.; Morillo, D.; Heras, I.; Morgades, M.; et al. Response to Novel Drugs before and after Allogeneic Stem Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol. Blood Marrow Transplant. 2019, 25, 1703–1712. [Google Scholar] [CrossRef]
- Chhabra, S.; Szabo, A.; Glisch, C.; George, G.; Narra, R.K.; Harrington, A.; Jerkins, J.H.; D’Souza, A.; Dhakal, B.; Pasquini, M.C.; et al. Relapse after Allogeneic Hematopoietic Cell Transplantation for Multiple Myeloma: Survival Outcomes and Factors Influencing Them. Biol. Blood Marrow Transplant. 2020, 26, 1288–1297. [Google Scholar] [CrossRef]
- Htut, M.; Dhakal, B.; Cohen, A.D.; Martin, T.; Berdeja, J.G.; Usmani, S.Z.; Agha, M.; Jackson, C.C.; Madduri, D.; Deraedt, W.; et al. Ciltacabtagene Autoleucel in Patients with Prior Allogeneic Stem Cell Transplant in the CARTITUDE-1 Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, 882–888. [Google Scholar] [CrossRef] [PubMed]
- John, L.; Sauer, S.; Hegenbart, U.; Dreger, P.; Hundemer, M.; Müller-Tidow, C.; Schmitt, A.; Schmitt, M.; Raab, M.S.; Schönland, S.O. Idecabtagene Vicleucel Is Well Tolerated and Effective in Relapsed/Refractory Myeloma Patients with Prior Allogeneic Stem Cell Transplantation. Transplant. Cell Ther. 2023, 29, 609.e1–609.e6. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Elsada, A.; Zalin-Miller, A.; Knott, C.; Caravotas, L. A registry study of relapsed or refractory multiple myeloma pre-exposed to three or more prior therapies including a proteasome inhibitor, an immunomodulatory agent and CD38-targeted monoclonal antibody therapy in England. eJHaem 2021, 2, 493–497. [Google Scholar] [CrossRef]
- Jagannath, S.; Lin, Y.; Goldschmidt, H.; Reece, D.; Nooka, A.; Senin, A.; Rodriguez-Otero, P.; Powles, R.; Matsue, K.; Shah, N.; et al. KarMMa-RW: Comparison of idecabtagene vicleucel with real-world outcomes in relapsed and refractory multiple myeloma. Blood Cancer J. 2021, 11, 116. [Google Scholar] [CrossRef]
- Merz, M.; Goldschmidt, H.; Hari, P.; Agha, M.; Diels, J.; Ghilotti, F.; Perualila, N.J.; Cabrieto, J.; Haefliger, B.; Sliwka, H.; et al. Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry. Cancers 2021, 13, 5996. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Vekemans, M.-C.; Anguille, S.; Depaus, J.; Meuleman, N.; Van de Velde, A.; Vande Broek, I.; Strens, D.; Van Hoorenbeeck, S.; Moorkens, E.J.; et al. Real-World Outcomes for Standard-of-Care Treatments in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2021, 138, 4075. [Google Scholar] [CrossRef]
- Tang, D.; Hari, P.; Ramasamy, K.; Weisel, K.; Joshi, P.; Liu, L.; Che, M.; Hernandez, G.; Abonour, R.; Hardin, J.W.; et al. Real-World Treatment Patterns and Clinical, Economic, and Humanistic Burden in Triple-Class Refractory Multiple Myeloma: Analysis of the Connect ® Multiple Myeloma (MM) Disease Registry. Blood 2021, 138, 117. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]

| 2013–2018 (n = 19) | 2019–2022 (n = 19) | All Patients (n = 38) | |
|---|---|---|---|
| Median age, years | 52.8 (38.4–64.4) | 58.1 (44.8–67.2) | 55.2 (38.4–67.2) |
| Sex | |||
| Male | 12 (63%) | 11 (58%) | 23 (61%) |
| Female | 7 (37%) | 8 (42%) | 15 (39%) |
| Median time from diagnosis to allo-HSCT, years | 3.7 (0.6–8.2) | 3.8 (0.3–13.6) | 3.8 (0.3–13.6) |
| Extramedullary disease | 2 (11%) | 6 (32%) | 8 (21%) |
| ISS stage | |||
| I | 7 (37%) | 4 (21%) | 11 (29%) |
| II | 5 (26%) | 5 (26%) | 10 (26%) |
| III | 3 (16%) | 6 (32%) | 9 (24%) |
| Unknown | 4 (21%) | 4 (21%) | 8 (21%) |
| Cytogenetic profile | |||
| Any high-risk CA | 8 (42%) | 12 (63%) | 20 (53%) |
| High-risk IMWG | 1 (5%) | 5 (26%) | 6 (16%) |
| Gain/Amp 1q | 7 (37%) | 10 (53%) | 17 (45%) |
| Complex karyotype | 4 (21%) | 0 (0%) | 4 (11%) |
| Median previous therapy regimens | 7 (4–13) | 7 (4–13) | 7 (4–13) |
| Previous ASCT | 18 (95%) | 18 (95%) | 36 (95%) |
| >1 ASCT | 9 (47%) | 10 (53%) | 19 (50%) |
| Tandem ASCT | 2 (11%) | 3 (16%) | 5 (13%) |
| Triple-class exposed | 9 (47%) | 19 (100%) | 28 (74%) |
| Triple-class refractory | 4 (21%) | 5 (26%) | 9 (24%) |
| 2013–2018 (n = 19) | 2019–2022 (n = 19) | All Patients (n = 38) | |
|---|---|---|---|
| Status at allo-HSCT | |||
| SCR | 0 (0%) | 2 (11%) | 2 (5%) |
| CR | 4 (21%) | 3 (16%) | 7 (18%) |
| VGPR | 5 (26%) | 4 (21%) | 9 (24%) |
| PR | 8 (42%) | 7 (37%) | 15 (39%) |
| SD | 0 (0%) | 3 (16%) | 3 (8%) |
| PD | 2 (11%) | 0 (0%) | 2 (5%) |
| Type of transplantation | |||
| MRD | 5 (26%) | 5 (26%) | 10 (26%) |
| MUD | 7 (37%) | 6 (32%) | 13 (34%) |
| Haplo | 5 (26%) | 7 (37%) | 12 (32%) |
| MMUD | 2 (11%) | 1 (5%) | 3 (8%) |
| Source of stem cells | |||
| BM | 6 (32%) | 0 (0%) | 6 (16%) |
| PB | 13 (68%) | 19 (100%) | 32 (84%) |
| Conditioning regimen | |||
| Myeloablative | 10 (53%) | 11 (58%) | 21 (55%) |
| Reduced intensity | 9 (47%) | 8 (42%) | 17 (45%) |
| Auto-allo protocol | 4 (21%) | 0 (0%) | 4 (11%) |
| TBI | 8 (42%) | 14 (74%) | 22 (58%) |
| GVHD prophylaxis | |||
| ATLG | 15 (79%) | 10 (53%) | 25 (66%) |
| CNI-MTX | 4 (21%) | 0 (0%) | 4 (11%) |
| CNI-MMF | 10 (53%) | 11 (58%) | 21 (55%) |
| PTCy-Tac-MMF | 5 (26%) | 8 (42%) | 13 (34%) |
| Maintenance therapy post allo-HSCT | 1 (5%) | 9 (47%) | 10 (26%) |
| DLI | 8 (42%) | 6 (32%) | 14 (37%) |
| Variable | Hazard Ratio | p-Value |
|---|---|---|
| Less than VGPR before allo-HSCT | 7.70 (1.22–48.65) | 0.03 |
| High-risk CA | 5.28 (1.13–24.82) | 0.04 |
| Allo-HSCT 2013-2018 | 0.54 (0.12–2.38) | 0.42 |
| Interval ASCT to allo-HSCT < 2 years | 2.19 (0.44–11.01) | 0.34 |
| One ASCT before allo-HSCT (versus two) | 0.65 (0.15–2.86) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strassl, I.; Nikoloudis, A.; Machherndl-Spandl, S.; Buxhofer-Ausch, V.; Binder, M.; Wipplinger, D.; Stiefel, O.; Kaynak, E.; Milanov, R.; Aichinger, C.; et al. Allogeneic Stem Cell Transplantation in Multiple Myeloma: Risk Factors and Outcomes in the Era of New Therapeutic Options—A Single-Center Experience. Cancers 2023, 15, 5738. https://doi.org/10.3390/cancers15245738
Strassl I, Nikoloudis A, Machherndl-Spandl S, Buxhofer-Ausch V, Binder M, Wipplinger D, Stiefel O, Kaynak E, Milanov R, Aichinger C, et al. Allogeneic Stem Cell Transplantation in Multiple Myeloma: Risk Factors and Outcomes in the Era of New Therapeutic Options—A Single-Center Experience. Cancers. 2023; 15(24):5738. https://doi.org/10.3390/cancers15245738
Chicago/Turabian StyleStrassl, Irene, Alexander Nikoloudis, Sigrid Machherndl-Spandl, Veronika Buxhofer-Ausch, Michaela Binder, Dagmar Wipplinger, Olga Stiefel, Emine Kaynak, Robert Milanov, Christoph Aichinger, and et al. 2023. "Allogeneic Stem Cell Transplantation in Multiple Myeloma: Risk Factors and Outcomes in the Era of New Therapeutic Options—A Single-Center Experience" Cancers 15, no. 24: 5738. https://doi.org/10.3390/cancers15245738
APA StyleStrassl, I., Nikoloudis, A., Machherndl-Spandl, S., Buxhofer-Ausch, V., Binder, M., Wipplinger, D., Stiefel, O., Kaynak, E., Milanov, R., Aichinger, C., Nocker, S., Bauer, T., Kreissl, S., Girschikofsky, M., Petzer, A., Weltermann, A., & Clausen, J. (2023). Allogeneic Stem Cell Transplantation in Multiple Myeloma: Risk Factors and Outcomes in the Era of New Therapeutic Options—A Single-Center Experience. Cancers, 15(24), 5738. https://doi.org/10.3390/cancers15245738






