The Anti- and Pro-Tumorigenic Role of Microbiota and Its Role in Anticancer Therapeutic Strategies
Abstract
:Simple Summary
Abstract
1. Introduction
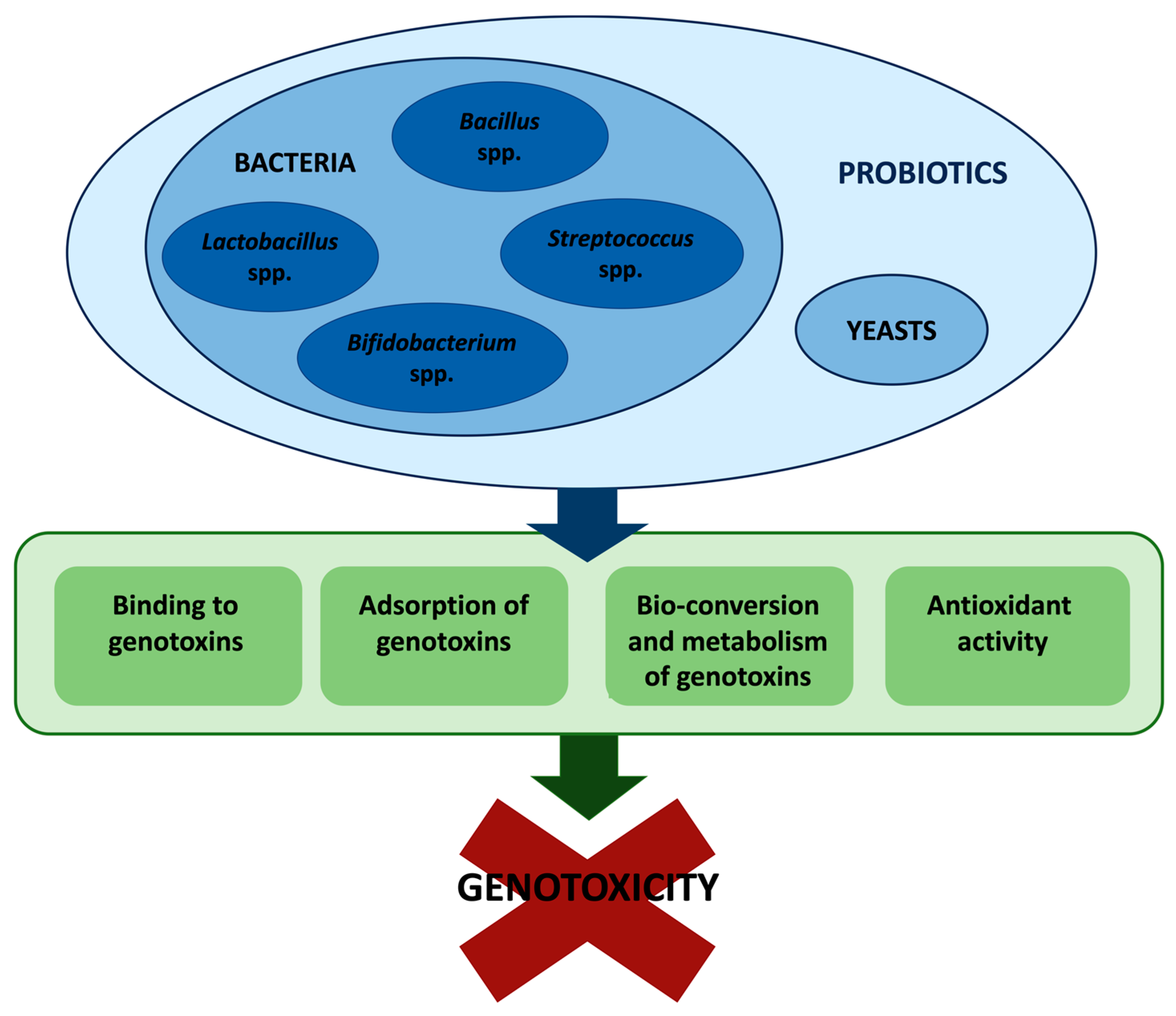
2. Antigenotoxic Effects of Gut Microbiota
2.1. Antigenotoxic Effects of Probiotics toward Heterocyclic Aromatic Amines (HAAs) and N-Nitrosamines
2.2. Antigenotoxic Effects of Probiotics toward Polycyclic Aromatic Hydrocarbons (PAHs)
3. Pro-carcinogenic Effects of Gut Microbiota
- (1)
- alterations of microbiota composition leading to immune evasion, chronic inflammation, and alteration of proliferative responses, which in turn may promote carcinogenesis;
- (2)
- generation of genotoxic metabolites through the bacterial activity of biotransformation;
- (3)
- direct production of bacterial genotoxins.
4. Bidirectional Interactions between Drugs and Microbiota
Anticancer Drugs
5. Effects of Local Microbiota on Tumor Microenvironment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The Role of Gut Microbiome in Cancer Genesis and Cancer Prevention. Health Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Gagnière, J. Gut Microbiota Imbalance and Colorectal Cancer. World J. Gastroenterol. 2016, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential Role of Intratumor Bacteria in Mediating Tumor Resistance to the Chemotherapeutic Drug Gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, V.; Gasperini, S.; Hrelia, P.; Tirri, M.; Marti, M.; Lenzi, M. Novel Psychoactive Phenethylamines: Impact on Genetic Material. Int. J. Mol. Sci. 2020, 21, 9616. [Google Scholar] [CrossRef]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and Its Consequences. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 87–110. [Google Scholar]
- Abid, Z.; Cross, A.J.; Sinha, R. Meat, Dairy, and Cancer. Am. J. Clin. Nutr. 2014, 100, 386S–393S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea Sativa Mill. Bark Extract Exhibits Chemopreventive Properties Triggering Extrinsic Apoptotic Pathway in Jurkat Cells. BMC Complement. Altern. Med. 2017, 17, 251. [Google Scholar] [CrossRef]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet Chestnut (Castanea Sativa Mill.) Bark Extract: Cardiovascular Activity and Myocyte Protection against Oxidative Damage. Oxid. Med. Cell Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fimognari, C.; Sestili, P.; Lenzi, M.; Cantelli-Forti, G.; Hrelia, P. Protective Effect of Creatine against RNA Damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2009, 670, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic Compounds and Their Possible Mechanisms of Action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, S.K.; Ranganatha, R.; Chakravarthy, S. High-Throughput Approaches for Genotoxicity Testing in Drug Development: Recent Advances. Int. J. High Throughput Screen. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Al-koshab, M.; Alabsi, A.M.; Mohd Bakri, M.; Ali-Saeed, R.; Selvi Naicker, M. Antitumor Activity of Ficus Deltoidea Extract on Oral Cancer: An In Vivo Study. J. Oncol. 2020, 2020, 5490468. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf (accessed on 15 October 2022).
- Krzyszczak, A.; Czech, B. Occurrence and Toxicity of Polycyclic Aromatic Hydrocarbons Derivatives in Environmental Matrices. Sci. Total Environ. 2021, 788, 147738. [Google Scholar] [CrossRef]
- Nowak, A.; Libudzisz, Z. Ability of Probiotic Lactobacillus Casei DN 114001 to Bind or/and Metabolise Heterocyclic Aromatic Amines in Vitro. Eur. J. Nutr. 2009, 48, 419–427. [Google Scholar] [CrossRef]
- Ambalam, P.; Dave, J.M.; Nair, B.M.; Vyas, B.R.M. In Vitro Mutagen Binding and Antimutagenic Activity of Human Lactobacillus Rhamnosus 231. Anaerobe 2011, 17, 217–222. [Google Scholar] [CrossRef]
- Caldini, G.; Trotta, F.; Villarini, M.; Moretti, M.; Pasquini, R.; Scassellati-Sforzolini, G.; Cenci, G. Screening of Potential Lactobacilli Antigenotoxicity by Microbial and Mammalian Cell-Based Tests. Int. J. Food Microbiol. 2005, 102, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Prete, R.; Tofalo, R.; Federici, E.; Ciarrocchi, A.; Cenci, G.; Corsetti, A. Food-Associated Lactobacillus Plantarum and Yeasts Inhibit the Genotoxic Effect of 4-Nitroquinoline-1-Oxide. Front. Microbiol. 2017, 8, 02349. [Google Scholar] [CrossRef] [Green Version]
- Caldini, G.; Trotta, F.; Cenci, G. Inhibition of 4-Nitroquinoline-1-Oxide Genotoxicity by Bacillus Strains. Res. Microbiol. 2002, 153, 165–171. [Google Scholar] [CrossRef]
- Cenci, G.; Rossi, J.; Trotta, F.; Caldini, G. Lactic Acid Bacteria Isolated from Dairy Products Inhibit Genotoxic Effect of 4-Nitroquinoline-1-Oxide in SOS-Chromotest. Syst. Appl. Microbiol. 2002, 25, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lankaputhra, W.E.V.; Shah, N.P. Antimutagenic Properties of Probiotic Bacteria and of Organic Acids. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 397, 169–182. [Google Scholar] [CrossRef]
- Afshar, P.; Roozbeh-Nasiraie, L.; Shokrzadeh, M.; Ghorbani-HasanSaraei, A.; Naghizadeh-Raeisi, S. Bio-Protective Effects of Lactobacillus Plantarum Subsp.Plantarum Against Aflatoxin B1 Genotoxicity on Human Blood Lymphocytes: A Native Probiotic Strain Isolated FromIranian Camel Milk. Curr. Med. Mycol. 2020, 6, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.; Wu, Q.K.; El-Nezami, H.; Juvonen, R.O.; Mykkänen, H.; Turner, P.C. Lactobacillus Rhamnosus Strain GG Reduces Aflatoxin B 1 Transport, Metabolism, and Toxicity in Caco-2 Cells. Appl. Environ. Microbiol. 2007, 73, 3958–3964. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Kuberski, S.; Libudzisz, Z. Probiotic Lactic Acid Bacteria Detoxify N-Nitrosodimethylamine. Food Addit. Contam. Part A 2014, 31, 1678–1687. [Google Scholar] [CrossRef]
- Hosono, A.; Wardojo, R.; Otani, H. Inhibitory Effects of Lactic Acid Bacteria from Fermented Milk on the Mutagenicities of Volatile Nitrosamines. Agric. Biol. Chem. 1990, 54, 1639–1643. [Google Scholar] [CrossRef]
- Bocci, A.; Sebastiani, B.; Trotta, F.; Federici, E.; Cenci, G. In Vitro Inhibition of 4-Nitroquinoline-1-Oxide Genotoxicity by Probiotic Lactobacillus Rhamnosus IMC501. J. Microbiol. Biotechnol. 2015, 25, 1680–1686. [Google Scholar] [CrossRef]
- Corsetti, A.; Caldini, G.; Mastrangelo, M.; Trotta, F.; Valmorri, S.; Cenci, G. Raw Milk Traditional Italian Ewe Cheeses as a Source of Lactobacillus Casei Strains with Acid-Bile Resistance and Antigenotoxic Properties. Int. J. Food Microbiol. 2008, 125, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Darsanaki, R.K.; Ghaemi, N.; Mirpour, M. Antimutagenic Activity of Lactobacillus spp. Isolated from Commercial Yoghurt versus Sodium Azide, Acrylic Amide, Potassium Permanganate and 2-Nitrofluorene. Minerva Biotechnol. Biomol. Res. 2016, 29, 24–29. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, L.; Liu, A.-P.; Guo, X.-H.; Ren, F.-Z. Analysis of Antigenotoxicity of Lactobacillus Salivarius by High Performance Liquid Chromatography. Chin. J. Anal. Chem. 2008, 36, 740–744. [Google Scholar] [CrossRef]
- Tsuda, H.; Hara, K.; Miyamoto, T. Binding of Mutagens to Exopolysaccharide Produced by Lactobacillus Plantarum Mutant Strain 301102S. J. Dairy Sci. 2008, 91, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Caldini, G.; Trotta, F.; Corsetti, A.; Cenci, G. Evidence for in Vitro Anti-Genotoxicity of Cheese Non-Starter Lactobacilli. Antonie Van Leeuwenhoek 2008, 93, 51–59. [Google Scholar] [CrossRef]
- Walia, S.; Keshani; Sood, S.; Kanwar, S.S. Exhibition of DNA-Bioprotective Activity by Microflora of Traditional Fermented Foods of North-Western Himalayas. Food Res. Int. 2014, 55, 176–180. [Google Scholar] [CrossRef]
- Nowak, A.; Czyżowska, A.; Stańczyk, M. Protective Activity of Probiotic Bacteria against 2-Amino-3-Methyl-3H-Imidazo[4,5-f]Quinoline (IQ) and 2-Amino-1-Methyl-6-Phenyl-1 H-Imidazo[4,5-b]Pyridine (PhIP)—An in Vitro Study. Food Addit. Contam. Part A 2015, 32, 1927–1938. [Google Scholar] [CrossRef]
- Xu, M.; Fu, L.; Zhang, J.; Wang, T.; Fan, J.; Zhu, B.; Dziugan, P.; Zhang, B.; Zhao, H. Potential of Inactivated Bifidobacterium Strain in Attenuating Benzo(A)Pyrene Exposure-Induced Damage in Colon Epithelial Cells In Vitro. Toxics 2020, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Cenci, G.; Caldini, G.; Trotta, F.; Bosi, P. In Vitro Inhibitory Activity of Probiotic Spore-Forming Bacilli against Genotoxins. Lett. Appl. Microbiol. 2008, 46, 331–337. [Google Scholar] [CrossRef]
- Isidori, M.; Parrella, A. Genotoxicity of Aqueous Extract from Heated Cooking Oils and Its Suppression by Lactobacilli. Food Sci. Technol. Int. 2009, 15, 267–273. [Google Scholar] [CrossRef]
- Janosch, D.; Dubbert, S.; Eiteljörge, K.; Diehl, B.W.K.; Sonnenborn, U.; Passchier, L.V.; Wassenaar, T.M.; von Bünau, R. Anti-Genotoxic and Anti-Mutagenic Activity of Escherichia Coli Nissle 1917 as Assessed by in Vitro Tests. Benef. Microbes 2019, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Dominici, L.; Villarini, M.; Trotta, F.; Federici, E.; Cenci, G.; Moretti, M. Protective Effects of Probiotic Lactobacillus Rhamnosus IMC501 in Mice Treated with PhIP. J. Microbiol. Biotechnol. 2014, 24, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Horie, H.; Zeisig, M.; Hirayama, K.; Midtvedt, T.; Möller, L.; Rafter, J. Probiotic Mixture Decreases DNA Adduct Formation in Colonic Epithelium Induced by the Food Mutagen 2-Amino-9H-Pyrido[2,3-b]Indole in a Human-Flora Associated Mouse Model. Eur. J. Cancer Prev. 2003, 12, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Xu, M.; Wang, T.; Dziugan, P.; Zhao, H.; Zhang, B. Detoxification of Oral Exposure to Benzo(a)Pyrene by Lactobacillus Plantarum CICC 23121 in Mice. Mol. Nutr. Food Res. 2021, 65, 2001149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, V.; Nagpal, R.; Kumar, A.; Gautam, S.K.; Behare, P.V.; Grover, C.R.; Aggarwal, P.K. Effect of Probiotic Fermented Milk and Chlorophyllin on Gene Expressions and Genotoxicity during AFB1-Induced Hepatocellular Carcinoma. Gene 2011, 490, 54–59. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Márquez-Márquez, R.; Reyes, A. Antigenotoxic Effect of Saccharomyces Cerevisiae on the Damage Produced in Mice Fed with Aflatoxin B1 Contaminated Corn. Food Chem. Toxicol. 2006, 44, 2058–2063. [Google Scholar] [CrossRef]
- Deabes, M.M.; Darwish, H.R.; Abdel-Aziz, K.B.; Farag, I.M.; Nada, S.A.; Tawfek, N.S. Protective Effects of Lactobacillus Rhamnosus GG on Aflatoxins-Induced Toxicities in Male Albino Mice. J. Environ. Anal. Toxicol. 2012, 2, 3. [Google Scholar] [CrossRef]
- González Pereyra, M.L.; Dogi, C.; Torres Lisa, A.; Wittouck, P.; Ortíz, M.; Escobar, F.; Bagnis, G.; Yaciuk, R.; Poloni, L.; Torres, A.; et al. Genotoxicity and Cytotoxicity Evaluation of Probiotic Saccharomyces cerevisiae RC016: A 60-Day Subchronic Oral Toxicity Study in Rats. J. Appl. Microbiol. 2014, 117, 824–833. [Google Scholar] [CrossRef]
- Khalil, A.A.; Abou-Gabal, A.E.; Abdellatef, A.A.; Khalid, A.E. Protective Role of Probiotic Lactic Acid Bacteria Against Dietary Fumonisin B1-Induced Toxicity and DNA-Fragmentation in Sprague-Dawley Rats. Prep. Biochem. Biotechnol. 2015, 45, 530–550. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Jiang, L.; Liu, R.; Zhang, L.; Lei, X.; Li, J.; Jiang, J.; Guo, H.; Fang, B.; et al. Lactobacillus salivarius REN Inhibits Rat Oral Cancer Induced by 4-Nitroquioline 1-Oxide. Cancer Prev. Res. 2013, 6, 686–694. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Cross, K.P.; Ponting, D.J. Developing Structure-Activity Relationships for N-Nitrosamine Activity. Comput. Toxicol. 2021, 20, 100186. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive Review of Polycyclic Aromatic Hydrocarbons in Water Sources, Their Effects and Treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Gómez-Martín, M.; Suárez, A.; González-Bernardo, O.; de los Reyes-Gavilán, C.G.; González, S. Xenobiotics Formed during Food Processing: Their Relation with the Intestinal Microbiota and Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Shoukat, S.; Aslam, M.Z.; Rehman, A.; Zhang, B. Screening of Bifidobacterium Strains to Bind with Benzo[a]Pyrene under Food Stress Factors and the Mechanism of the Process. J. Food Process. Preserv. 2019, 43, 13956. [Google Scholar] [CrossRef]
- Limeiras, S.M.A.; Ogo, F.M.; Genez, L.A.L.; Carreira, C.M.; Oliveira, E.J.T.; Pessatto, L.R.; Neves, S.C.; Pesarini, J.R.; Schweich, L.C.; Silva, R.A.; et al. Prevention of DNA Damage and Anticarcinogenic Activity of Activia® in a Preclinical Model. Genet. Mol. Res. 2017, 16, 9492. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium Nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.S. Association of Streptococcus Bovis Bacteremia With Colonic Neoplasia and Extracolonic Malignancy. Arch. Surg. 2004, 139, 760. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, Y.; Huycke, M.M. Commensal-Infected Macrophages Induce Dedifferentiation and Reprogramming of Epithelial Cells during Colorectal Carcinogenesis. Oncotarget 2017, 8, 102176–102190. [Google Scholar] [CrossRef]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-Driven Mechanisms at Different Stages of Cancer Development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef] [PubMed]
- Alaish, S.M.; Smith, A.D.; Timmons, J.; Greenspon, J.; Eyvazzadeh, D.; Murphy, E.; Shea-Donahue, T.; Cirimotich, S.; Mongodin, E.; Zhao, A.; et al. Gut Microbiota, Tight Junction Protein Expression, Intestinal Resistance, Bacterial Translocation and Mortality Following Cholestasis Depend on the Genetic Background of the Host. Gut Microbes 2013, 4, 292–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, T.; Akira, S. Toll-Like Receptor Signaling and Its Inducible Proteins. Microbiol. Spectr. 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zyla, J.; He, L.; Moura-Alves, P.; Steinle, H.; Saikali, P.; Lozza, L.; Nieuwenhuizen, N.; Weiner, J.; Mollenkopf, H.; et al. Cellular Stress Promotes NOD1/2-dependent Inflammation via the Endogenous Metabolite Sphingosine-1-phosphate. EMBO J. 2021, 40, e106272. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Morin, P.J.; Maouyo, D.; Sears, C.L. Bacteroides Fragilis Enterotoxin Induces C-Myc Expression and Cellular Proliferation. Gastroenterology 2003, 124, 392–400. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium Nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via Its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Chandra, H.; Sharma, K.K.; Tuovinen, O.H.; Sun, X.; Shukla, P. Pathobionts: Mechanisms of Survival, Expansion, and Interaction with Host with a Focus on Clostridioides difficile. Gut Microbes 2021, 13, 1979882. [Google Scholar] [CrossRef]
- Wang, X.; Allen, T.D.; May, R.J.; Lightfoot, S.; Houchen, C.W.; Huycke, M.M. Enterococcus Faecalis Induces Aneuploidy and Tetraploidy in Colonic Epithelial Cells through a Bystander Effect. Cancer Res. 2008, 68, 9909–9917. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, Y.; Moore, D.R.; Nimmo, S.L.; Lightfoot, S.A.; Huycke, M.M. 4-Hydroxy-2-Nonenal Mediates Genotoxicity and Bystander Effects Caused by Enterococcus Faecalis–Infected Macrophages. Gastroenterology 2012, 142, 543–551.e7. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, Y.; Huycke, M.M. Commensal Bacteria Drive Endogenous Transformation and Tumour Stem Cell Marker Expression through a Bystander Effect. Gut 2015, 64, 459–468. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, N.; Park, J.H.; Kim, Y.S.; Lee, J.; Kim, H.W.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Lee, D.H.; et al. Comparisons of Gut Microbiota Among Healthy Control, Patients with Conventional Adenoma, Sessile Serrated Adenoma, and Colorectal Cancer. J. Cancer Prev. 2017, 22, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Kanno, S.; Nosho, K.; Sukawa, Y.; Mitsuhashi, K.; Kurihara, H.; Igarashi, H.; Takahashi, T.; Tachibana, M.; Takahashi, H.; et al. Association of Fusobacterium Nucleatum with Clinical and Molecular Features in Colorectal Serrated Pathway. Int. J. Cancer 2015, 137, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium Nucleatum in Colorectal Carcinoma Tissue and Patient Prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Eisele, Y.; Mallea, P.M.; Gigic, B.; Stephens, W.Z.; Warby, C.A.; Buhrke, K.; Lin, T.; Boehm, J.; Schrotz-King, P.; Hardikar, S.; et al. Fusobacterium Nucleatum and Clinicopathologic Features of Colorectal Cancer: Results from the ColoCare Study. Clin. Colorectal. Cancer 2021, 20, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiao, X.; Zeng, M.; Fan, Z.; Li, X.; Yuan, Y.; Zhang, Q.; Xia, Y. Clinical Significance of Fusobacterium Nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis. Cancer Manag. Res. 2022, 14, 3021–3036. [Google Scholar] [CrossRef] [PubMed]
- al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.E.; Commane, D.M. Modelling the Role of Microbial P-Cresol in Colorectal Genotoxicity. Gut Microbes 2019, 10, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyzer, G.; Stams, A.J.M. The Ecology and Biotechnology of Sulphate-Reducing Bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen Sulfide Induces Direct Radical-Associated DNA Damage. Mol. Cancer Res. 2007, 5, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.E.; Williams, K.B.; Whitehead, T.R.; Heuman, D.M.; Hylemon, P.B. Development and Application of a Polymerase Chain Reaction Assay for the Detection and Enumeration of Bile Acid 7α-Dehydroxylating Bacteria in Human Feces. Clin. Chim. Acta 2003, 331, 127–134. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary Bile Acids: An Underrecognized Cause of Colon Cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Behar, J. Physiology and Pathophysiology of the Biliary Tract: The Gallbladder and Sphincter of Oddi—A Review. ISRN Physiol. 2013, 2013, 837630. [Google Scholar] [CrossRef]
- Putze, J.; Hennequin, C.; Nougayrède, J.-P.; Zhang, W.; Homburg, S.; Karch, H.; Bringer, M.-A.; Fayolle, C.; Carniel, E.; Rabsch, W.; et al. Genetic Structure and Distribution of the Colibactin Genomic Island among Members of the Family Enterobacteriaceae. Infect. Immun. 2009, 77, 4696–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faïs, T.; Delmas, J.; Barnich, N.; Bonnet, R.; Dalmasso, G. Colibactin: More Than a New Bacterial Toxin. Toxins 2018, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Velilla, J.A.; Volpe, M.R.; Kenney, G.E.; Walsh, R.M.; Balskus, E.P.; Gaudet, R. Structural Basis of Colibactin Activation by the ClbP Peptidase. Nat. Chem. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Jiang, Y.; Villalta, P.W.; Stornetta, A.; Boudreau, P.D.; Carrá, A.; Brennan, C.A.; Chun, E.; Ngo, L.; Samson, L.D.; et al. The Human Gut Bacterial Genotoxin Colibactin Alkylates DNA. Science 2019, 363, 7785. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.R.; Zha, L.; Balskus, E.P. Natural Product Discovery from the Human Microbiome. J. Biol. Chem. 2017, 292, 8546–8552. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.R.; Velilla, J.A.; Daniel-Ivad, M.; Yao, J.J.; Stornetta, A.; Villalta, P.W.; Huang, H.-C.; Bachovchin, D.A.; Balbo, S.; Gaudet, R.; et al. A Small Molecule Inhibitor Prevents Gut Bacterial Genotoxin Production. Nat. Chem. Biol. 2022. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The Biology of the Cytolethal Distending Toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Spanò, S.; Ugalde, J.E.; Galán, J.E. Delivery of a Salmonella Typhi Exotoxin from a Host Intracellular Compartment. Cell Host Microbe 2008, 3, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedor, Y.; Vignard, J.; Nicolau-Travers, M.-L.; Boutet-Robinet, E.; Watrin, C.; Salles, B.; Mirey, G. From Single-Strand Breaks to Double-Strand Breaks during S-Phase: A New Mode of Action of the Escherichia coli Cytolethal Distending Toxin. Cell Microbiol. 2013, 15, 12028. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sharipo, A.; Chaves-Olarte, E.; Masucci, M.G.; Levitsky, V.; Thelestam, M.; Frisan, T. The Haemophilus Ducreyi Cytolethal Distending Toxin Activates Sensors of DNA Damage and Repair Complexes in Proliferating and Non-Proliferating Cells. Cell Microbiol. 2002, 4, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; van Pelt, W.; Neefjes, J. Increased Colon Cancer Risk after Severe Salmonella Infection. PLoS ONE 2018, 13, e0189721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duijster, J.W.; Hansen, J.V.; Franz, E.; Neefjes, J.J.C.; Frisch, M.; Mughini-Gras, L.; Ethelberg, S. Association between Salmonella Infection and Colon Cancer: A Nationwide Registry-Based Cohort Study. Epidemiol. Infect. 2021, 149, e56. [Google Scholar] [CrossRef]
- Brigotti, M.; Alfieri, R.; Sestili, P.; Bonelli, M.; Petronini, P.G.; Guidarelli, A.; Barbieri, L.; Stirpe, F.; Sperti, S. Damage to Nuclear DNA Induced by Shiga Toxin 1 and Ricin in Human Endothelial Cells. FASEB J. 2002, 16, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Sestili, P.; Alfieri, R.; Carnicelli, D.; Martinelli, C.; Barbieri, L.; Stirpe, F.; Bonelli, M.; Petronini, P.G.; Brigotti, M. Shiga Toxin 1 and Ricin Inhibit the Repair of H2O2-Induced DNA Single Strand Breaks in Cultured Mammalian Cells. DNA Repair. 2005, 4, 271–277. [Google Scholar] [CrossRef]
- Lee, K.-S.; Jeong, Y.-J.; Lee, M.-S. Escherichia Coli Shiga Toxins and Gut Microbiota Interactions. Toxins 2021, 13, 416. [Google Scholar] [CrossRef]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The Gastrointestinal Microbiota as a Site for the Biotransformation of Drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The Microbial Pharmacists within Us: A Metagenomic View of Xenobiotic Metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef]
- Takeno, S.; Hirano, Y.; Kitamura, A.; Sakai, T. Comparative Developmental Toxicity and Metabolism of Nitrazepam in Rats and Mice. Toxicol. Appl. Pharmacol. 1993, 121, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S.; et al. Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local Bacteria Affect the Efficacy of Chemotherapeutic Drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.F.; Figg, W.D.; Sparreboom, A. Pharmacogenetics of Irinotecan Metabolism and Transport: An Update. Toxicol. Vitr. 2006, 20, 163–175. [Google Scholar] [CrossRef]
- Hong, B.-Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-Induced Oral Mucositis Is Associated with Detrimental Bacterial Dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The Influence of Gut Microbiota Dysbiosis to the Efficacy of 5-Fluorouracil Treatment on Colorectal Cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut Microbial Metabolites Facilitate Anticancer Therapy Efficacy by Modulating Cytotoxic CD8+ T Cell Immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Picard, M.; Yonekura, S.; Slowicka, K.; Petta, I.; Rauber, C.; Routy, B.; Richard, C.; Iebba, V.; Tidjani Alou, M.; Becharef, S.; et al. Ileal Immune Tonus Is a Prognosis Marker of Proximal Colon Cancer in Mice and Patients. Cell Death Differ. 2021, 28, 1532–1547. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut Microbiota Is Critical for the Induction of Chemotherapy-Induced Pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Ueda, K.; Yonekura, S.; Ogasawara, N.; Matsunaga, Y.; Hoshino, R.; Kurose, H.; Chikui, K.; Uemura, K.; Nakiri, M.; Nishihara, K.; et al. The Impact of Antibiotics on Prognosis of Metastatic Renal Cell Carcinoma in Japanese Patients Treated With Immune Checkpoint Inhibitors. Anticancer Res. 2019, 39, 6265–6271. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Ogura, K.; Kato, A.; Takubo, H.; Watabe, T. A Possible Mechanism of Eighteen Patient Deaths Caused by Interactions of Sorivudine, a New Antiviral Drug, with Oral 5-Fluorouracil Prodrugs. J. Pharmacol. Exp. Ther. 1998, 287, 791–799. [Google Scholar] [PubMed]
- Rigby, R.J.; Carr, J.; Orgel, K.; King, S.L.; Lund, P.K.; Dekaney, C.M. Intestinal Bacteria Are Necessary for Doxorubicin-Induced Intestinal Damage but Not for Doxorubicin-Induced Apoptosis. Gut Microbes 2016, 7, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Culp, E.; Perry, J.; Lau, J.T.; MacNeil, L.T.; Surette, M.G.; Wright, G.D. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect. Dis. 2018, 4, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.L.; Canova, M.J.; Radhi, I.J.; Koteva, K.; Kireeva, I.; Waglechner, N.; Wright, G.D. Bacterial Inactivation of the Anticancer Drug Doxorubicin. Chem. Biol. 2012, 19, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [Green Version]
- Mégraud, F. A Humble Bacterium Sweeps This Year’s Nobel Prize. Cell 2005, 123, 975–976. [Google Scholar] [CrossRef] [Green Version]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein–Barr Virus: More than 50 Years Old and Still Providing Surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Wei, Y.; Kuang, D.-M. Influence of Gut and Intratumoral Microbiota on the Immune Microenvironment and Anti-Cancer Therapy. Pharmacol. Res. 2021, 174, 105966. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, 4552. [Google Scholar] [CrossRef] [PubMed]
- Deepak KG, K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor Microenvironment: Challenges and Opportunities in Targeting Metastasis of Triple Negative Breast Cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Huang, L.; Hu, D.; Zeng, S.; Han, Y.; Shen, H. The Role of the Tumor Microbe Microenvironment in the Tumor Immune Microenvironment: Bystander, Activator, or Inhibitor? J. Exp. Clin. Cancer Res. 2021, 40, 327. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Liwinski, T.; Zheng, D.; Elinav, E. The Microbiome and Cytosolic Innate Immune Receptors. Immunol. Rev. 2020, 297, 207–224. [Google Scholar] [CrossRef]
- Javaheri, A.; Kruse, T.; Moonens, K.; Mejías-Luque, R.; Debraekeleer, A.; Asche, C.I.; Tegtmeyer, N.; Kalali, B.; Bach, N.C.; Sieber, S.A.; et al. Helicobacter Pylori Adhesin HopQ Engages in a Virulence-Enhancing Interaction with Human CEACAMs. Nat. Microbiol. 2017, 2, 16189. [Google Scholar] [CrossRef] [Green Version]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [Green Version]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type–Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium Persistence and Antibiotic Response in Colorectal Cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zheng, W.; Yang, K.; Harris, K.G.; Ni, K.; Xue, L.; Lin, W.; Chang, E.B.; Weichselbaum, R.R.; Fu, Y.-X. Intratumoral Accumulation of Gut Microbiota Facilitates CD47-Based Immunotherapy via STING Signaling. J. Exp. Med. 2020, 217, 2282. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef] [Green Version]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing Human Lung Tissue Microbiota and Its Relationship to Epidemiological and Clinical Features. Genome Biol. 2016, 17, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the Microbiome and TP53 in Human Lung Cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell Rep. 2018, 24, 3528–3538. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.J.; Ingle, J.N.; Tang, X.; Chia, N.; Jeraldo, P.R.; Walther-Antonio, M.R.; Kandimalla, K.K.; Johnson, S.; Yao, J.Z.; Harrington, S.C.; et al. A Comprehensive Analysis of Breast Cancer Microbiota and Host Gene Expression. PLoS ONE 2017, 12, e0188873. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Srikham, K.; Daengprok, W.; Niamsup, P.; Thirabunyanon, M. Characterization of Streptococcus Salivarius as New Probiotics Derived from Human Breast Milk and Their Potential on Proliferative Inhibition of Liver and Breast Cancer Cells and Antioxidant Activity. Front. Microbiol. 2021, 12, 797445. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated with Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrázek, J.; Mekadim, C.; Kučerová, P.; Švejstil, R.; Salmonová, H.; Vlasáková, J.; Tarasová, R.; Čížková, J.; Červinková, M. Melanoma-Related Changes in Skin Microbiome. Folia. Microbiol. 2019, 64, 435–442. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A Commensal Strain of Staphylococcus epidermidis Protects against Skin Neoplasia. Sci. Adv. 2018, 4, 4502. [Google Scholar] [CrossRef] [PubMed]
| Probiotic(s) | Strain(s) | Experimental Protocol(s) | Effect(s) | Test(s) | Reference |
|---|---|---|---|---|---|
| Lb. casei | DN 114001 | Incubation of bacterial cells (109 cfu/mL) with IQ (25 μg/mL) for 24 h in MRS broth or for 168 h in phosphate buffer | ↓ Comet tail (both co-incubations) | Comet assay | [21] |
| Incubation of bacterial cells (109 cfu/mL) with MelQx (25 μg/mL) or PhIP (25 μg/mL) for 24 h in MRS broth or for 168 h in phosphate buffer | ↓ Comet tail (only co-incubation in PBS | ||||
| Lb. rhamnosus | 231 | Incubation of bacterial cells (8 × 1011 cfu/mL) with MeIQx (10 μg/mL) (time not indicated) | ↓ Revertant colonies | Ames test with S. typhimurium TA98 and TA100 strains | [22] |
| Lb. casei Lb. acidophilus Lb. delbrueckii subsp. bulgaricus Lb. rhamnosus Lb. plantarum | 17, unspecified 15, unspecified 24, unspecified 6, unspecified 3, unspecified | Incubation of bacterial cells (108–109 cells/mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [23] |
| ↓ Tail moment | Comet assay on Caco-2 cells | ||||
| ↓ Revertant colonies | Ames test with S. typhimurium TA100 strain | ||||
| Lb. plantarum Debaryomyces hansenii Wickerhamomyces anomalus Pichia fermentans Torulaspora delbrueckii Hanseniaspora uvarum Metschnikowiaaff fructicola Metschnikowia raukaufii Candida apicola Meyerozyma guilliermondii Saccharomyces boulardii | 05, 013, N14, C9O4, C9S2 21B, CF1 LAB1, LAB30, LAB32, LAB40, LAB49, LAB62 LT21, LT52, LT53, LT99, LT100, ATCC 14917TM WCSF, 1IMC 510R®, IMC 513R® LG2, LG15 LUL14, TO8 TO1, TO10 TO2, TO3 TO5 RIB1, RIB3 LAM3 UV10 PR1 Codex© | Incubation of bacterial or yeast cells (108–109 cells /mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [24] |
| Bacillus subtilis Bacillus megaterium Bacillus firmus Bacillus pumilus | ATCC 9799, ATCC 6051, ATCC 23857, ATCC 33677 ATCC 99 ATCC 17060 ATCC 7065 | Incubation of bacterial cells (108–109 cells /mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [25] |
| Lb. delbrueckii subsp. bulgaricus Lb. casei Lb. rhamnosus Lb. acidophilus Lb. plantarum Bifidobacterium bifidum | V2Z2, V2Z3, V2Z4, V2Z5, V2Z6, V2Z8, V2Z9, V5Z5, V5Z6, V5Z7, V5Z11, V2c, V5X5, 6a, 6b, 2b, 5a, 5b, 5c, 1a, 1b, J87, J88, J89 5H1, 5H2, 5H3, 5H4, 5H5, 5H6, 5H7, 5H8, 5H9, 5H10, C1, C2, C3, V5Z4, V5Z9, V5Z10, 2a J10, J30, J42, J54, J61, J62 J71, J72, J76, J77, A1, A2, A3, A4, A5, A7, A8, A9, A11, A43, A44 J1, J25, J40 J91, J92 | Incubation of bacterial cells (108–109 cells /mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [26] |
| Lb. acidophilus Bifidobacterium bifidum Bifidobacterium infanti Bifidobacterium adolescentis Bifidobacterium breve Bifidobacterium longum | 2400, 2401, 2404, 2405, 2049, 2415 1900, 1901 1912 1920 1930 1941 | Incubation of bacterial cells (concentration not indicated) with NF, AMPIP, AMPI (0.5 μg/plate) or AFB1 (0.05 μg/plate) for 3 h | ↓ Revertant colonies | Ames test with S. typhimurium TA98 and TA100 strains, with and without S9 mix as a metabolic activation system | [27] |
| Lb. plantarum subsp. plantarum | NIMBB003 | Treatment of human lymphocytes with AFB1 (10 μM) and bacterial cells (107, 109, 1011 cfu/mL) | ↓ MN frequency | Micronucleus assay in human lymphocytes | [28] |
| Lb. rhamnosus | GG | Incubation of AFB1 (150 μM) with bacterial cells (1 × 1010 and 5 × 1010) for 72 h | ↓ DNA fragmentation induced by 25(OH)2D3 | DNA fragmentation in Caco-2 cells | [29] |
| Lb. rhamnosus Lb. brevis Lb. casei | 0908, 0900 0945 DN 114001, 0919 | Treatment of HL60 cells with cell-free supernatants of bacteria (109 cfu/mL; cultivated in MRS for 24 h or in phosphate buffer for 168 h) and NMDA (10 μg/mL) for 1 h | ↓ Comet tail (both co-incubations) | Comet assay | [30] |
| Streptococcus faecalis subsp. liquefaciens Streptococcus lactis Lb. casei subsp. casei Lb. casei subsp. rhamnosus Leuconostoc paramesenteroides Streptococcus lactis subsp. diacetylactis Streptococcus cremoris | R-9, R-11, R-19, R-32, R-55 R-24 R-12, R-35, R-52, R-68 R-33 R-5, R-6, R-8, R-10, R-13, R-21, R-23, R-26, R-27, R-29, R-31, R-40, R-45, R-49, R-51, R-53, R-62, R-64 R-63, R-22, R-43 R-2, R-14, R-17, R-48 | Incubation of lyophilized bacterial cells (5 mg) with NDEA (60 μM) for 1 h | ↓ Revertant colonies | Ames test with S. typhimurium TA98 strain and S9 mix as a metabolic activation system | [31] |
| Leuconostoc paramesenteroides Streptococcus lactis subsp. diacetylactis Streptococcus cremoris | R-62, R-8 R-63 R-48 | Incubation of lyophilized bacterial cells (3, 5, 7 mg) with NMDA (60 μM), NPYR (50 μM), or NPIP (50 μM) for 1 h | ↓ Revertant colonies (only for NMDA) | ||
| Lb. rhamnosus | IMC501 | Incubation of bacterial cells (109 cells/mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [32] |
| Lb. casei | MSA1, MSA24, MSA21, MSA13, ATCC 393T, MSA4, MSA23, MSA8, MSA15, MSA11, MSA10, MSA25, MSA12, MSA6, MSA22, MSA20, MSA17, MSA7, MSA19, MSA3, MSA9, MSA18, MSA16, MSA14, MSA5 | Incubation of bacterial cells (105–109 cfu/mL) with 4-NQO (0.1 mM) | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [33] |
| Lb. plantarum Lb. casei Lb. brevis |
Unspecified
Unspecified Unspecified | Incubation of bacterial cells with NF (concentrations and time were not indicated) | ↓ Revertant colonies | Ames test with S. typhimurium TA100 strain, with and without S9 mix as a metabolic activation system | [34] |
| Lb. salivarius | FDB89 | Incubation of bacterial cells (108–109 cfu/mL) and 4-NQO (20 mg/L) for 3 h | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [35] |
| Lb. plantarum | 301102 | Incubation of fermented milk, prepared with Lb. plantarum 301102 or 301102S (mutant strain), or lyophilized exopolysaccharide (EPS) solutions (0.01, 0.1, and 1.0 mg/mL) with Trp-P-1 (0.1 mg/mL) for 30 min | ↓ Revertant colonies (only with Lb. plantarum 301102S or lyophilized EPS) | Ames test with S. typhimurium TA98 strain and S9 mix as a metabolic activation system | [36] |
| Lb. casei Lb. plantarum Lb. rhamnosus Lb. brevis Lb. spp | C33, 306, 32C, 364, 66C, H5, 357, 362, 369G, 349, 350, 365, 347, 410, 394, 408, 300, 342, 398, 88b, 455, 417, 447, 53Be, 400, 353, 88, 391, 13A 66B, 337, 371, 4Ab, 62B, 8A, 45A, 36D, 301, 303, 8c, 56, 329, 48Ab, 336, 366, ZAR61, 61B, 43, 19B 94, 442, 25B, 14A, 93, 85 7A, 38Db, 38D, 41, 39D sp. 434, sp. 432, sp. 428 | Incubation of bacterial cells (105–109 cfu/mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [37] |
| Enterococcus faecium Bacillus coagulans Lb. plantarum Saccharomyces cerevisiae Pichia anamola Cryptococcus albidus | AdF1, AdF2, AdF3, AdF11 AdF4 | Incubation of bacterial or yeast cells (108–109 cells/mL) with 4-NQO (0.1 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [38] |
| AdF5, Adf6, Adf7, Adf9, Adf10 Sc04, Sc08, SC12, Sc20 Sc17 Sc18 | ↓ Revertant colonies | Ames test with S. typhimurium TA100 strain | |||
| Lb. rhamnosus Lb. casei | 0900, 0908 0919 | Treatment of Caco-2 cells with bacterial cells (1 × 109 cfu/mL) for 1 h prior to IQ or PhiP (50 μg/mL) exposure for 10 min | ↓ Comet tail | Comet assay in Caco-2 cells | [39] |
| Bifidobacterium animalis subsp. lactis | BI-04 | Treatment of Caco-2 cells with inactivated bacterial cells (about 5 × 108 cfu/mL) and BaP (50 μM) for 4 h | ↓ Comet length, ↓tail moment, ↓tail length, ↓ olive tail moment | Comet assay in Caco-2 cells | [40] |
| Bacillus clausii Bacillus subtilis Bacillus lentus Bacillus pumilus Bacillus firmus Bacillus megaterium Bacillus sp. | O⁄C, N⁄R, SIN, T, DSM 8716T, DSM 9783, DSM 2512 LPM, ATCC 6051T, ATCC 33677, ATCC 23857, ATCC 9799 E2, V4, ATCC 10841T ATCC 7061T, ATCC 7065 ATCC 14575T, ATCC 17060 ATCC 14946 718 | Incubation of bacterial cells (108–109 cells/mL) with 4-NQO (0.1 mM), AFB1 (0.05 mM) or MeIQ (0.16 mM) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37, with or without S9 mix as a metabolic activation system | [41] |
| Lb. acidophilus Lb. casei Lb. delbrueckii subsp. bulgaricus Lb. fermentum Lb. plantarum Lb. rhamnosus Bifidobacterium longum | J76 5H10 J87 Unspecified J25 J54 Unspecified | Incubation of bacterial cells (108–109 cells/mL) with aqueous extracts of heated oils and their dilution (1:1 and 1:3) for 150 min | ↓ IFSOS | SOS chromotest with E. coli PQ37 | [42] |
| E. coli | Nissle 1917 | Treatment of Caco-2 cells with bacterial cells (109–1010 cfu/mL) and 4-NQO (20 μg/mL) for 150 min or BaP (100 μg/mL) for 90 min | ↓ Comet tail | Comet assay in Caco-2 cells | [43] |
| Incubation of bacterial cells (109 cfu/mL) with 4-NQO (20 μg/mL) or BaP (100 μg/mL) for 20 min | ↓ Revertant colonies | Ames test with S. Typhimurium TA100 and E. coli WP2 strains, with and without S9 mix as a metabolic activation system |
| Probiotic(s) | Experimental Protocol(s) | Experimental Model | Effect(s) | Test(s) | References |
|---|---|---|---|---|---|
| Lb. rhamnosus (IMC501) | Administration of bacterial cells (109 cells/mL, 10 mL/kg b.w.) for 10 days before PhIP administration (100 mg/kg b.w.) | Male CD-1 mice | ↓ Tail length | Comet assay on peripheral blood | [44] |
| Streptococcus faecalis (T-110) Clostridium butyricum (TO-A) Bacillus mesentericus (TO-A) | Administration of Streptococcus faecalis (108 cfu/g), Clostridium bothrium (107 cfu/g) and Bacillus mesentericus (106 cfu/g) for 2 weeks before AAC (40 mg/kg b.w.) administration once a day for 3 days | HFA mice | ↓ DNA adduct formation | 32P-postlabelling assay on colonic epithelium | [45] |
| Lb. plantarum (CICC 23121) | Administration of bacterial cells (5 × 1010 cfu/mL) and BaP (50 mg/kg) twice a week for 28 days | Clean-grade Kunming mice | ↓ Tail length | Comet assay on peripheral blood cells | [46] |
| Fermented milk (FM) supplemented with Lb. rhamnosus (GG) and Lb. casei (strain shirota) | (1) Administration of AFB1 (450 μg/kg b.w) from week 4 to week 10 and of FM (108 cfu/g) from week 4 to week 25 (2) Administration of AFB1 (450 μg/kg b.w.) from week 4 to week 10 and of FM (108 cfu/g) from week 10 to week 25 (3) Administration of AFB1 (450 μg/kg b.w.) from week 4 to week 10 and of FM (108 cfu/g) from week 1 to week 25 | Male Wistar rats | ↓ DNA damage scoring (cells with comet) | Comet assay on liver cells | [47] |
| Saccharomyces cerevisiae (unspecified strain) | Administration of yeast cells (108 viable cells) and corn contaminated with AFB1 (400 and 800 μg/kg) for 6 weeks | Male CD-1 mice | ↓ Frequency of MNNE | Micronucleus test on mice erythrocytes | [48] |
| Lb. rhamnosus (GG) | Administration of bacterial cells (1 × 1010 cfu) 2h before AFB1, AFB2, AFG1, AFG2 (0.7 mg/kg b.w.) administration every day for 7 consecutive days | Male Swiss Albino mice | ↓ Structural and numerical chromosome aberrations, ↑ mitotic activity, ↑ meiotic activity | Micronucleus assay in mice bone marrow cells and in mice spermatocytes | [49] |
| Saccharomyces cerevisiae (RC016) | (1) Administration of AFB1 (40 μg/kg) + AFG1 (20 μg/kg) and yeast cells (108 viable cells) daily for 60 days (2) Administration of AFB1 (100 μg/kg) + AFG1 (50 μg/kg) and yeast cells (108 viable cells) daily for 60 days | Inbred male Wistar rats | ↓ % MNPCE | Micronucleus test on rat erythrocytes | [50] |
| No difference of tail moment | Comet assay on rat lymphocytes | ||||
| Pediococcus acidilactici (NNRL B-5627) Lb. delbrueckii subsp. lactis (DSM 20076) | Administration of bacterial cells (1010 cfu/mL) and fumonisin B1 (100 and 200 mg/kg) once a day for 4 weeks | Sprague-Dawley rats | ↓ DNA fragmentation | Analysis of DNA fragmentation on blood cells | [51] |
| Lb. salivarius (REN) | (1) Administration of bacterial cells (5 × 106, 5 × 108, or 5 × 1010 cfu/kg b.w.) once a day from week 1 to week 32 and 4-NQO (20 ppm) from week 2 to week 9 (2) Administration of 4-NQO (20 ppm) from week 2 to week 9 and bacterial cells (5 × 106, 5 × 108, or 5 × 1010 cfu/kg b.w.) once a day from week 10 to week 32 | Male F344 rats | ↓ 8-OHdG levels | Measurement of 8-OHdG levels on rat tongue epithelium | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, G.; Zeppa, S.D.; Agostini, D.; Attisani, G.; Stefanelli, C.; Ferrini, F.; Sestili, P.; Fimognari, C. The Anti- and Pro-Tumorigenic Role of Microbiota and Its Role in Anticancer Therapeutic Strategies. Cancers 2023, 15, 190. https://doi.org/10.3390/cancers15010190
Greco G, Zeppa SD, Agostini D, Attisani G, Stefanelli C, Ferrini F, Sestili P, Fimognari C. The Anti- and Pro-Tumorigenic Role of Microbiota and Its Role in Anticancer Therapeutic Strategies. Cancers. 2023; 15(1):190. https://doi.org/10.3390/cancers15010190
Chicago/Turabian StyleGreco, Giulia, Sabrina Donati Zeppa, Deborah Agostini, Giuseppe Attisani, Claudio Stefanelli, Fabio Ferrini, Piero Sestili, and Carmela Fimognari. 2023. "The Anti- and Pro-Tumorigenic Role of Microbiota and Its Role in Anticancer Therapeutic Strategies" Cancers 15, no. 1: 190. https://doi.org/10.3390/cancers15010190








