Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
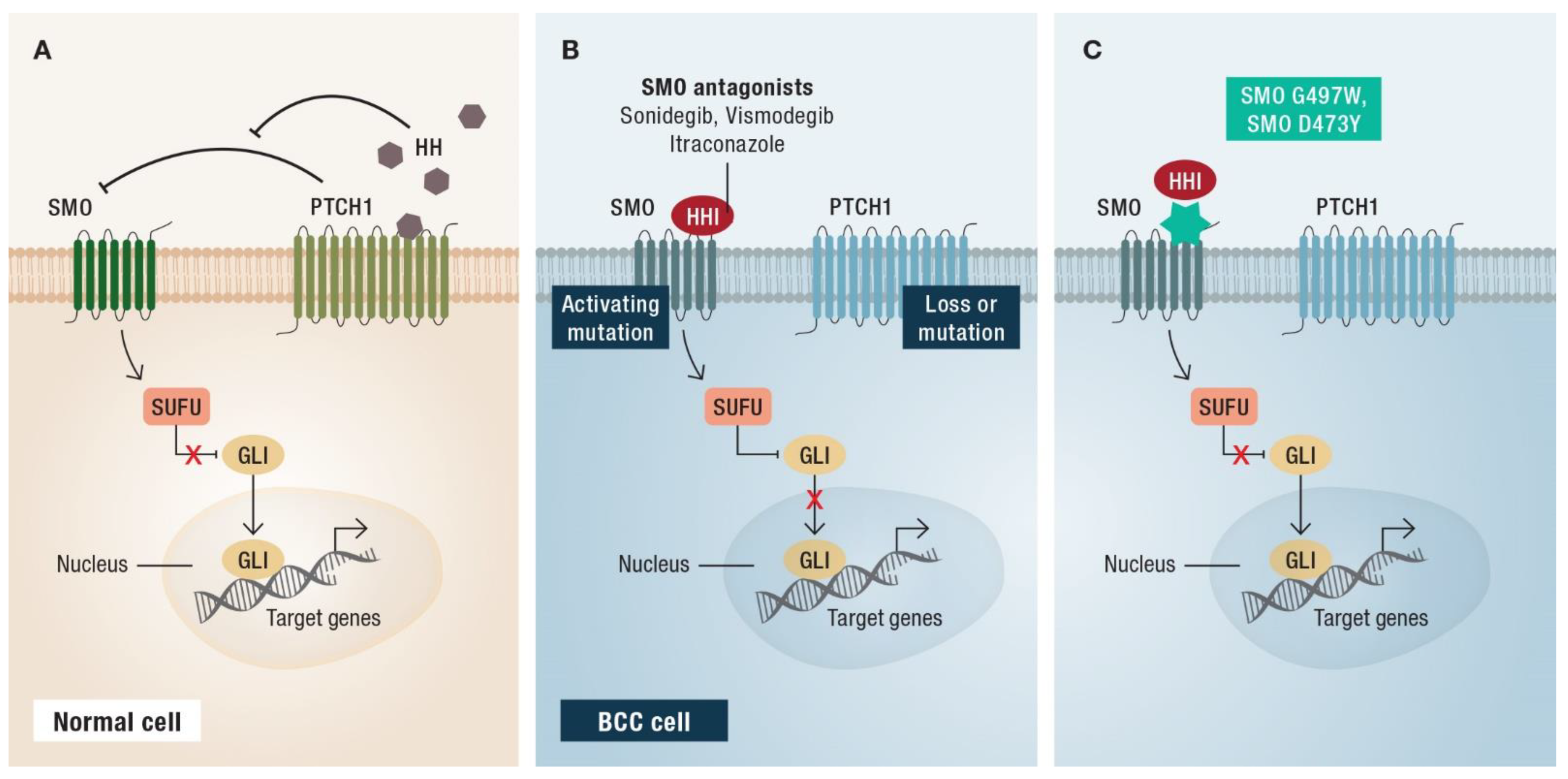
2. First-Line Therapy: Hedgehog Inhibitors
3. Targeted Treatment with HHIs—How to Balance Efficacy and Toxicity
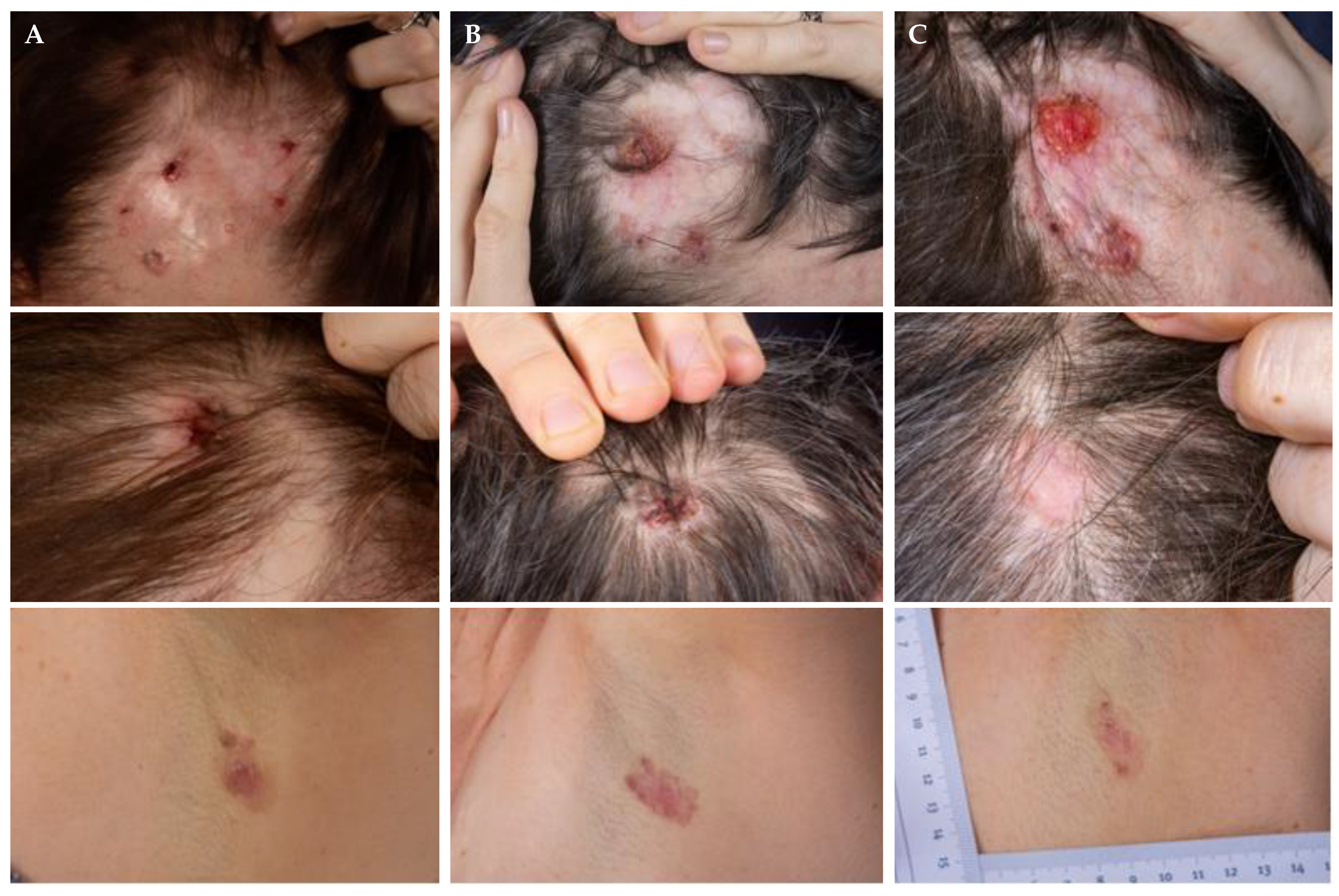
3.1. On-Off Treatment Schedules
3.2. Dose Reduction
3.3. Re-Challenge after Relapse without Other Intercurrent Treatments
3.4. Maintenance of Remission
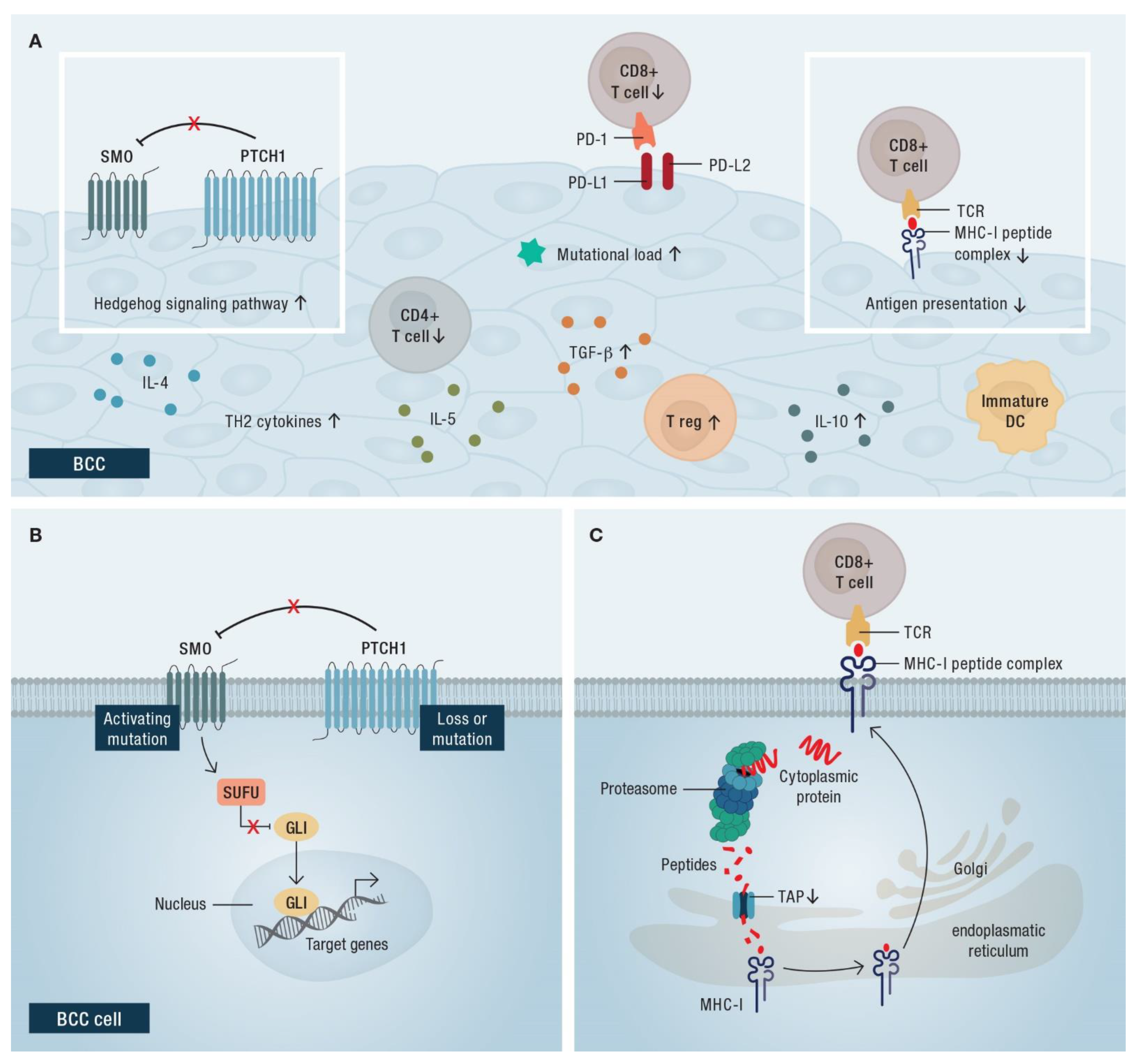
3.5. Resistance Mechanisms of HHIs
4. Immune Checkpoint Blockade with Anti-PD-1-Blocking Antibodies
4.1. Efficacy of PD-1 Inhibitors in BCC
4.2. Rationale for the Use of Immune Checkpoint Blockade in BCC
5. What Follows Immunotherapy?
5.1. Re-Challenge with HHI after Immune Checkpoint Blockade
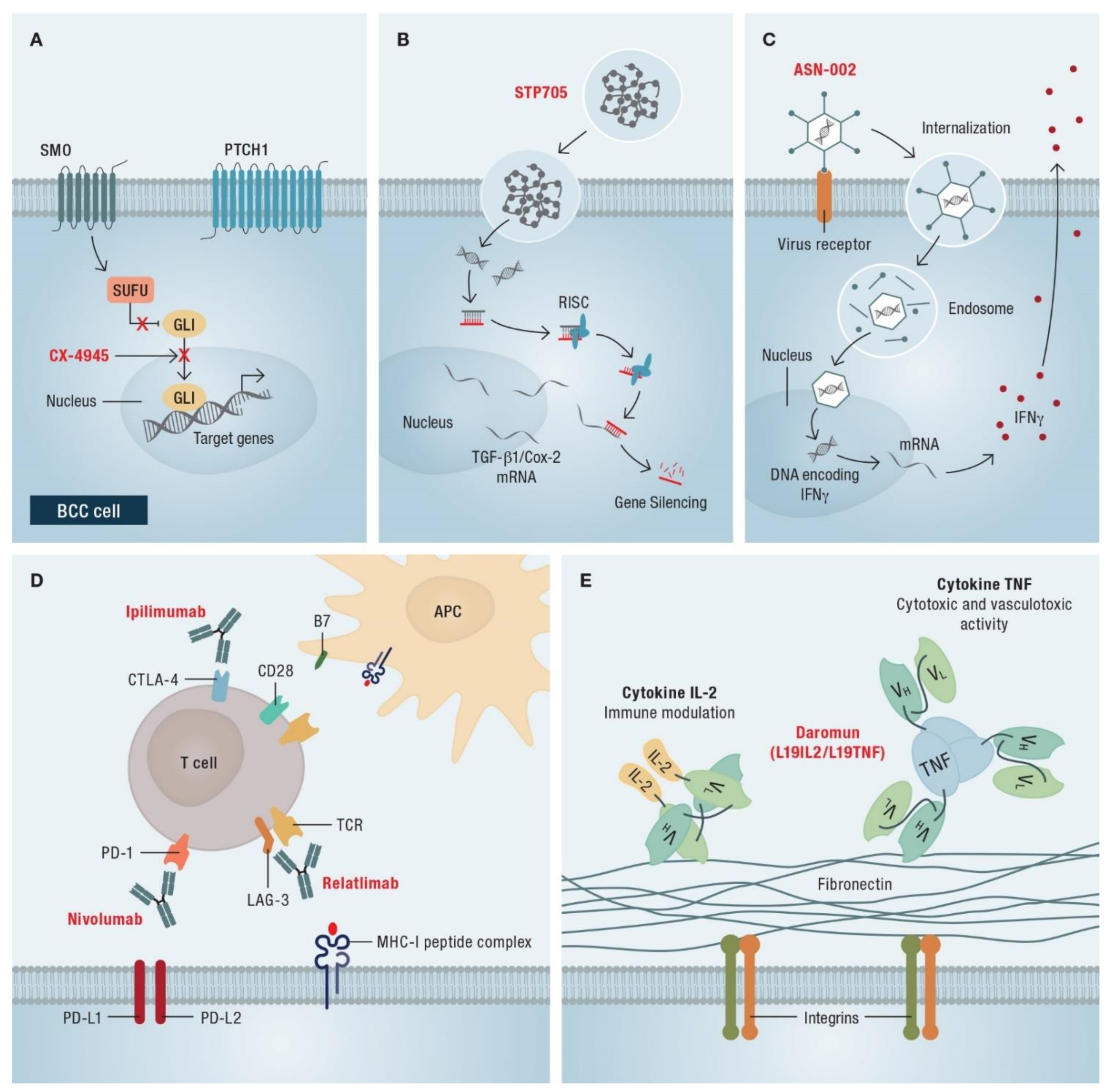
5.2. Combination Therapies
6. Ongoing Studies and Future Developments
6.1. Combination of HHI and Immunotherapy
6.2. Current Research
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olencki, T.; Rodgers, P. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Lear, J.T.; Corner, C.; Dziewulski, P.; Fife, K.; Ross, G.L.; Varma, S.; Harwood, C.A. Challenges and new horizons in the management of advanced basal cell carcinoma: A UK perspective. Br. J. Cancer 2014, 111, 1476–1481. [Google Scholar] [CrossRef]
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef]
- Johnson, R.L.; Rothman, A.L.; Xie, J.; Goodrich, L.V.; Bare, J.W.; Bonifas, J.M.; Quinn, A.G.; Myers, R.M.; Cox, D.R.; Epstein, E.H., Jr.; et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996, 272, 1668–1671. [Google Scholar] [CrossRef]
- Reifenberger, J.; Wolter, M.; Knobbe, C.B.; Köhler, B.; Schönicke, A.; Scharwächter, C.; Kumar, K.; Blaschke, B.; Ruzicka, T.; Reifenberger, G. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br. J. Dermatol. 2005, 152, 43–51. [Google Scholar] [CrossRef]
- Xie, J.; Murone, M.; Luoh, S.M.; Ryan, A.; Gu, Q.; Zhang, C.; Bonifas, J.M.; Lam, C.W.; Hynes, M.; Goddard, A.; et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 1998, 391, 90–92. [Google Scholar] [CrossRef]
- Low, J.A.; de Sauvage, F.J. Clinical experience with Hedgehog pathway inhibitors. J. Clin. Oncol. 2010, 28, 5321–5326. [Google Scholar] [CrossRef]
- Pan, S.; Wu, X.; Jiang, J.; Gao, W.; Wan, Y.; Cheng, D.; Han, D.; Liu, J.; Englund, N.P.; Wang, Y.; et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS Med. Chem. Lett. 2010, 1, 130–134. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Public Assessment Report: Erivedge. November 2016. Available online: https://www.ema.europa.eu/en/documents/overview/erivedge-epar-summary-public_en.pdf (accessed on 9 February 2022).
- European Medicines Agency. European Public Assessment Report: Odomzo. September 2015. Available online: https://www.ema.europa.eu/en/documents/overview/odomzo-epar-summary-public_en.pdf (accessed on 9 February 2022).
- Lacouture, M.E.; Dréno, B.; Ascierto, P.A.; Dummer, R.; Basset-Seguin, N.; Fife, K.; Ernst, S.; Licitra, L.; Neves, R.I.; Peris, K.; et al. Characterization and Management of Hedgehog Pathway Inhibitor-Related Adverse Events in Patients with Advanced Basal Cell Carcinoma. Oncologist 2016, 21, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Woltsche, N.; Pichler, N.; Wolf, I.; Di Meo, N.; Zalaudek, I. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: A single centre experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e144–e145. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Poblete-Lopez, C.; Vidimos, A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: A retrospective case series. J. Am. Acad. Dermatol. 2020, 82, 1539–1542. [Google Scholar] [CrossRef]
- Villani, A.; Costa, C.; Fabbrocini, G.; Cappello, M.; Scalvenzi, M. Reply to: Comparison of daily dosing vs. Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma (laBCC) and basal cell nevus syndrome: A retrospective case series. J. Am. Acad. Dermatol. 2020, 83, e201–e202. [Google Scholar] [CrossRef]
- Villani, A.; Costa, C.; Fabbrocini, G.; Ruggiero, A.; Scalvenzi, M. Dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor sonidegib to manage adverse effects: A retrospective case series. J. Am. Acad. Dermatol. 2021, 84, e211–e212. [Google Scholar] [CrossRef]
- Dréno, B.; Kunstfeld, R.; Hauschild, A.; Fosko, S.; Zloty, D.; Labeille, B.; Grob, J.-J.; Puig, S.; Gilberg, F.; Bergström, D.; et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017, 18, 404–412. [Google Scholar] [CrossRef]
- Scalvenzi, M.; Costa, C.; Cappello, M.; Villani, A. Reply to Woltsche N. et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: A single-centre experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e145–e147. [Google Scholar] [CrossRef]
- Tronconi, M.C.; Solferino, A.; Giordano, L.; Borroni, R.; Mancini, L.; Santoro, A. Tailored Toxicity-Driven Administration of Vismodegib in Patients with Multiple or Locally Advanced Basal Cell Carcinoma: A Pilot Analysis. Front. Oncol. 2020, 10, 563404. [Google Scholar] [CrossRef]
- Villani, A.; Costa, C.; Fabbrocini, G.; Scalvenzi, M. Drug holiday regimen for vismodegib treatment in patients with multiple primary basal cell carcinomas. Dermatol. Ther. 2020, 33, e13707. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Lear, J.T.; Migden, M.R.; Lewis, K.D.; Chang, A.L.S.; Guminski, A.; Gutzmer, R.; Dirix, L.; Combemale, P.; Stratigos, A.; Plummer, R.; et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Lewis, K.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; Marmur, E.; Rudin, C.M.; et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J. Am. Acad. Dermatol. 2015, 72, 1021–1026.e8. [Google Scholar] [CrossRef]
- Gutzmer, R.; Robert, C.; Loquai, C.; Schadendorf, D.; Squittieri, N.; Arntz, R.; Martelli, S.; Dummer, R. Assessment of various efficacy outcomes using ERIVANCE-like criteria in patients with locally advanced basal cell carcinoma receiving sonidegib: Results from a preplanned sensitivity analysis. BMC Cancer 2021, 21, 1244. [Google Scholar] [CrossRef]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef]
- Gutzmer, R.; Schulze, H.J.; Hauschild, A.; Leiter, U.; Meier, F.; Haferkamp, S.; Ulrich, C.; Wahl, R.U.; Berking, C.; Herbst, R.; et al. Effectiveness, safety and utilization of vismodegib in locally advanced basal cell carcinoma under real-world conditions in Germany—The non-interventional study NIELS. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1678–1685. [Google Scholar] [CrossRef]
- Herms, F.; Baroudjian, B.; Delyon, J.; Laly, P.; Tetu, P.; Lebbe, C.; Basset-Seguin, N. Sonidegib in the Treatment of Locally Advanced Basal Cell Carcinoma: A retrospective single-center study in France. In Proceedings of the Virtual European Academy of Dermatology and Venereology Congress, Virtual, 29 September–2 October 2021. [Google Scholar]
- Leow, L.J.; Teh, N. Clinical clearance of complex basal cell carcinoma in patients receiving sonidegib: A case series. Dermatol. Ther. 2022, 35, e15217. [Google Scholar] [CrossRef]
- Villani, A.; Fabbrocini, G.; Scalvenzi, M. Sonidegib treatment in patients with locally advanced basal cell carcinoma. Dermatol. Ther. 2022, 35, e15348. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Gutzmer, R.; Loquai, C.; Robert, C.; Dréno, B.; Guminski, A.; Lewis, K.; Arntz, R.; Martelli, S.; Squittieri, N.; Kheterpal, M. Key Clinical Adverse Events in Patients with Advanced Basal Cell Carcinoma Treated with Sonidegib or Vismodegib: A Post Hoc Analysis. Dermatol. Ther. 2021, 11, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Calzavara Pinton, P.; Licitra, L.; Peris, K.; Santoro, A.; Ascierto, P.A. Vismodegib in the treatment of basal cell carcinoma: Indications for clinical practice. Future Oncol. 2015, 11, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Ray, H. Safety and Tolerability of Sonic Hedgehog Pathway Inhibitors in Cancer. Drug Saf. 2019, 42, 263–279. [Google Scholar] [CrossRef] [PubMed]
- EMA. Summary of Product Characteristics, Odomzo 200 mg Hard Capsules. Available online: https://www.ema.europa.eu/en/documents/product-information/odomzo-epar-product-information_en.pdf (accessed on 7 February 2022).
- EMA. Summary of Product Characteristics, Erivedge 150 mg Hard Capsules. Available online: https://www.ema.europa.eu/en/documents/product-information/erivedge-epar-product-information_en.pdf (accessed on 7 February 2022).
- Odomzo® US Prescribing Information 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205266s006lbl.pdf (accessed on 6 July 2022).
- Sharma, M.R.; Karrison, T.G.; Kell, B.; Wu, K.; Turcich, M.; Geary, D.; Kang, S.P.; Takebe, N.; Graham, R.A.; Maitland, M.L.; et al. Evaluation of food effect on pharmacokinetics of vismodegib in advanced solid tumor patients. Clin. Cancer Res. 2013, 19, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Guminski, A.; Gutzmer, R.; Dirix, L.; Lewis, K.D.; Combemale, P.; Herd, R.M.; Kudchadkar, R.; Trefzer, U.; Gogov, S.; et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015, 16, 716–728. [Google Scholar] [CrossRef]
- Basset-Seguin, N.; Hauschild, A.; Grob, J.-J.; Kunstfeld, R.; Dréno, B.; Mortier, L.; Ascierto, P.A.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): A pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015, 16, 729–736. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332. [Google Scholar] [CrossRef]
- Lewis, K.; Dummer, R.; Farberg, A.S.; Guminski, A.; Squittieri, N.; Migden, M. Effects of Sonidegib Following Dose Reduction and Treatment Interruption in Patients with Advanced Basal Cell Carcinoma During 42-Month BOLT Trial. Dermatol. Ther. 2021, 11, 2225–2234. [Google Scholar] [CrossRef]
- Daly, J.; Willis, K.; Small, R.; Green, J.; Welch, N.; Kealy, M.; Hughes, E. A hierarchy of evidence for assessing qualitative health research. J. Clin. Epidemiol. 2007, 60, 43–49. [Google Scholar] [CrossRef]
- Herms, F.; Lambert, J.; Grob, J.J.; Haudebourg, L.; Bagot, M.; Dalac, S.; Dutriaux, C.; Guillot, B.; Jeudy, G.; Mateus, C.; et al. Follow-Up of Patients with Complete Remission of Locally Advanced Basal Cell Carcinoma After Vismodegib Discontinuation: A Multicenter French Study of 116 Patients. J. Clin. Oncol. 2019, 37, 3275–3282. [Google Scholar] [CrossRef]
- Scalvenzi, M.; Cappello, M.; Costa, C.; Fabbrocini, G.; Luciano, M.; Villani, A. Low-Dose Vismodegib as Maintenance Therapy After Locally Advanced Basal Cell Carcinoma Complete Remission: High Efficacy with Minimal Toxicity. Dermatol. Ther. 2020, 10, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Ally, M.S.; Chanana, A.M.; Mackay-Wiggan, J.M.; Aszterbaum, M.; Lindgren, J.A.; Ulerio, G.; Rezaee, M.R.; Gildengorin, G.; Marji, J.; et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: Final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1720–1731. [Google Scholar] [CrossRef]
- Alfieri, S.; Marceglia, S.; Filippini, D.M.; Bergamini, C.; Resteghini, C.; Cavalieri, S.; Peris, K.; Sollena, P.; Piccerillo, A.; Gualdi, G.; et al. 1065P A retrospective multicenter Italian analysis of the effect of longer vismodegib intake in 68 basal cell carcinoma patients who achieved clinical complete remission. Ann. Oncol. 2021, 32, S886. [Google Scholar] [CrossRef]
- Doan, H.Q.; Chen, L.; Nawas, Z.; Lee, H.H.; Silapunt, S.; Migden, M. Switching Hedgehog inhibitors and other strategies to address resistance when treating advanced basal cell carcinoma. Oncotarget 2021, 12, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.A.; Pop, O.T.; Ighilahriz, M.; Padioleau, I.; Rajabi, F.; Sharpe, H.J.; Poulalhon, N.; Dreno, B.; Khammari, A.; Delord, M.; et al. Frequency and Genomic Aspects of Intrinsic Resistance to Vismodegib in Locally Advanced Basal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Pricl, S.; Cortelazzi, B.; Dal Col, V.; Marson, D.; Laurini, E.; Fermeglia, M.; Licitra, L.; Pilotti, S.; Bossi, P.; Perrone, F. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol. Oncol. 2015, 9, 389–397. [Google Scholar] [CrossRef]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.B.; Dlugosz, A.A.; Haverty, P.M.; et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef]
- Zhao, X.; Ponomaryov, T.; Ornell, K.J.; Zhou, P.; Dabral, S.K.; Pak, E.; Li, W.; Atwood, S.X.; Whitson, R.J.; Chang, A.L.; et al. RAS/MAPK Activation Drives Resistance to Smo Inhibition, Metastasis, and Tumor Evolution in Shh Pathway-Dependent Tumors. Cancer Res. 2015, 75, 3623–3635. [Google Scholar] [CrossRef]
- Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; Li, J.R.; Kim, G.; Rezaee, M.; Ally, M.S.; Kim, J.; Yao, C.; Chang, A.L.; et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 2015, 27, 342–353. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef]
- Danial, C.; Sarin, K.Y.; Oro, A.E.; Chang, A.L. An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib. Clin. Cancer Res. 2016, 22, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Zargari, O.; Azimi, S.Z.; Geranmayeh, S. Inoperable infiltrative basal cell carcinoma successfully treated with vismodegib. Dermatol. Ther. 2017, 30, e12509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.A.; Li, A.S.; Chang, A.L. Patient with Gorlin syndrome and metastatic basal cell carcinoma refractory to smoothened inhibitors. JAMA Dermatol. 2014, 150, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Apicelli, A.J., 3rd; Pavlopoulos, T.V. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2018, 4, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.C.; Moffat, A.; Brotherton, R.; Pague, A.; Zhu, G.A.; Chang, A.L.S. An exploratory open-label, investigator-initiated study to evaluate the efficacy and safety of combination sonidegib and buparlisib for advanced basal cell carcinomas. J. Am. Acad. Dermatol. 2018, 78, 1011–1013. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Phase 2 study of cemiplimab in patients with locally advanced basal cell carcinoma after hedgehog inhibitor therapy: Long-term follow-up. In Proceedings of the virtual European Association of Dermato Oncology congress, Seville, Spain, 21–23 April 2022. [Google Scholar]
- Chang, A.L.S.; Tran, D.C.; Cannon, J.G.D.; Li, S.; Jeng, M.; Patel, R.; Van der Bokke, L.; Pague, A.; Brotherton, R.; Rieger, K.E.; et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. J. Am. Acad. Dermatol. 2019, 80, 564–566. [Google Scholar] [CrossRef]
- Walter, A.; Barysch, M.J.; Behnke, S.; Dziunycz, P.; Schmid, B.; Ritter, E.; Gnjatic, S.; Kristiansen, G.; Moch, H.; Knuth, A.; et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin. Cancer Res. 2010, 16, 3562–3570. [Google Scholar] [CrossRef]
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. Immunother. Cancer 2017, 5, 23. [Google Scholar] [CrossRef]
- Chang, J.; Zhu, G.A.; Cheung, C.; Li, S.; Kim, J.; Chang, A.L. Association Between Programmed Death Ligand 1 Expression in Patients with Basal Cell Carcinomas and the Number of Treatment Modalities. JAMA Dermatol. 2017, 153, 285–290. [Google Scholar] [CrossRef]
- Hua, L.A.; Kagen, C.N.; Carpenter, R.J.; Goltz, R.W. HLA and beta 2-microglobulin expression in basal and squamous cell carcinomas of the skin. Int. J. Dermatol. 1985, 24, 660–663. [Google Scholar] [CrossRef]
- Kooy, A.J.; Prens, E.P.; Van Heukelum, A.; Vuzevski, V.D.; Van Joost, T.; Tank, B. Interferon-gamma-induced ICAM-1 and CD40 expression, complete lack of HLA-DR and CD80 (B7.1), and inconsistent HLA-ABC expression in basal cell carcinoma: A possible role for interleukin-10? J. Pathol. 1999, 187, 351–357. [Google Scholar] [CrossRef]
- Corinti, S.; Albanesi, C.; la Sala, A.; Pastore, S.; Girolomoni, G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 2001, 166, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Elamin, I.; Zecević, R.D.; Vojvodić, D.; Medenica, L.; Pavlović, M.D. Cytokine concentrations in basal cell carcinomas of different histological types and localization. Acta Dermatovenerol. Alp. Pannonica Adriat. 2008, 17, 55–59. [Google Scholar] [PubMed]
- Gambichler, T.; Skrygan, M.; Kaczmarczyk, J.M.; Hyun, J.; Tomi, N.S.; Sommer, A.; Bechara, F.G.; Boms, S.; Brockmeyer, N.H.; Altmeyer, P.; et al. Increased expression of TGF-beta/Smad proteins in basal cell carcinoma. Eur. J. Med. Res. 2007, 12, 509–514. [Google Scholar]
- Otsuka, A.; Dreier, J.; Cheng, P.F.; Nägeli, M.; Lehmann, H.; Felderer, L.; Frew, I.J.; Matsushita, S.; Levesque, M.P.; Dummer, R. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin. Cancer Res. 2015, 21, 1289–1297. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Kim, J.; Tang, J.Y.; Gong, R.; Kim, J.; Lee, J.J.; Clemons, K.V.; Chong, C.R.; Chang, K.S.; Fereshteh, M.; Gardner, D.; et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 2010, 17, 388–399. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.; Spaunhurst, K.; Montoya, J.; Khodosh, R.; Chandra, K.; Fu, T.; Gilliam, A.; Molgo, M.; Beachy, P.A.; et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J. Clin. Oncol. 2014, 32, 745–751. [Google Scholar] [CrossRef]
- Liu, M.; Liang, G.; Zheng, H.; Zheng, N.; Ge, H.; Liu, W. Triazoles bind the C-terminal domain of SMO: Illustration by docking and molecular dynamics simulations the binding between SMO and triazoles. Life Sci. 2019, 217, 222–228. [Google Scholar] [CrossRef]
- Yoon, J. Vismodegib dose reduction effective when combined with itraconazole for the treatment of advanced basal cell carcinoma. JAAD Case Rep. 2021, 7, 107–109. [Google Scholar] [CrossRef]
- Ramelyte, E.; Restivo, G.; Imhof, L.; Nageli, M.C.; Dummer, R. How to break resistance to hedgehog inhibitors in advanced basal cell carcinoma? Br. J. Dermatol. 2021, 184, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.T.; Fernandez-Lopez, E.; Silk, A.W.; Dummer, R.; Bhatia, S. Immunologic Characteristics of Nonmelanoma Skin Cancers: Implications for Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 398–407. [Google Scholar] [CrossRef] [PubMed]

| Patients with laBCC | Sonidegib 200 mg QD [25] Central View RECIST-like | Vismodegib 150 mg QD [26] Central View RECIST |
|---|---|---|
| n = 66 | n = 63 | |
| ORR, n (%); 95% CI | 40 (60.6); 47.8–72.4 | 30 (47.6); 35.5–60.6 |
| CR, n (%) | 14 (21.2) | 14 (22.2) |
| PR, n (%) | 26 (39.4) | 16 (25.4) |
| SD, n (%) | 20 (30.3) | 22 (34.9) |
| PD, n (%) | 1 (1.5) | 8 (12.7) |
| Unknown, n (%) | 5 (7.6) | 3 (4.8) |
| DOR, median, months | 26.1 | 9.5 |
| MIKIE [20] n = 229 | STEVIE [42] n = 1215 | ERIVANCE [43] n = 104 | |
|---|---|---|---|
| AEs leading to discontinuation | 23% Group A: 23%, Group B: 30% | 31% | 21% |
| AE ≥ grade 3 | 31% | 44% | 56% |
| mDOT (weeks) | 71 | 38 | 56 |
| Reference | Study Type | Quality of Evidence * | Number of Patients | HHI | Treatment Schedule |
|---|---|---|---|---|---|
| On-off Treatment | |||||
| Dréno et al. 2017 [20] | RCT, phase II | 1 | 116 | vismodegib (150 mg/day) | 12 weeks on, 8 weeks off |
| 113 | 24 weeks on followed by 8 weeks off, 8 weeks on | ||||
| Scalvenzi et al. 2019 [21] | retrospective case series | 4 | 10 | vismodegib (150 mg/day) | 4–16 weeks off |
| Tronconi et al. 2020 [22] | retrospective case series | 4 | 8 | vismodegib (150 mg/day) | 4 weeks on, 2 weeks off |
| Villani et al. 2020 [23] | retrospective case series | 4 | 4 | vismodegib (150 mg/day) | 8–12 weeks off |
| Dose Reduction | |||||
| Woltsche et al. 2019 [16] | retrospective case series | 4 | 13 | vismodegib (150 mg/day) | 1/2–1/7 |
| Wong et al. 2020 [17] | retrospective case series | 4 | 8 | vismodegib (150 mg/day) | Mo–Fr, 2 days off |
| Villani et al. 2020 [18] | retrospective case series | 4 | 8 | vismodegib (150 mg/day) | 1/2 |
| Villani et al. 2021 [19] | retrospective case series | 4 | 9 | sonidegib (200 mg/day) | after 12–24 weeks 1/1 switch to 1/2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heppt, M.V.; Gebhardt, C.; Hassel, J.C.; Alter, M.; Gutzmer, R.; Leiter, U.; Berking, C. Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives. Cancers 2022, 14, 4547. https://doi.org/10.3390/cancers14194547
Heppt MV, Gebhardt C, Hassel JC, Alter M, Gutzmer R, Leiter U, Berking C. Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives. Cancers. 2022; 14(19):4547. https://doi.org/10.3390/cancers14194547
Chicago/Turabian StyleHeppt, Markus V., Christoffer Gebhardt, Jessica C. Hassel, Mareike Alter, Ralf Gutzmer, Ulrike Leiter, and Carola Berking. 2022. "Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives" Cancers 14, no. 19: 4547. https://doi.org/10.3390/cancers14194547
APA StyleHeppt, M. V., Gebhardt, C., Hassel, J. C., Alter, M., Gutzmer, R., Leiter, U., & Berking, C. (2022). Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives. Cancers, 14(19), 4547. https://doi.org/10.3390/cancers14194547









