Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications
Abstract
Simple Summary
Abstract
1. Introduction
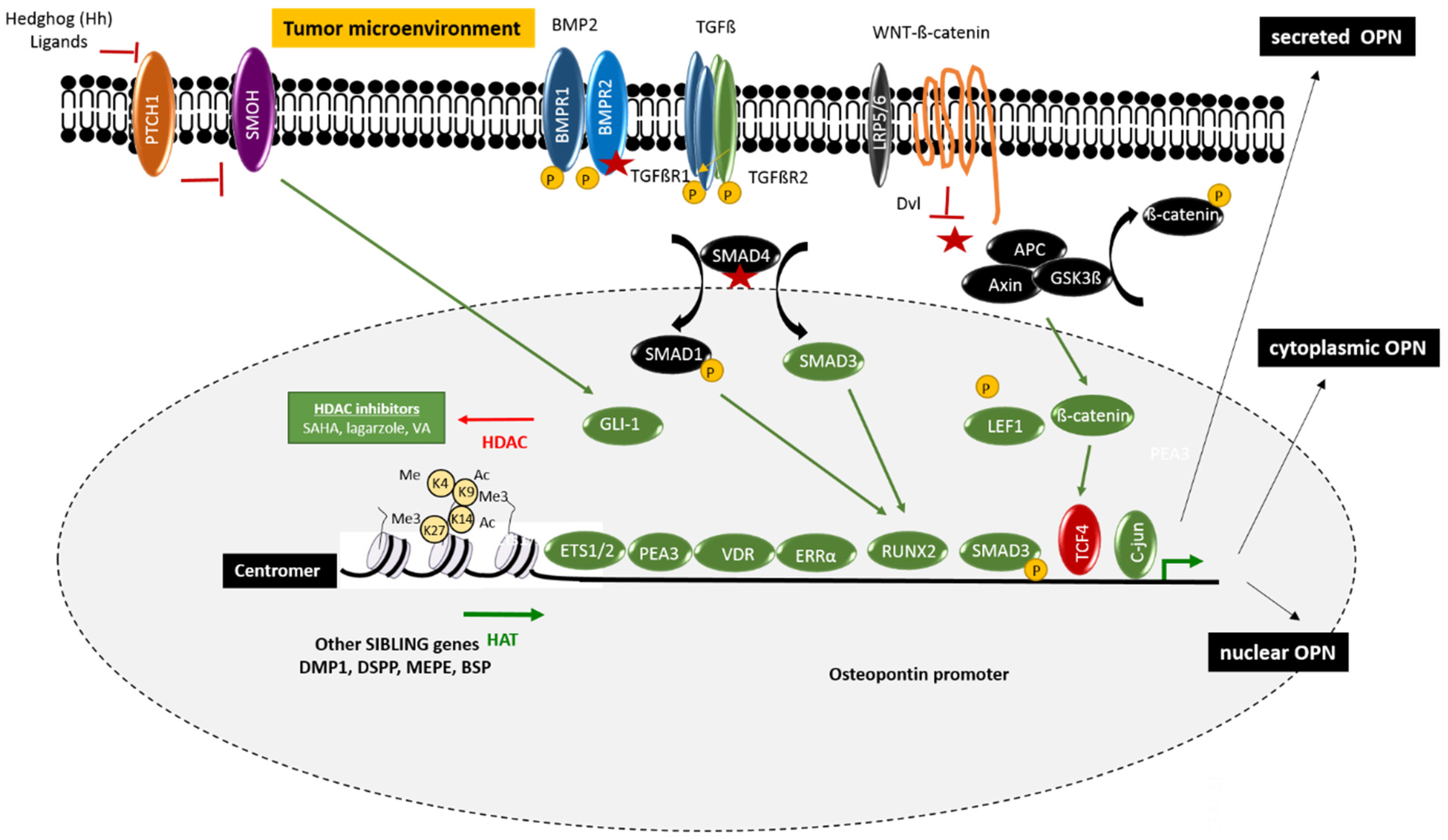
2. Regulation of Osteopontin
2.1. Epigenetic Regulators
2.1.1. Promoter Methylation, Histone Modifiers, and Chromatin Remodeling
2.1.2. Non-Coding RNAs
2.2. Gene Expression and Signaling Pathways
2.2.1. Wnt/Wingless Pathway
2.2.2. Bone Morphogen Protein/Transforming Growth Factor Pathway
2.2.3. Vitamin D Receptor (VDR) Pathway
2.2.4. Nuclear Receptor Pathway
2.2.5. Hedgehog Pathway
2.3. Alternative Splicing, Alternative Translation, and Protein Localization
2.4. Post-Translational Modifications
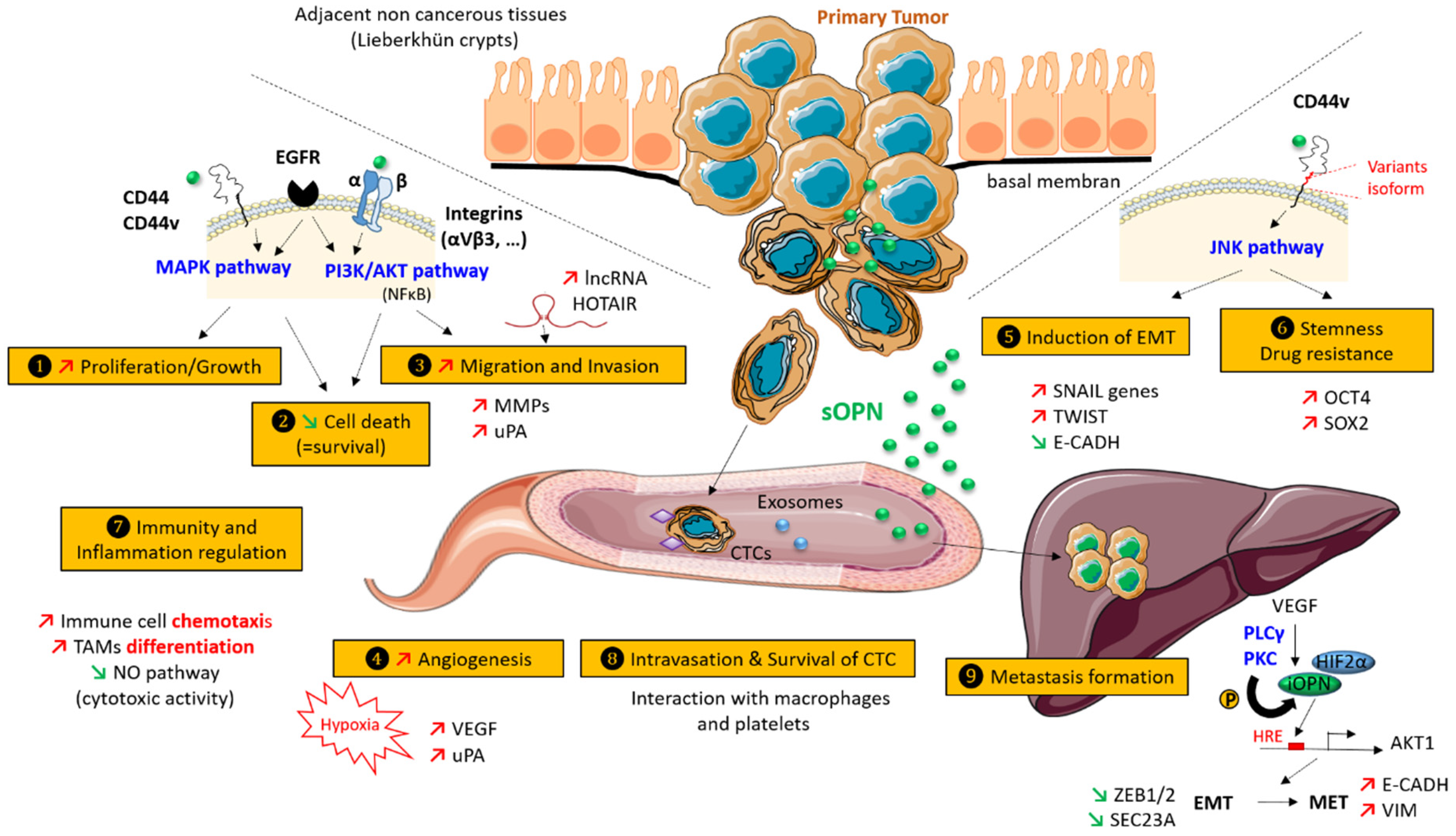
3. Role of Osteopontin in CRC
3.1. Increased Proliferation and Cell Growth
3.2. Inhibition of Cell Death
3.3. Migration and Invasion
3.4. Hypoxia and Neo-Angiogenesis
3.5. Induction of Epithelial to Mesenchymal Transition (EMT)
3.6. Stemness and Drug-Resistance
3.7. Immunity and Inflammation
3.8. Survival, Protection, and Intravasation of Circulating Tumor Cells (CTCs)
3.9. Metastasis Formation
4. Potential Applications of OPN as a Biomarker
4.1. Screening and Early Detection
4.2. Prognostic Value in CRC Patients
4.3. Evaluation of Recurrence Rate after Surgery
4.4. Potential Immune Marker
5. Potential Applications of OPN as a Therapeutic Target
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Zhong, W.; Yu, Z.; Zhan, J.; Yu, T.; Lin, Y.; Xia, Z.-S.; Yuan, Y.-H.; Chen, Q.-K. Association of Serum Levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and Disease Characteristics in Colorectal Cancer. Pathol. Oncol. Res. POR 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Chen, T.; Irby, R.; Quackenbush, J.; Chambers, A.F.; Szabo, M.; Cantor, A.; Coppola, D.; Yeatman, T.J. Osteopontin Identified as Lead Marker of Colon Cancer Progression, Using Pooled Sample Expression Profiling. J. Natl. Cancer Inst. 2002, 94, 513–521. [Google Scholar] [CrossRef]
- Coppola, D.; Szabo, M.; Boulware, D.; Muraca, P.; Alsarraj, M.; Chambers, A.F.; Yeatman, T.J. Correlation of Osteopontin Protein Expression and Pathological Stage across a Wide Variety of Tumor Histologies. Clin. Cancer Res. 2004, 10, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Mistretta, D.; Chambers, A.F.; Krishna, S.; Porter, J.F.; Raghuram, S.; Rittling, S.R. Transcriptional Regulation of Osteopontin and the Metastatic Phenotype: Evidence for a Ras-Activated Enhancer in the Human OPN Promoter. Clin. Exp. Metastasis 2003, 20. [Google Scholar] [CrossRef] [PubMed]
- Bellahcène, A.; Castronovo, V.; Ogbureke, K.U.E.; Fisher, L.W.; Fedarko, N.S. Small Integrin-Binding Ligand N-Linked Glycoproteins (SIBLINGs): Multifunctional Proteins in Cancer. Nat. Rev. Cancer 2008, 8, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The Role of Osteopontin in the Progression of Solid Organ Tumour. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Wirth, D.F.; Hynes, R.O. Transformed Mammalian Cells Secrete Specific Proteins and Phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Oldberg, A.; Franzén, A.; Heinegård, D. Cloning and Sequence Analysis of Rat Bone Sialoprotein (osteopontin) cDNA Reveals an Arg-Gly-Asp Cell-Binding Sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 8819–8823. [Google Scholar] [CrossRef]
- Craig, A.M.; Smith, J.H.; Denhardt, D.T. Osteopontin, a Transformation-Associated Cell Adhesion Phosphoprotein, Is Induced by 12-O-Tetradecanoylphorbol 13-Acetate in Mouse Epidermis. J. Biol. Chem. 1989, 264, 9682–9689. [Google Scholar] [CrossRef]
- Patarca, R.; Freeman, G.J.; Singh, R.P.; Wei, F.Y.; Durfee, T.; Blattner, F.; Regnier, D.C.; Kozak, C.A.; Mock, B.A.; Morse, H.C., 3rd; et al. Structural and Functional Studies of the Early T Lymphocyte Activation 1 (Eta-1) Gene. Definition of a Novel T Cell-Dependent Response Associated with Genetic Resistance to Bacterial Infection. J. Exp. Med. 1989, 170, 145–161. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Tummala, P.; Castillo, A.B.; Zhang, F.; Jacobs, C.R. The Epigenetic Mechanism of Mechanically Induced Osteogenic Differentiation. J. Biomech. 2010, 43, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Ren, A.; Qiu, Y.; Zhang, Y. Epigenetic Regulation of Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 235–246. [Google Scholar] [CrossRef]
- Huynh, N.C.-N.; Everts, V.; Ampornaramveth, R.S. Histone Deacetylases and Their Roles in Mineralized Tissue Regeneration. Bone Rep. 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Janson, W.T.; Jenkins, L.J.; Chionh, F.; Mariadason, J.M. Aberrant DNA Methylation in Colorectal Cancer: What Should We Target? Trends Cancer 2017, 3, 698–712. [Google Scholar] [CrossRef]
- Haller, F.; Zhang, J.D.; Moskalev, E.A.; Braun, A.; Otto, C.; Geddert, H.; Riazalhosseini, Y.; Ward, A.; Balwierz, A.; Schaefer, I.-M.; et al. Combined DNA Methylation and Gene Expression Profiling in Gastrointestinal Stromal Tumors Reveals Hypomethylation of SPP1 as an Independent Prognostic Factor. Int. J. Cancer 2015, 136, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-J.; Tsou, Y.-A.; Chen, H.-L.; Huang, H.-J.; Wu, S.-C.; Cheng, W.T.K.; Chen, C.Y.-C.; Chen, C.-M. Osteoponin Promoter Controlled by DNA Methylation: Aberrant Methylation in Cloned Porcine Genome. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Sato, A.; Saito, Y.; Sugiyama, K.; Sakasegawa, N.; Muramatsu, T.; Fukuda, S.; Yoneya, M.; Kimura, M.; Ebinuma, H.; Hibi, T.; et al. Suppressive Effect of the Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid (SAHA) on Hepatitis C Virus Replication. J. Cell. Biochem. 2013, 114, 1987–1996. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Yan, H.; Qi, H.; Deng, C.; Ye, T.; Zhou, S.; Li, F.-R. Role of Histone Deacetylase Inhibitors in the Aging of Human Umbilical Cord Mesenchymal Stem Cells. J. Cell. Biochem. 2013, 114, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Lund, R.J.; Emani, M.R.; Barbaric, I.; Kivinen, V.; Jones, M.; Baker, D.; Gokhale, P.; Nykter, M.; Lahesmaa, R.; Andrews, P.W. Karyotypically Abnormal Human ESCs Are Sensitive to HDAC Inhibitors and Show Altered Regulation of Genes Linked to Cancers and Neurological Diseases. Stem Cell Res. 2013, 11, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Paino, F.; la Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; De Rosa, A.; Laino, L.; Altucci, L.; Papaccio, G. Histone Deacetylase Inhibition with Valproic Acid Downregulates Osteocalcin Gene Expression in Human Dental Pulp Stem Cells and Osteoblasts: Evidence for HDAC2 Involvement. Stem Cells Dayt. Ohio 2014, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, P.; Ge, J.; Cheng, J.; Dong, W.; Yuan, H.; Du, Y.; Yang, M.; Sun, R.; Jiang, H. Histone Deacetylase 8 Suppresses Osteogenic Differentiation of Bone Marrow Stromal Cells by Inhibiting Histone H3K9 Acetylation and RUNX2 Activity. Int. J. Biochem. Cell Biol. 2014, 54, 68–77. [Google Scholar] [CrossRef]
- Cai, M.; Bompada, P.; Atac, D.; Laakso, M.; Groop, L.; De Marinis, Y. Epigenetic Regulation of Glucose-Stimulated Osteopontin (OPN) Expression in Diabetic Kidney. Biochem. Biophys. Res. Commun. 2016, 469, 108–113. [Google Scholar] [CrossRef]
- Xu, J.; Yu, B.; Hong, C.; Wang, C.-Y. KDM6B Epigenetically Regulates Odontogenic Differentiation of Dental Mesenchymal Stem Cells. Int. J. Oral Sci. 2013, 5, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Matsui, Y.; Higashimoto, M.; Kawaguchi, Y.; Seki, S.; Motomura, H.; Hori, T.; Yahara, Y.; Kanamori, M.; Kimura, T. Myxoid Liposarcoma-Associated EWSR1-DDIT3 Selectively Represses Osteoblastic and Chondrocytic Transcription in Multipotent Mesenchymal Cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.D.; Garrison, J.; Guo, H.; Mi, Z.; Markovic, J.; Kim, V.M.; Kuo, P.C. Micro-RNA-181a Regulates Osteopontin Dependent Metastatic Function In Hepatocellular Cancer Cell Lines. Surgery 2010, 148, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-X.; Xia, Y.-H.; Xue, T.-C.; Ye, S.-L. Suppression of microRNA-96 Expression Inhibits the Invasion of Hepatocellular Carcinoma Cells. Mol. Med. Rep. 2012, 5, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, N.; Yang, Y.; Qiu, X. Regulation of Osteosarcoma Cell Invasion through Osteopontin Modification by miR-4262. Tumor Biol. 2016, 37, 6493–6499. [Google Scholar] [CrossRef]
- Taipaleenmäki, H.; Farina, N.H.; van Wijnen, A.J.; Stein, J.L.; Hesse, E.; Stein, G.S.; Lian, J.B. Antagonizing miR-218-5p Attenuates Wnt Signaling and Reduces Metastatic Bone Disease of Triple Negative Breast Cancer Cells. Oncotarget 2016, 7, 79032–79046. [Google Scholar] [CrossRef]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 Inhibits Tumor Growth and Bone Metastasis in Prostate Cancer by Direct Regulation of Osteogenic Transcription Factor RUNX2. Cell Death Dis. 2017, 8, e2572. [Google Scholar] [CrossRef]
- Arffa, M.L.; Zapf, M.A.; Kothari, A.N.; Chang, V.; Gupta, G.N.; Ding, X.; Al-Gayyar, M.M.; Syn, W.; Elsherbiny, N.M.; Kuo, P.C.; et al. Epigallocatechin-3-Gallate Upregulates miR-221 to Inhibit Osteopontin-Dependent Hepatic Fibrosis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Huang, G.; Guo, H.; Guo, C.; Li, H.; Ye, S.; Ling, S.; Jiang, L.; Tian, Y.; Lin, T. Circulating miR-221 Directly Amplified from Plasma Is a Potential Diagnostic and Prognostic Marker of Colorectal Cancer and Is Correlated with p53 Expression. J. Gastroenterol. Hepatol. 2010, 25, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Watanabe, K.; Hara, E.S.; Ono, M.; Kuboki, T.; Calderwood, S.K. OstemiR: A Novel Panel of MicroRNA Biomarkers in Osteoblastic and Osteocytic Differentiation from Mesencymal Stem Cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Fu, J.; Zhou, W.; Li, W.; Dong, S.; Yu, S.; Hu, Z.; Wang, H.; Xie, Z. MicroRNA-125b Regulates Osteogenic Differentiation of Mesenchymal Stem Cells by Targeting Cbfβ in Vitro. Biochimie 2014, 102. [Google Scholar] [CrossRef]
- Shirafkan, N.; Mansoori, B.; Mohammadi, A.; Shomali, N.; Ghasbi, M.; Baradaran, B. MicroRNAs as Novel Biomarkers for Colorectal Cancer: New Outlooks. Biomed. Pharmacother. 2018, 97, 1319–1330. [Google Scholar] [CrossRef]
- Žlajpah, M.; Boštjančič, E.; Zidar, N. (Epi)genetic Regulation of Osteopontin in Colorectal Cancerogenesis. Epigenomics 2020, 12, 1389–1403. [Google Scholar] [CrossRef]
- Iacona, J.R.; Lutz, C.S. miR-146a-5p: Expression, Regulation, and Functions in Cancer. WIREs RNA 2019, 10, e1533. [Google Scholar] [CrossRef] [PubMed]
- Bleau, A.-M.; Redrado, M.; Nistal-Villan, E.; Villalba, M.; Exposito, F.; Redin, E.; de Aberasturi, A.L.; Larzabal, L.; Freire, J.; Gomez-Roman, J.; et al. miR-146a Targets c-Met and Abolishes Colorectal Cancer Liver Metastasis. Cancer Lett. 2018, 414, 257–267. [Google Scholar] [CrossRef]
- El Bairi, K.; Tariq, K.; Himri, I.; Jaafari, A.; Smaili, W.; Kandhro, A.H.; Gouri, A.; Ghazi, B. Decoding Colorectal Cancer Epigenomics. Cancer Genet. 2018, 220, 49–76. [Google Scholar] [CrossRef]
- Baretti, M.; Azad, N.S. The Role of Epigenetic Therapies in Colorectal Cancer. Curr. Probl. Cancer 2018, 42, 530–547. [Google Scholar] [CrossRef]
- Hijiya, N.; Setoguchi, M.; Matsuura, K.; Higuchi, Y.; Akizuki, S.; Yamamoto, S. Cloning and Characterization of the Human Osteopontin Gene and Its Promoter. Biochem. J. 1994, 303, 255–262. [Google Scholar] [CrossRef]
- Wang, D.; Yamamoto, S.; Hijiya, N.; Benveniste, E.N.; Gladson, C.L. Transcriptional Regulation of the Human Osteopontin Promoter: Functional Analysis and DNA-Protein Interactions. Oncogene 2000, 19, 5801–5809. [Google Scholar] [CrossRef][Green Version]
- The Cancer Genome Atlas Network. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Rohde, F.; Rimkus, C.; Friederichs, J.; Rosenberg, R.; Marthen, C.; Doll, D.; Holzmann, B.; Siewert, J.-R.; Janssen, K.-P. Expression of Osteopontin, a Target Gene of de-Regulated Wnt Signaling, Predicts Survival in Colon Cancer. Int. J. Cancer 2007, 121, 1717–1723. [Google Scholar] [CrossRef]
- Ravindranath, A.; O’Connell, A.; Johnston, P.G.; El-Tanani, M.K. The Role of LEF/TCF Factors in Neoplastic Transformation. Curr. Mol. Med. 2008, 8. [Google Scholar] [CrossRef]
- Martinez, C.; Churchman, M.; Freeman, T.; Ilyas, M. Osteopontin Provides Early Proliferative Drive and May Be Dependent upon Aberrant c-Myc Signalling in Murine Intestinal Tumours. Exp. Mol. Pathol. 2010, 88, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Rennoll, S.; Yochum, G. Regulation of MYC Gene Expression by Aberrant Wnt/β-Catenin Signaling in Colorectal Cancer. World J. Biol. Chem. 2015, 6, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Chua, M.-S.; Choi, Y.-L.; Yeh, W.; Kim, Y.H.; Azzi, R.; Adams, G.A.; Sainani, K.; van de Rijn, M.; So, S.K.; et al. Comparative Profiling of Primary Colorectal Carcinomas and Liver Metastases Identifies LEF1 as a Prognostic Biomarker. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ashida, R.; Tominaga, K.; Sasaki, E.; Watanabe, T.; Fujiwara, Y.; Oshitani, N.; Higuchi, K.; Mitsuyama, S.; Iwao, H.; Arakawa, T. AP-1 and Colorectal Cancer. Inflammopharmacology 2005, 13, 113–125. [Google Scholar] [CrossRef]
- El-Tanani, M.; Platt-Higgins, A.; Rudland, P.S.; Campbell, F.C. Ets Gene PEA3 Cooperates with β-Catenin-Lef-1 and c-Jun in Regulation of Osteopontin Transcription. J. Biol. Chem. 2004, 279, 20794–20806. [Google Scholar] [CrossRef] [PubMed]
- Wai, P.Y.; Mi, Z.; Gao, C.; Guo, H.; Marroquin, C.; Kuo, P.C. Ets-1 and Runx2 Regulate Transcription of a Metastatic Gene, Osteopontin, in Murine Colorectal Cancer Cells. J. Biol. Chem. 2006, 281, 18973–18982. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.S.; Osman, W.M. Relationship between Osteopontin and β-Catenin Immunohistochemical Expression and Prognostic Parameters of Colorectal Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 1503–1514. [Google Scholar] [PubMed]
- Shi, X.; Bai, S.; Li, L.; Cao, X. Hoxa-9 Represses Transforming Growth Factor-β-Induced Osteopontin Gene Transcription. J. Biol. Chem. 2001, 276, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Georges, R.; Adwan, H.; Zhivkova, M.; Eyol, E.; Bergmann, F.; Berger, M.R. Regulation of Osteopontin and Related Proteins in Rat CC531 Colorectal Cancer Cells. Int. J. Oncol. 2010, 37, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sase, T.; Suzuki, T.; Miura, K.; Shiiba, K.; Sato, I.; Nakamura, Y.; Takagi, K.; Onodera, Y.; Miki, Y.; Watanabe, M.; et al. Runt-Related Transcription Factor 2 in Human Colon Carcinoma: A Potent Prognostic Factor Associated with Estrogen Receptor. Int. J. Cancer 2012, 131, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Li, S.; Jiang, W.; Zhu, J.; Liu, J.; Zhao, S. miR-539 Inhibits Human Colorectal Cancer Progression by Targeting RUNX2. Biomed. Pharmacother. 2017, 95, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-C.; Lin, J.-K. Liquiritigenin Inhibits Colorectal Cancer Proliferation, Invasion, and Epithelial-to-Mesenchymal Transition by Decreasing Expression of Runt-Related Transcription Factor 2. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 139–146. [Google Scholar] [CrossRef]
- Xu, H.; McCann, M.; Zhang, Z.; Posner, G.H.; Bingham, V.; El-Tanani, M.; Campbell, F.C. Vitamin D Receptor Modulates the Neoplastic Phenotype through Antagonistic Growth Regulatory Signals. Mol. Carcinog. 2009, 48, 758–772. [Google Scholar] [CrossRef]
- Matusiak, D.; Murillo, G.; Carroll, R.E.; Mehta, R.G.; Benya, R.V. Expression of Vitamin D Receptor and 25-Hydroxyvitamin D3-1α-Hydroxylase in Normal and Malignant Human Colon. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Sheinin, Y.; Kaserer, K.; Wrba, F.; Wenzl, E.; Kriwanek, S.; Peterlik, M.; Cross, H.S. In Situ mRNA Hybridization Analysis and Immunolocalization of the Vitamin D Receptor in Normal and Carcinomatous Human Colonic Mucosa: Relation to Epidermal Growth Factor Receptor Expression. Virchows Arch. 2000, 437, 501–507. [Google Scholar] [CrossRef]
- Lechner, D.; Kállay, E.; Cross, H.S. 1α,25-Dihydroxyvitamin D3 Downregulates CYP27B1 and Induces CYP24A1 in Colon Cells. Mol. Cell. Endocrinol. 2007, 263, 55–64. [Google Scholar] [CrossRef]
- Labudzynskyi, D.; Shymanskyy, I.; Veliky, M. Role of Vitamin D3 in Regulation of Interleukin-6 and Osteopontin Expression in Liver of Diabetic Mice. Eur Rev Med Pharmacol Sci. 2016, 20, 2916–2919. [Google Scholar]
- Zagani, R.; Hamzaoui, N.; Cacheux, W.; de Reyniès, A.; Terris, B.; Chaussade, S.; Romagnolo, B.; Perret, C.; Lamarque, D. Cyclooxygenase-2 Inhibitors Down-Regulate Osteopontin and Nr4a2—New Therapeutic Targets for Colorectal Cancers. Gastroenterology 2009, 137, 1358–1366. [Google Scholar] [CrossRef]
- Boudjadi, S.; Bernatchez, G.; Beaulieu, J.-F.; Carrier, J.C. Control of the Human Osteopontin Promoter by ERRα in Colorectal Cancer. Am. J. Pathol. 2013, 183, 266–276. [Google Scholar] [CrossRef]
- Das, S.; Harris, L.G.; Metge, B.J.; Liu, S.; Riker, A.I.; Samant, R.S.; Shevde, L.A. The Hedgehog Pathway Transcription Factor GLI1 Promotes Malignant Behavior of Cancer Cells by Up-Regulating Osteopontin. J. Biol. Chem. 2009, 284, 22888–22897. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, J.; Harvey, E.; Athwal, V.; Berry, A.; Rowe, C.; Oakley, F.; Moles, A.; Mann, D.A.; Bobola, N.; Sharrocks, A.D.; et al. Osteopontin Is a Novel Downstream Target of SOX9 with Diagnostic Implications for Progression of Liver Fibrosis in Humans. Hepatology 2012, 56, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Gimba, E.R.; Tilli, T.M. Human Osteopontin Splicing Isoforms: Known Roles, Potential Clinical Applications and Activated Signaling Pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.-K. Osteopontin Splice Variants and Polymorphisms in Cancer Progression and Prognosis. Biochim. Biophys. Acta BBA-Rev. Cancer 2017, 1868, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Shaughnessy, E.; Hurley, J.K.; Vanpatten, K.A.; Pestano, G.A.; He, B.; Weber, G.F. Osteopontin-c Is a Selective Marker of Breast Cancer. Int. J. Cancer 2008, 122, 889–897. [Google Scholar] [CrossRef]
- Zduniak, K.; Ziolkowski, P.; Ahlin, C.; Agrawal, A.; Agrawal, S.; Blomqvist, C.; Fjällskog, M.-L.; Weber, G.F. Nuclear Osteopontin-c Is a Prognostic Breast Cancer Marker. Br. J. Cancer 2015, 112, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, Z.; Gu, Y.; Jiang, W.G.; Cheng, S. Prognostic Value of Osteopontin Splice Variant-c Expression in Breast Cancers: A Meta-Analysis. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Shen, H.; Weber, G.F. The Osteopontin-c Splice Junction Is Important for Anchorage-Independent Growth. Mol. Carcinog. 2014, 53, 480–487. [Google Scholar] [CrossRef]
- Boguslawska, J.; Sokol, E.; Rybicka, B.; Czubaty, A.; Rodzik, K.; Piekielko-Witkowska, A. microRNAs Target SRSF7 Splicing Factor to Modulate the Expression of Osteopontin Splice Variants in Renal Cancer Cells. Gene 2016, 595, 142–149. [Google Scholar] [CrossRef]
- Chang, S.; Huang, J.; Niu, H.; Wang, J.; Si, Y.; Bai, Z.; Cheng, S.; Ding, W. Epigenetic Regulation of Osteopontin Splicing Isoform c Defines Its Role as a Microenvironmental Factor to Promote the Survival of Colon Cancer Cells from 5-FU Treatment. Cancer Cell Int. 2020, 20, 452. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Kim, H.-J.; Kim, J.-H.; Garcia, V.A.; Cantor, H. Alternative Translation of Osteopontin Generates Intracellular and Secreted Isoforms That Mediate Distinct Biological Activities in Dendritic Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Intracellular Osteopontin (iOPN) and Immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Lee, W.; Arora, P.; Cheifetz, S.; McCulloch, C.; Sodek, J. Single Cell Analysis of Intracellular Osteopontin in Osteogenic Cultures of Fetal Rat Calvarial Cells. J. Cell. Physiol. 1997, 170, 88–100. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, H.-J.; Jun, J.-I.; Oh, Y.; Choi, S.-G.; Kim, H.; Chung, C.-W.; Kim, I.-K.; Park, I.-S.; Chae, H.-J.; et al. Intracellular Cleavage of Osteopontin by Caspase-8 Modulates Hypoxia/reoxygenation Cell Death through p53. Proc. Natl. Acad. Sci. USA 2009, 106, 15326–15331. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Moon, M.C.; Harding, G.E.J.; Zahradka, P. Osteopontin Localizes to the Nucleus of 293 Cells and Associates with Polo-like Kinase-1. Am. J. Physiol.-Cell Physiol. 2007, 292, C919–C926. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Baba, O.; Butler, W.T. Post-Translational Modifications of Sibling Proteins and Their Roles in Osteogenesis and Dentinogenesis. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2004, 15, 126–136. [Google Scholar] [CrossRef]
- Christensen, B.; Kazanecki, C.C.; Petersen, T.E.; Rittling, S.R.; Denhardt, D.T.; Sørensen, E.S. Cell Type-Specific Post-Translational Modifications of Mouse Osteopontin Are Associated with Different Adhesive Properties. J. Biol. Chem. 2007, 282, 19463–19472. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (Osteopontin): An Early Component of Type-1 (Cell-Mediated) Immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Weber, G.F.; Zawaideh, S.; Hikita, S.; Kumar, V.A.; Cantor, H.; Ashkar, S. Phosphorylation-Dependent Interaction of Osteopontin with Its Receptors Regulates Macrophage Migration and Activation. J. Leukoc. Biol. 2002, 72, 752–761. [Google Scholar] [CrossRef]
- Kazanecki, C.C.; Kowalski, A.J.; Ding, T.; Rittling, S.R.; Denhardt, D.T. Characterization of Anti-Osteopontin Monoclonal Antibodies: Binding Sensitivity to Post-Translational Modifications. J. Cell. Biochem. 2007, 102, 925–935. [Google Scholar] [CrossRef]
- Castellano, G.; Malaponte, G.; Mazzarino, M.C.; Figini, M.; Marchese, F.; Gangemi, P.; Travali, S.; Stivala, F.; Canevari, S.; Libra, M. Activation of the Osteopontin/Matrix Metalloproteinase-9 Pathway Correlates with Prostate Cancer Progression. Clin. Cancer Res. 2008, 14, 7470–7480. [Google Scholar] [CrossRef] [PubMed]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The Integrin α9β1 Binds to a Novel Recognition Sequence (SVVYGLR) in the Thrombin-Cleaved Amino-Terminal Fragment of Osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Sakai, F.; Kon, S.; Morimoto, J.; Kimura, C.; Yamazaki, H.; Okazaki, I.; Seki, N.; Fujii, T.; Uede, T. Essential Role of the Cryptic Epitope SLAYGLR within Osteopontin in a Murine Model of Rheumatoid Arthritis. J. Clin. Investig. 2003, 112, 181–188. [Google Scholar] [CrossRef]
- Christensen, B.; Kläning, E.; Nielsen, M.S.; Andersen, M.H.; Sørensen, E.S. C-Terminal Modification of Osteopontin Inhibits Interaction with the αVβ3-Integrin. J. Biol. Chem. 2012, 287, 3788–3797. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Jiang, C.; Zhou, H.; Gu, L.; Liu, Y.; Xu, Q. SPP1, Analyzed by Bioinformatics Methods, Promotes the Metastasis in Colorectal Cancer by Activating EMT Pathway. Biomed. Pharmacother. 2017, 91, 1167–1177. [Google Scholar] [CrossRef]
- Ishigamori, R.; Komiya, M.; Takasu, S.; Mutoh, M.; Imai, T.; Takahashi, M. Osteopontin Deficiency Suppresses Intestinal Tumor Development in Apc-Deficient Min Mice. Int. J. Mol. Sci. 2017, 18, 1058. [Google Scholar] [CrossRef]
- Harada, N.; Mizoi, T.; Kinouchi, M.; Hoshi, K.; Ishii, S.; Shiiba, K.; Sasaki, I.; Matsuno, S. Introduction of Antisense CD44s cDNA down-Regulates Expression of Overall CD44 Isoforms and Inhibits Tumor Growth and Metastasis in Highly Metastatic Colon Carcinoma Cells. Int. J. Cancer 2001, 91, 67–75. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Roddey, G.; McDonnell, T.J.; Shen, Y.; Cleary, K.R.; Stephens, L.C. Increased Apoptosis Accompanies Neoplastic Development in the Human Colorectum. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1999–2006. [Google Scholar]
- Huang, R.; Quan, Y.; Chen, J.; Wang, T.; Xu, M.; Ye, M.; Yuan, H.; Zhang, C.; Liu, X.; Min, Z. Osteopontin Promotes Cell Migration and Invasion, and Inhibits Apoptosis and Autophagy in Colorectal Cancer by Activating the p38 MAPK Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 1851–1864. [Google Scholar] [CrossRef]
- Cao, D.-X. Osteopontin as Potential Biomarker and Therapeutic Target in Gastric and Liver Cancers. World J. Gastroenterol. 2012, 18, 3923. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, S.; Gao, F.; Liu, Z.; Lu, M.; Peng, S.; Zhang, T.; Zhang, F. Osteopontin Enhances the Expression of HOTAIR in Cancer Cells via IRF1. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2014, 1839, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Österlund, P.; Pignon, J.P. Angiogenesis Inhibition in the Second-Line Treatment of Metastatic Colorectal cancer—A Definite Conclusion? Semin. Oncol. 2017, 44, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, G.; Hauff, K.; Gasser, M.; Waaga-Gasser, A.; Grimmig, T.; Flentje, M.; Polat, B. Hypoxia Induces Differential Expression Patterns of Osteopontin and CD44 in Colorectal Carcinoma. Oncol. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Lin, K.-J.; Bai, A.-P.; Wang, W.-X.; Meng, X.-K.; Su, X.-L.; Hou, M.-X.; Dong, P.-D.; Zhang, J.-J.; Wang, Z.-Y.; et al. Osteopontin Knockdown Suppresses the Growth and Angiogenesis of Colon Cancer Cells. World J. Gastroenterol. WJG 2014, 20, 10440–10448. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-S.; Chen, W.-S.; Chen, L.-L.; Chen, C.-C.; Hsu, Y.-T.; Chua, K.V.; Wang, H.-D.; Huang, T.-S. Osteopontin–integrin Engagement Induces HIF-1α–TCF12-Mediated Endothelial-Mesenchymal Transition to Exacerbate Colorectal Cancer. Oncotarget 2017, 9, 4998–5015. [Google Scholar] [CrossRef]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial-mesenchymal Transition Spectrum Quantification and Its Efficacy in Deciphering Survival and Drug Responses of Cancer Patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-Mesenchymal Transitions: The Importance of Changing Cell State in Development and Disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Boesch, M.; Spizzo, G.; Seeber, A. Concise Review: Aggressive Colorectal Cancer: Role of Epithelial Cell Adhesion Molecule in Cancer Stem Cells and Epithelial-to-Mesenchymal Transition. Stem Cells Transl. Med. 2018, 7, 495–501. [Google Scholar] [CrossRef]
- Kothari, A.N.; Arffa, M.L.; Chang, V.; Blackwell, R.H.; Syn, W.-K.; Zhang, J.; Mi, Z.; Kuo, P.C. Osteopontin—A Master Regulator of Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 39. [Google Scholar] [CrossRef]
- Ng, L.; Wan, T.; Chow, A.; Iyer, D.; Man, J.; Chen, G.; Yau, T.C.-C.; Lo, O.; Foo, C.-C.; Poon, J.T.-C.; et al. Osteopontin Overexpression Induced Tumor Progression and Chemoresistance to Oxaliplatin through Induction of Stem-Like Properties in Human Colorectal Cancer. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef]
- Rao, G.; Du, L.; Chen, Q. Osteopontin, a Possible Modulator of Cancer Stem Cells and Their Malignant Niche. Oncoimmunology 2013, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 Is a Marker of Constitutive and Reprogrammed Cancer Stem Cells Driving Colon Cancer Metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Bouygues, A.; Ouaret, D.; Ferrand, N.; Chouaib, S.; Thiery, J.-P.; Muchardt, C.; Sabbah, M.; Larsen, A.K. Chronic Chemotherapeutic Stress Promotes Evolution of Stemness and WNT/beta-Catenin Signaling in Colorectal Cancer Cells: Implications for Clinical Use of WNT-Signaling Inhibitors. Oncotarget 2015, 6, 18518–18533. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Kumar, D.; Chakraborty, G.; Gorain, M.; Kundu, G.C. Functional Characterization of Stromal Osteopontin in Melanoma Progression and Metastasis. PLoS ONE 2013, 8, e69116. [Google Scholar] [CrossRef]
- Iida, T.; Wagatsuma, K.; Hirayama, D. Hiroshi Nakase Is Osteopontin a Friend or Foe of Cell Apoptosis in Inflammatory Gastrointestinal and Liver Diseases? Int. J. Mol. Sci. 2017, 19, 7. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H. The Significant Prognostic Value of Circulating Tumor Cells in Colorectal Cancer: A Systematic Review and Meta-Analysis. Curr. Probl. Cancer 2018, 42, 95–106. [Google Scholar] [CrossRef]
- Cayrefourcq, L.; Mazard, T.; Joosse, S.; Solassol, J.; Ramos, J.; Assenat, E.; Schumacher, U.; Costes, V.; Maudelonde, T.; Pantel, K.; et al. Establishment and Characterization of a Cell Line from Human Circulating Colon Cancer Cells. Cancer Res. 2015, 75, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; von Au, A.; Schnölzer, M.; Hackert, T.; Zöller, M. CD44v6-Competent Tumor Exosomes Promote Motility, Invasion and Cancer-Initiating Cell Marker Expression in Pancreatic and Colorectal Cancer Cells. Oncotarget 2016, 7, 55409–55436. [Google Scholar] [CrossRef]
- Jørgensen, M.; Bæk, R.; Pedersen, S.; Søndergaard, E.K.L.; Kristensen, S.R.; Varming, K. Extracellular Vesicle (EV) Array: Microarray Capturing of Exosomes and Other Extracellular Vesicles for Multiplexed Phenotyping. J. Extracell. Vesicles 2013, 2, 20920. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Wen, Y.; Jeong, S.; Xia, Q.; Kong, X. Role of Osteopontin in Liver Diseases. Int. J. Biol. Sci. 2016, 12, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pan, C.; Hu, H.; Zheng, S.; Ding, L. Osteopontin-Enhanced Hepatic Metastasis of Colorectal Cancer Cells. PLoS ONE 2012, 7, e47901. [Google Scholar] [CrossRef]
- Wang, X.-B.; Qi, Q.-R.; Wu, K.-L.; Xie, Q.-Z. Role of Osteopontin in Decidualization and Pregnancy Success. Reproduction 2018, 155, 423–432. [Google Scholar] [CrossRef]
- Jia, R.; Liang, Y.; Chen, R.; Liu, G.; Wang, H.; Tang, M.; Zhou, X.; Wang, H.; Yang, Y.; Wei, H.; et al. Osteopontin Facilitates Tumor Metastasis by Regulating Epithelial–mesenchymal Plasticity. Cell Death Dis. 2016, 7, e2564. [Google Scholar] [CrossRef]
- Limani, P.; Linecker, M.; Schneider, M.A.; Kron, P.; Tschuor, C.; Kachaylo, E.; Ungethuem, U.; Nicolau, C.; Lehn, J.-M.; Graf, R.; et al. The Allosteric Hemoglobin Effector ITPP Inhibits Metastatic Colon Cancer in Mice. Ann. Surg. 2017, 266, 746–753. [Google Scholar] [CrossRef][Green Version]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.M.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 315, 2576. [Google Scholar] [CrossRef]
- Wild, N.; Andres, H.; Rollinger, W.; Krause, F.; Dilba, P.; Tacke, M.; Karl, J. A Combination of Serum Markers for the Early Detection of Colorectal Cancer. Clin. Cancer Res. 2010, 16, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krause, F.; Rolny, V.; Strobl, M.; Morgenstern, D.; Datz, C.; Chen, H.; Brenner, H. Evaluation of a 5-Marker Blood Test for Colorectal Cancer Early Detection in a Colorectal Cancer Screening Setting. Clin. Cancer Res. 2016, 22, 1725–1733. [Google Scholar] [CrossRef]
- Dressen, K.; Hermann, N.; Manekeller, S.; Walgenbach-Bruenagel, G.; Schildberg, F.A.; Hettwer, K.; Uhlig, S.; Kalff, J.C.; Hartmann, G.; Holdenrieder, S. Diagnostic Performance of a Novel Multiplex Immunoassay in Colorectal Cancer. Anticancer Res. 2017, 37, 2477–2486. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liang, F.; Zhang, B.; Yan, W.; Zhang, J. The Impact of Osteopontin on Prognosis and Clinicopathology of Colorectal Cancer Patients: A Systematic Meta-Analysis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 Immune Checkpoint Controls CD8+ T Cell Activation and Tumor Immune Evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef] [PubMed]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef]
- Klement, J.D.; Poschel, D.B.; Lu, C.; Merting, A.D.; Yang, D.; Redd, P.S.; Liu, K. Osteopontin Blockade Immunotherapy Increases Cytotoxic T Lymphocyte Lytic Activity and Suppresses Colon Tumor Progression. Cancers 2021, 13, 1006. [Google Scholar] [CrossRef]
- Bandopadhyay, M.; Bulbule, A.; Butti, R.; Chakraborty, G.; Ghorpade, P.; Ghosh, P.; Gorain, M.; Kale, S.; Kumar, D.; Kumar, S.; et al. Osteopontin as a Therapeutic Target for Cancer. Expert Opin. Ther. Targets 2014, 18, 883–895. [Google Scholar] [CrossRef]
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human Osteopontin: Potential Clinical Applications in Cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef]
- Talbot, L.J.; Mi, Z.; Bhattacharya, S.D.; Kim, V.; Guo, H.; Kuo, P.C. Pharmacokinetic Characterization of an RNA Aptamer against Osteopontin and Demonstration of in Vivo Efficacy in Reversing Growth of Human Breast Cancer Cells. Surgery 2011, 150, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.I.F.; Gunasekharan, V.K.; Ravindranath, A.; O’Connell, C.; Johnston, P.G.; El-Tanani, M.K. Osteopontin as a Target for Cancer Therapy. Front. Biosci. 2008, 13, 4361–4372. [Google Scholar] [CrossRef]
- Weber, G.F. The Metastasis Gene Osteopontin: A Candidate Target for Cancer Therapy. Biochim. Biophys. Acta 2001, 1552, 61–85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amilca-Seba, K.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers 2021, 13, 3793. https://doi.org/10.3390/cancers13153793
Amilca-Seba K, Sabbah M, Larsen AK, Denis JA. Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers. 2021; 13(15):3793. https://doi.org/10.3390/cancers13153793
Chicago/Turabian StyleAmilca-Seba, Katyana, Michèle Sabbah, Annette K. Larsen, and Jérôme A. Denis. 2021. "Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications" Cancers 13, no. 15: 3793. https://doi.org/10.3390/cancers13153793
APA StyleAmilca-Seba, K., Sabbah, M., Larsen, A. K., & Denis, J. A. (2021). Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers, 13(15), 3793. https://doi.org/10.3390/cancers13153793







