Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Characteristics of HER2-Amplified CRC
3. Diagnostic Procedures of HER2-Amplified CRC
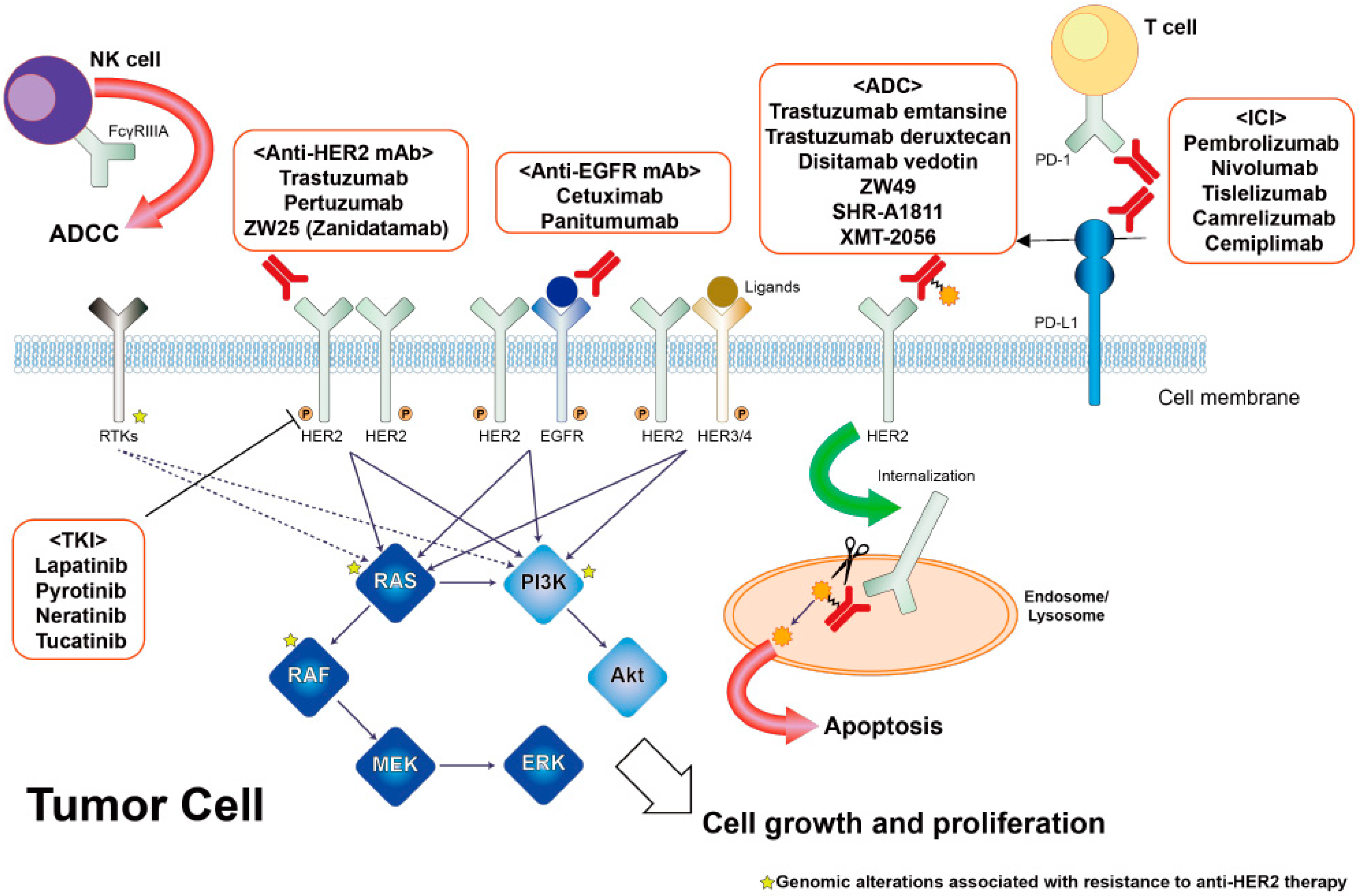
4. Efficacy of HER2-Targeted Treatment for Patients with HER2-Amplified mCRC in Clinical Trials
5. Resistance Mechanism against HER2-Targeted Treatment in HER2-Amplified mCRC
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marx, A.H.; Burandt, E.C.; Choschzick, M.; Simon, R.; Yekebas, E.; Kaifi, J.T.; Mirlacher, M.; Atanackovic, D.; Bokemeyer, C.; Fiedler, W.; et al. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum. Pathol. 2010, 41, 1577–1585. [Google Scholar] [CrossRef]
- Ingold Heppner, B.; Behrens, H.M.; Balschun, K.; Haag, J.; Krüger, S.; Becker, T.; Röcken, C. HER2/neu testing in primary colorectal carcinoma. Br. J. Cancer 2014, 111, 1977–1984. [Google Scholar] [CrossRef]
- Richman, S.D.; Southward, K.; Chambers, P.; Cross, D.; Barrett, J.; Hemmings, G.; Taylor, M.; Wood, H.; Hutchins, G.; Foster, J.M.; et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: Analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J. Pathol. 2016, 238, 562–570. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, J.; Hong, Y.S.; Kim, D.; Kim, J.E.; Kim, S.Y.; Kim, K.P.; Yoon, Y.K.; Kim, D.; Chun, S.M. HER2 Amplification and Cetuximab Efficacy in Patients with Metastatic Colorectal Cancer Harboring Wild-type RAS and BRAF. Clin. Color. Cancer 2017, 16, e147–e152. [Google Scholar] [CrossRef]
- Sawada, K.; Nakamura, Y.; Yamanaka, T.; Kuboki, Y.; Yamaguchi, D.; Yuki, S.; Yoshino, T.; Komatsu, Y.; Sakamoto, N.; Okamoto, W.; et al. Prognostic and Predictive Value of HER2 Amplification in Patients with Metastatic Colorectal Cancer. Clin. Color. Cancer 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Raghav, K.; Loree, J.M.; Morris, J.S.; Overman, M.J.; Yu, R.; Meric-Bernstam, F.; Menter, D.; Korphaisarn, K.; Kee, B.; Muranyi, A.; et al. Validation of HER2 Amplification as a Predictive Biomarker for Anti–Epidermal Growth Factor Receptor Antibody Therapy in Metastatic Colorectal Cancer. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Bertotti, A.; Papp, E.; Jones, S.; Adleff, V.; Anagnostou, V.; Lupo, B.; Sausen, M.; Phallen, J.; Hruban, C.A.; Tokheim, C.; et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015, 526, 263–267. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Amatu, A.; Porcu, L.; Ghezzi, S.; Lonardi, S.; Leone, F.; Bergamo, F.; Fenocchio, E.; Martinelli, E.; Borelli, B.; et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019, 24, 1395–1402. [Google Scholar] [CrossRef]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A Molecularly Annotated Platform of Patient-Derived Xenografts (“Xenopatients”) Identifies HER2 as an Effective Therapeutic Target in Cetuximab-Resistant Colorectal Cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 Signaling Causes Resistance to the EGFR-Directed Therapeutic Antibody Cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Bando, H.; Nakamura, Y.; Hasegawa, H.; Kuwaki, T.; Okamoto, W.; Taniguchi, H.; Aoyagi, Y.; Miki, I.; Uchigata, H.; et al. Trajectory for the Regulatory Approval of a Combination of Pertuzumab Plus Trastuzumab for Pre-treated HER2-positive Metastatic Colorectal Cancer Using Real-world Data. Clin. Color. Cancer 2022, in press. [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 340, 1546–1558. [Google Scholar] [CrossRef]
- Muzny, D.M.; Bainbridge, M.N.; Chang, K.; Dinh, H.H.; Drummond, J.A.; Fowler, G.; Kovar, C.L.; Lewis, L.R.; Morgan, M.B.; Newsham, I.F.; et al. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar]
- Sjöblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. CMS classification of CRCas a predictive factor for chemotherapeutic efficacy against metastatic CRC. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef]
- Stintzing, S.; Wirapati, P.; Lenz, H.-J.; Neureiter, D.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Ou, F.-S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of Consensus Molecular Subtype on Survival in Patients with Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2019, 37, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.H.; Aussó, S.; Lopez-Doriga, A.; Cordero, D.; Guinó, E.; Soler, R.S.; Barenys, M.; De Oca, J.; Capella, G.; Salazar, R.; et al. Comprehensive analysis of copy number aberrations in microsatellite stable colon cancer in view of stromal component. Br. J. Cancer 2017, 117, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.-A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 124, 1358–1373. [Google Scholar] [CrossRef]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef]
- Battaglin, F.; Ou, F.-S.; Qu, X.; Bertagnolli, M.M.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Zemla, T.J.; Blanke, C.D.; et al. Predictive and prognostic value of HER2 gene expression and HER2 amplification in patients with metastatic colorectal cancer (mCRC) enrolled in CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2020, 38, 4086. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sawada, K.; Fujii, S.; Yoshino, T. HER2-targeted therapy should be shifted towards an earlier line for patients with anti-EGFR-therapy naive, HER2-amplified metastatic colorectal cancer. ESMO Open 2019, 4, e000530. [Google Scholar] [CrossRef]
- Fujii, S.; Magliocco, A.M.; Kim, J.; Okamoto, W.; Kim, J.E.; Sawada, K.; Nakamura, Y.; Kopetz, S.; Park, W.-Y.; Tsuchihara, K.; et al. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified Metastatic Colorectal Cancer Allowing for Screening by Next-Generation Sequencing Panel. JCO Precis. Oncol. 2020, 4, 6–19. [Google Scholar] [CrossRef]
- Siravegna, G.; Sartore-Bianchi, A.; Nagy, R.J.; Raghav, K.; Odegaard, J.I.; Lanman, R.B.; Trusolino, L.; Marsoni, S.; Siena, S.; Bardelli, A. Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3046–3053. [Google Scholar] [CrossRef]
- Leto, S.M.; Sassi, F.; Catalano, I.; Torri, V.; Migliardi, G.; Zanella, E.R.; Throsby, M.; Bertotti, A.; Trusolino, L. Sustained Inhibition of HER3 and EGFR Is Necessary to Induce Regression of HER2-Amplified Gastrointestinal Carcinomas. Clin. Cancer Res. 2015, 21, 5519–5531. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Gupta, R.; Garrett-Mayer, E.; Halabi, S.; Mangat, P.K.; D’Andre, S.D.; Meiri, E.; Shrestha, S.; Warren, S.L.; Ranasinghe, S.; Schilsky, R.L. Pertuzumab plus trastuzumab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 amplification or overexpression: Results from the TAPUR Study. J. Clin. Oncol. 2020, 38, 132. [Google Scholar] [CrossRef]
- Strickler, J.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.; Hubbard, J.; Coveler, A.; et al. LBA-2 Primary analysis of MOUNTAINEER: A phase 2 study of tucatinib and trastuzumab for HER2-positive mCRC. Ann. Oncol. 2022, 33, S375–S376. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Raghav, K.; Masuishi, T.; Yamaguchi, K.; Nishina, T.; Elez, E.; Rodriguez, J.; Chau, I.; Di Bartolomeo, M.; Kawakami, H.; et al. 386O Exploratory biomarker analysis of DESTINY-CRC01, a phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd, DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC). Ann. Oncol. 2021, 32, S532. [Google Scholar] [CrossRef]
- Yagisawa, M.; Sawada, K.; Nakamura, Y.; Fujii, S.; Yuki, S.; Komatsu, Y.; Yoshino, T.; Sakamoto, N.; Taniguchi, H. Prognostic Value and Molecular Landscape of HER2 Low-Expressing Metastatic Colorectal Cancer. Clin. Color. Cancer 2021, 20, 113–120.e1. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Hamilton, E.; Hanna, D.; Beeram, M.; Lee, K.-W.; Kang, Y.-K.; Chaves, J.; Lee, J.-Y.; Goodwin, R.; Vaklavas, C.; et al. Safety, anti-tumour activity, and biomarker results of the HER2-targeted bispecific antibody ZW25 in HER2-expressing solid tumours. Ann. Oncol. 2019, 30, ix22. [Google Scholar] [CrossRef]
- Jhaveri, K.; Han, H.; Dotan, E.; Oh, D.-Y.; Ferrario, C.; Tolcher, A.; Lee, K.-W.; Liao, C.-Y.; Kang, Y.-K.; Kim, Y.H.; et al. 460MO Preliminary results from a phase I study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody-drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. Ann. Oncol. 2022, 33, S749–S750. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Gong, J.; Zhang, X.; Peng, Z.; Sheng, X.; Mao, C.; Fan, Q.; Bai, Y.; Ba, Y.; et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody–MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 2021, 24, 913–925. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Bekaii-Saab, T.; Tabernero, J.; Siena, S.; Yoshino, T.; Norwood, K.G.; Adelberg, D.E.; Ward, J.; Yang, S.; Strickler, J.H.; et al. 438TiP MOUNTAINEER-03: Phase III study of tucatinib, trastuzumab, and mFOLFOX6 as first-line treatment in HER2+ metastatic colorectal cancer (trial in progress). Ann. Oncol. 2022, 33, S734–S735. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Meng, Q.; Sun, H.; Zhang, X.; Yun, J.; Li, B.; Wu, S.; Li, X.; Yang, H.; Zhu, H.; et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
| IHC | IHC Pattern | Classification | Eligibility |
|---|---|---|---|
| Surgical specimen | Complete lateral or circumferential membrane staining with strong intensity and within >10% of tumor cells | Positive IHC (3+) | Eligible (tissue +) |
| Biopsy specimen | Tumor cells with complete lateral of circumferential membrane staining with strong intensity irrespective of percentage of tumor cells stained | ||
| Surgical specimen | Incomplete lateral or circumferential membrane staining with weak/moderate intensity and within >10% of tumor cells or complete lateral or circumferential membrane staining with strong intensity and within <10% of tumor cells | Equivocal IHC (2+) | Not eligible |
| Biopsy specimen | Tumor cells with weak to moderate complete circumferential or weak to moderate lateral membrane staining irrespective of percentage of tumor cells stained | ||
| Surgical specimen | Segmental of granular staining in any cellularity | Negative IHC (1+) | Not eligible |
| Biopsy specimen | Tumor cells with faint or barely perceptible membrane staining irrespective of percentage of tumor cells stained | ||
| Surgical specimen | No staining is observed or incomplete membrane staining with faint/barely perceptible intensity and within ≤10% of tumor cells | Negative IHC (0+) | Not eligible |
| Biopsy specimen | No staining in any tumor cell | ||
| FISH | HER2/CEP17 ratio | ||
| Surgical or biopsy specimen | ≥2 | Positive | Eligible (tissue +) |
| <2 | Negative | Not eligible | |
| Plasma NGS | HER2 status by Gurdant360 | ||
| ctDNA | HER2 amplification (and KRAS/NRAS wild type) | Positive | Eligible (blood +) |
| No HER2 amplification | Negative | Not eligible |
| Clinical Trial | n | PFS | ||
|---|---|---|---|---|
| HERACLES [29,31] | (weeks) | |||
| qPCR (tissue) | CN ≥ 9.45 | 18 | 29 | HR 0.67 (95% CI 0.6–0.8), p = 0.0001 |
| CN < 9.45 | 9 | 16 | ||
| NGS (plasma) | ApCN ≥ 25.82 | 15 | 22.5 | p = 0.0347 |
| ApCN < 25.82 | 13 | 14.8 | ||
| TRIUMPH [14] | (months) | |||
| NGS (tissue) | CN ≥ 68.7 | 9 | 6.2 | HR 0.28 (95% CI 0.11–0.74) |
| CN < 68.7 | 20 | 2.2 | ||
| NGS (plasma) | ApCN ≥ 16.7 | 13 | 5.6 | HR 0.14 (95% CI 0.05–0.39) |
| ApCN < 16.7 | 16 | 1.6 | ||
| DESTINY-CRC01 [38] | (months) | |||
| NGS (plasma) | ApCN ≥ 30.9 | 24 | 10.9 | |
| ApCN < 30.9 | 28 | 4.1 |
| Phase | NCT Number | Title | Status | Treatment | n | HER2 Status |
|---|---|---|---|---|---|---|
| III | NCT05253651 | A Study of Tucatinib With Trastuzumab and mFOLFOX6 Versus Standard of Care Treatment in First-line HER2+ Metastatic Colorectal Cancer (MOUNTAINEER-03) | Recruiting | Tucatinib in combination with Trastuzumab and mFOLFOX6 | 400 | HER2+ disease as determined by a tissue-based assay |
| II | NCT03365882 | S1613, Trastuzumab and Pertuzumab or Cetuximab and Irinotecan Hydrochloride in Treating Patients With Locally Advanced or Metastatic HER2/Neu Amplified Colorectal Cancer That Cannot Be Removed by Surgery | Active, not recruiting | Trastuzumab + Pertuzumab | 240 | IHC 3+ or IHC 2+ and ISH with HER2/CEP17 ratio ≥2.0 |
| II | NCT03929666 | A Safety and Efficacy Study of ZW25 (Zanidatamab) Plus Combination Chemotherapy in HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | Recruiting | ZW25 (zanidatamab) + chemothrapy | 362 | IHC 3+ or HER2 amplification (based upon central assessment) |
| II | NCT04380012 | A Clinical Study of Pyrotinib in Patients With HER2-positive Advanced Colorectal Cancer | Recruiting | Pyrotinib + Trastuzumab | 40 | IHC 3+ or 2+ in more than 50% of cells, confirmed by SISH or FISH with HER2/CEP17 ratio ≥ 2.0 |
| II | NCT04744831 | Trastuzumab Deruxtecan in Participants With HER2-overexpressing Advanced or Metastatic Colorectal Cancer (DESTINY-CRC02) | Active, not recruiting | T-DXd | 122 | IHC 3+ or IHC 2+/ISH + |
| II | NCT05193292 | Camrelizumab Combined With Trastuzumab and Chemotherapy in Patients With HER2-positive Advanced Colorectal Cancer | Not yet recruiting | Camrelizumab + Trastuzumab + Chemotherapy | 77 | HERACLES diagnostic criteria or NGS sequencing of tumor tissue/blood samples showed HER2 amplification. |
| II | NCT05350917 | Study of Tislelizumab Combined With DisitamabVedotin and Pyrotinib Maleate in HER2-positive or Mutated Advanced Colorectal Cancer Who Failed Standard Therapy | Not yet recruiting | Tislelizumab combined with Disitamab Vedotin and Pyrotinib | 20 | - |
| II | NCT05333809 | Pembrolizumab and Disitamab Vedotin in HER2-expressing Metastatic Colorectal Cancer | Not yet recruiting | Pembrolizumab + Disitamab Vedotin | 30 | IHC 3+ or IHC 2+ |
| II | NCT05356897 | Tucatinib Combined With Trastuzumab and TAS-102 for the Treatment of HER2 Positive Metastatic Colorectal Cancer in Molecularly Selected Patients, 3T Study | Not yet recruiting | Tucatinib combined with Trastuzumab and TAS-102 | 30 | HER2 3+ or IHC 2+/FISH or CISH with signal ratio > 2.2 or gene copy number > 6 or HER2 amplification by NGS |
| I/II | NCT04278144 | A First-in-human Study Using BDC-1001 as a Single Agent and in Combination With Nivolumab in Advanced HER2-Expressing Solid Tumors | Recruiting | BDC-1001+/−Nivolumab | 390 | - |
| I | NCT03821233 | A Dose Finding Study of ZW49 in Patients With HER2-Positive Cancers | Recruiting | ZW49 | 174 | HER2 high |
| I | NCT04460456 | A Study of SBT6050 Alone and in Combination With PD-1 Inhibitors in Subjects With Advanced HER2 Expressing Solid Tumors | Active, not recruiting | SBT6050 + Pembrolizumab or Cemiplimab | 58 | IHC 3+ or 2+ |
| I | NCT04704661 | Testing the Combination of Two Anti-cancer Drugs, DS-8201a and AZD6738, for The Treatment of Patients With Advanced Solid Tumors Expressing the HER2 Protein or Gene, The DASH Trial | Recruiting | T-DXd and Ceralasertib (AZD6738) | 15 | IHC 1–3+ or HER2 amplification based on FISH or NGS |
| I | NCT04513223 | A Phase I Study of SHR-A1811 in Patients With Selected HER2 Expressing Tumors | Recruiting | SHR-A1811 | 114 | - |
| I | NCT05514717 | A Study of XMT-2056 in Advanced/Recurrent Solid Tumors That Express HER2 | Not yet recruiting | XMT-2056 | 144 | HER2+ will be determined by institutional practice (e.g., IHC, ISH, or NGS) |
| Phase | NCT Number | Title | Status | Treatment | n | HER2 Status |
|---|---|---|---|---|---|---|
| I | NCT04319757 | ACE1702 in Subjects With Advanced or Metastatic HER2-expressing Solid Tumors | Recruiting | ACE1702 | 36 | IHC 3+ or 2+ |
| I | NCT03740256 | Binary Oncolytic Adenovirus in Combination With HER2-Specific Autologous CAR VST, Advanced HER2 Positive Solid Tumors (VISTA) | Recruiting | HER2 specific CAR-T cell + CAdVEC | 45 | IHC 2+ or above |
| I | NCT04660929 | CAR macrophages for the Treatment of HER2-Overexpressing Solid Tumors | Recruiting | CT-0508 | 18 | HER2 positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, A.; Nakamura, Y. Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer. Cancers 2023, 15, 183. https://doi.org/10.3390/cancers15010183
Yoshikawa A, Nakamura Y. Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer. Cancers. 2023; 15(1):183. https://doi.org/10.3390/cancers15010183
Chicago/Turabian StyleYoshikawa, Ayumu, and Yoshiaki Nakamura. 2023. "Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer" Cancers 15, no. 1: 183. https://doi.org/10.3390/cancers15010183
APA StyleYoshikawa, A., & Nakamura, Y. (2023). Molecular Basis of HER2-Targeted Therapy for HER2-Positive Colorectal Cancer. Cancers, 15(1), 183. https://doi.org/10.3390/cancers15010183





