Comparing the Effectiveness of a Wearable Activity Tracker in Addition to Counseling and Counseling Only to Reinforce Leisure-Time Physical Activity among Breast Cancer Patients: A Randomized Controlled Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
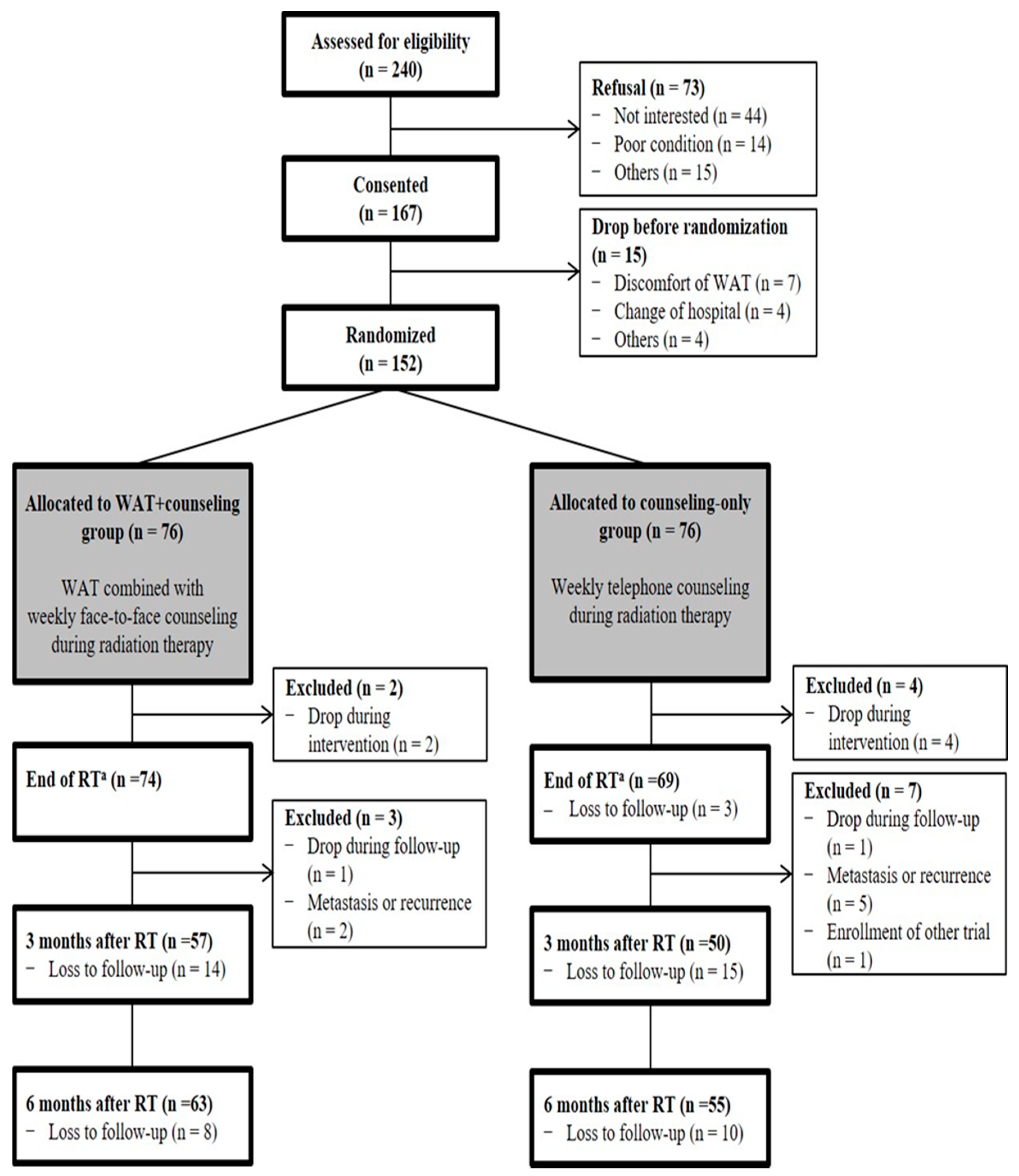
2.1. Trial Design and Participants
2.2. Intervention
2.3. Outcomes
2.4. Other Variables
2.5. Sample Size
2.6. Randomization and Blinding
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
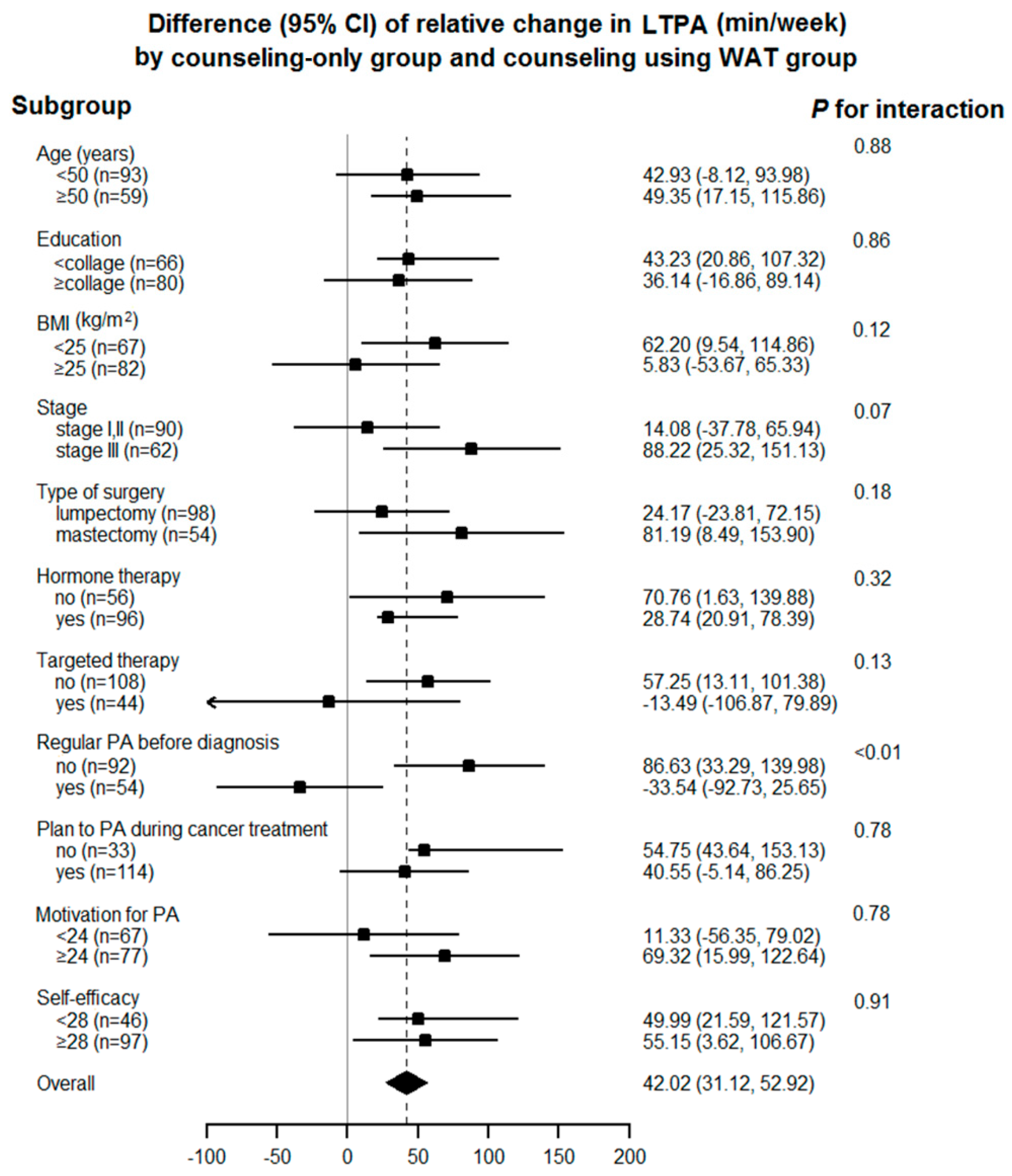
3.2. Relative Change in Self-Reported Leisure-Time Physical Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Nat. Canc. Inst. 2017, 109, 1–22. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical activity and survival after breast cancer diagnosis. Jama 2005, 293, 2479–2486. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Anton, P.M.; Hopkins-Price, P.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; Mocharnuk, R.; McAuley, E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2015, 149, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Speck, R.M.; Courneya, K.S.; Mâsse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Survivor. Res. Pract. 2010, 4, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Caballero-García, A.; Martínez, A.C.; Asensio, M.P.L.; Fernández-Lázaro, C.I. Physical activity in oncology patients with breast cancer: Non-pharmacological sports-based medical therapy? Systematic review. Arch. Med. Deporte 2020, 37, 8. [Google Scholar]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Alfano, C.M.; Smith, A.W.; Wang, C.-Y.; Neuhouser, M.L.; Duggan, C.; Bernstein, L.; Baumgartner, K.B.; Baumgartner, R.N.; Ballard-Barbash, R.; et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003, 97, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Andrykowski, M.A.; Beacham, A.O.; Jacobsen, P.B. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol. Biomarkers. Prev. 2007, 16, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Stalsberg, R.; Eikemo, T.A.; Lundgren, S.; Reidunsdatter, R.J. Physical activity in long-term breast cancer survivors—A mixed-methods approach. Breast (Edinburgh, Scotland) 2019, 46, 126–135. [Google Scholar] [CrossRef]
- Irwin, M.L.; McTiernan, A.; Bernstein, L.; Gilliland, F.D.; Baumgartner, R.; Baumgartner, K.; Ballard-Barbash, R. Physical activity levels among breast cancer survivors. Med. Sci. Sports Exer. 2004, 36, 1484–1491. [Google Scholar]
- McCabe, M.S.; Bhatia, S.; Oeffinger, K.C.; Reaman, G.H.; Tyne, C.; Wollins, D.S.; Hudson, M.M. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. J. Clin. Oncol. 2013, 31, 631–640. [Google Scholar] [CrossRef]
- Moore, S.C.; Patel, A.V.; Matthews, C.E.; Berrington de Gonzalez, A.; Park, Y.; Katki, H.A.; Linet, M.S.; Weiderpass, E.; Visvanathan, K.; Helzlsouer, K.J.; et al. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med. 2012, 9, 1001335. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sport. Exer. 2014, 46, 1744–1751. [Google Scholar] [CrossRef]
- Samuel, S.R.; Gandhi, A.R.; Kumar, K.V.; Saxena, P.P. Pedometer-based Exercise Interventions for Patients with Breast Cancer Receiving Chemotherapy-A Systematic Review. Ind. J. Palliat. Care 2020, 26, 105–109. [Google Scholar]
- Casla, S.; Lopez-Tarruella, S.; Jerez, Y.; Marquez-Rodas, I.; Galvao, D.A.; Newton, R.U.; Cubedo, R.; Calvo, I.; Sampedro, J.; Barakat, R.; et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: A randomized controlled trial. Breast Cancer Res. Treat. 2015, 153, 371–382. [Google Scholar] [CrossRef]
- Kwasnicka, D.; Dombrowski, S.U.; White, M.; Sniehotta, F. Theoretical explanations for maintenance of behaviour change: A systematic review of behaviour theories. Health Psychol. Rev. 2016, 10, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, K.J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; May, A.M.; van Overmeeren, E.J.; Simons, M.; Monninkhof, E.M. The Effect of Physical Activity Interventions Comprising Wearables and Smartphone Applications on Physical Activity: A Systematic Review and Meta-analysis. Sports Med. 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Coronel, B.D.; Coakes, C.E.; Mainous, A.G., 3rd. Is There a Benefit to Patients Using Wearable Devices Such as Fitbit or Health Apps on Mobiles? A Systematic Review. Am. J. Med. 2019, 132, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.J.; Lewis, Z.H.; Mayrsohn, B.G.; Rowland, J.L. Behavior change techniques implemented in electronic lifestyle activity monitors: A systematic content analysis. J. Med. Inter. Res. 2014, 16, e192. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Ungar, N.; Sieverding, M.; Weidner, G.; Ulrich, C.M.; Wiskemann, J. A self-regulation-based intervention to increase physical activity in cancer patients. Psychol. Health Medi. 2016, 21, 163–175. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Davis, K.K.; Rogers, R.J.; King, W.C.; Marcus, M.D.; Helsel, D.; Rickman, A.D.; Wahed, A.S.; Belle, S.H. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss: The IDEA Randomized Clinical Trial. Jama 2016, 316, 1161–1171. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Lansing, J.E.; DeShaw, K.J.; Peyer, K.L.; Bai, Y.; Perez, M.; Phillips, L.A.; Welk, G.J. Evaluating Motivational Interviewing and Habit Formation to Enhance the Effect of Activity Trackers on Healthy Adults’ Activity Levels: Randomized Intervention. JMIR Mhealth Uhealth 2019, 7, e10988. [Google Scholar] [CrossRef]
- Sallis, R.; Franklin, B.; Joy, L.; Ross, R.; Sabgir, D.; Stone, J. Strategies for promoting physical activity in clinical practice. Prog. Cardiovasc. Dis. 2015, 57, 375–386. [Google Scholar] [CrossRef]
- Sullivan, A.N.; Lachman, M.E. Behavior Change with Fitness Technology in Sedentary Adults: A Review of the Evidence for Increasing Physical Activity. Front. Public Health 2016, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.M.; Nguyen, N.H.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Vallance, J.K.; Milton, S.; Friedenreich, C.M.; English, D.R. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer 2019, 125, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Geraerts, I.; Demeyer, H.; Van der Gucht, E.; Dams, L.; de Kinkelder, C.; Dukers-van Althuis, S.; Van Kampen, M.; Devoogdt, N. Physical activity levels after treatment for breast cancer: Two-year follow-up. Breast (Edinburgh, Scotland) 2018, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-J.; Liu, C.-W. Understanding Older Adult’s Technology Adoption and Withdrawal for Elderly Care and Education: Mixed Method Analysis from National Survey. J. Med. Inter. Res. 2017, 19, 374. [Google Scholar] [CrossRef] [PubMed]
- Sushames, A.; Edwards, A.; Thompson, F.; McDermott, R.; Gebel, K. Validity and Reliability of Fitbit Flex for Step Count, Moderate to Vigorous Physical Activity and Activity Energy Expenditure. PLoS ONE 2016, 11, e0161224. [Google Scholar] [CrossRef]
- Mohr, D.C.; Ho, J.; Duffecy, J.; Reifler, D.; Sokol, L.; Burns, M.N.; Jin, L.; Siddique, J. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: A randomized trial. Jama 2012, 307, 2278–2285. [Google Scholar] [CrossRef]
- Matthews, C.E.; Wilcox, S.; Hanby, C.L.; Der Ananian, C.; Heiney, S.P.; Gebretsadik, T.; Shintani, A. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support. Care Cancer. 2007, 15, 203–211. [Google Scholar] [CrossRef]
- Donovan, R.J.; Jones, S.; Holman, C.D.; Corti, B. Assessing the reliability of a stage of change scale. Health Educ. Res. 1998, 13, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Gell, N.M.; Grover, K.W.; Humble, M.; Sexton, M.; Dittus, K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support. Care Cancer. 2017, 25, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Kathryn, J.D.; Laura, E.; Yang, B.; Jeni, L.; Maria, P.; Greg, W. Methods for Activity Monitor Validation Studies: An Example With the Fitbit Charge. J. Measur. Physical Behaviour 2018, 1, 130–135. [Google Scholar]
- Marshall, S.J.; Levy, S.S.; Tudor-Locke, C.E.; Kolkhorst, F.W.; Wooten, K.M.; Ji, M.; Macera, C.A.; Ainsworth, B.E. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am. J. Prevent. Med. 2009, 36, 410–415. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sport. Exer. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. Jama 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C. Steps to Better Cardiovascular Health: How Many Steps Does It Take to Achieve Good Health and How Confident Are We in This Number? Curr. Cardiovasc. Risk Rep. 2010, 4, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Internat. J. Behavior. Nutr. Physic. Activ. 2011, 8, 80. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sport. Exer. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, C.; Min, J.; Kang, D.W.; Kim, J.Y.; Yang, H.I.; Park, J.; Lee, M.K.; Lee, M.Y.; Park, I.; et al. Development of the Korean Global Physical Activity Questionnaire: Reliability and validity study. Glob. Health Promot. 2019, 27, 44–55. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Pub. Health 2014, 14, 1255. [Google Scholar] [CrossRef]
- Brooke, S.M.; An, H.S.; Kang, S.K.; Noble, J.M.; Berg, K.E.; Lee, J.M. Concurrent Validity of Wearable Activity Trackers Under Free-Living Conditions. J. Strength Cond. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Hill, K.D.; Lautenschlager, N.T.; Thøgersen-Ntoumani, C.; Lewin, G.; Boyle, E.; Howie, E. Reliability and validity of two fitness tracker devices in the laboratory and home environment for older community-dwelling people. BMC Geriatrics 2018, 18, 103. [Google Scholar] [CrossRef]
- Dishman, R.K.; Ickes, W. Self-motivation and adherence to therapeutic exercise. J. Behavior. Med. 1981, 4, 421–438. [Google Scholar] [CrossRef]
- Nieto-Riveiro, L.; Groba, B.; Miranda, M.C.; Concheiro, P.; Pazos, A.; Pousada, T.; Pereira, J. Technologies for participatory medicine and health promotion in the elderly population. Medicine 2018, 97, e10791. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Banasik, J.L. Exercise self-efficacy. Clin. Excell. Nurse Pract. 2001, 5, 134–143. [Google Scholar] [CrossRef]
- Boyle, T.; Vallance, J.K.; Ransom, E.K.; Lynch, B.M. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support. Care Cancer 2016, 24, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Cadmus-Bertram, L.A.; Marcus, B.H.; Patterson, R.E.; Parker, B.A.; Morey, B.L. Randomized Trial of a Fitbit-Based Physical Activity Intervention for Women. Am. J. Prev. Med. 2015, 49, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef]
- Erdmier, C.; Hatcher, J.; Lee, M. Wearable device implications in the healthcare industry. J. Med. Eng. Technol. 2016, 40, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.D.; Lawler, S.P.; Brakenridge, C.L.; Reeves, M.M.; Eakin, E.G. Telephone, print, and Web-based interventions for physical activity, diet, and weight control among cancer survivors: A systematic review. J. Cancer Surviv. 2015, 9, 660–682. [Google Scholar] [CrossRef]
- Grimmett, C.; Corbett, T.; Brunet, J.; Shepherd, J.; Pinto, B.M.; May, C.R.; Foster, C. Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 37. [Google Scholar] [CrossRef]
- Pinto, B.M.; Ciccolo, J.T. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011, 186, 367–387. [Google Scholar]
- Moshki, M.; Dehnoalian, A.; Alami, A. Effect of Precede-Proceed Model on Preventive Behaviors for Type 2 Diabetes Mellitus in High-Risk Individuals. Clin. Nurs. Res. 2017, 26, 241–253. [Google Scholar] [CrossRef]
- Greaney, M.L.; Riebe, D.; Ewing Garber, C.; Rossi, J.S.; Lees, F.D.; Burbank, P.A.; Nigg, C.R.; Ferrone, C.L.; Clark, P.G. Long-Term Effects of a Stage-Based Intervention for Changing Exercise Intentions and Behavior in Older Adults. The Gerontologist 2008, 48, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Centis, E.; Trento, M.; Dei Cas, A.; Pontiroli, A.E.; De Feo, P.; Bruno, A.; Sasdelli, A.S.; Arturi, F.; Strollo, F.; Vigili De’ Kreutzenberg, S.; et al. Stage of change and motivation to healthy diet and habitual physical activity in type 2 diabetes. Acta Diabetologica 2014, 51, 559–566. [Google Scholar] [CrossRef]
- van der Bij, A.K.; Laurant, M.G.; Wensing, M. Effectiveness of physical activity interventions for older adults: A review. Am. J. Prev. Med. 2002, 22, 120–133. [Google Scholar] [CrossRef]
- Huy, C.; Schmidt, M.E.; Vrieling, A.; Chang-Claude, J.; Steindorf, K. Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. Eur. J. Cancer 2012, 48, 297–304. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Fogleman, A.; Trammell, R.; Hopkins-Price, P.; Vicari, S.; Rao, K.; Edson, B.; Verhulst, S.; Courneya, K.S.; Hoelzer, K. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: Pilot randomized trial. Integrat. Cancer Ther. 2013, 12, 323–335. [Google Scholar] [CrossRef]
- Marcus, B.H.; Dubbert, P.M.; Forsyth, L.H.; McKenzie, T.L.; Stone, E.J.; Dunn, A.L.; Blair, S.N. Physical activity behavior change: Issues in adoption and maintenance. Health Psychol. 2000, 19, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, N.; Buys, R.; Fourneau, I.; Dewit, T.; Cornelissen, V. Exploring physical activity behaviour - needs for and interest in a technology-delivered, home-based exercise programme among patients with intermittent claudication. Vasa 2018, 47, 109–117. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Maxwell-Smith, C.; Kamarova, S.; Lamb, S.; Millar, L.; Cohen, P.A. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Supp. Care Cancer 2018, 26, 1289–1295. [Google Scholar] [CrossRef]
- Parschau, L.; Fleig, L.; Koring, M.; Lange, D.; Knoll, N.; Schwarzer, R.; Lippke, S. Positive experience, self-efficacy, and action control predict physical activity changes: A moderated mediation analysis. Brit. J. Health Psychol. 2013, 18, 395–406. [Google Scholar] [CrossRef]
- Bravata, D.M.; Smith-Spangler, C.; Sundaram, V.; Gienger, A.L.; Lin, N.; Lewis, R.; Stave, C.D.; Olkin, I.; Sirard, J.R. Using pedometers to increase physical activity and improve health: A systematic review. Jama 2007, 298, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J.J. Goal-setting protocol in adherence to exercise by Italian adults. Percept. Motor Skills 2002, 94, 453–458. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, S.; Spruijt-Metz, D.; Farias, A.J. Associations Among Wearable Activity Tracker Use, Exercise Motivation, and Physical Activity in a Cohort of Cancer Survivors: Secondary Data Analysis of the Health Information National Trends Survey. JMIR Cancer 2021, 7, e24828. [Google Scholar] [CrossRef] [PubMed]

| Counseling-Only Group (N = 76) | WAT+Counseling Group (N = 76) | p-Value | |
|---|---|---|---|
| Age (years) | 46.8 ± 7.6 | 47.3 ± 8.5 | 0.67 |
| Marital status (married) | 63 (82.9) | 59 (77.6) | 0.42 |
| Educational level (≥college) | 44 (57.9) | 39 (51.3) | 0.42 |
| Employment status before diagnosis (employed) a | 46 (60.5) | 47 (61.8) | 0.87 |
| Drinker for the past 1 year (yes) | 38 (50.0) | 30 (39.5) | 0.19 |
| Smoker for the past 1 year (yes) | 6 (7.9) | 5 (6.6) | 0.75 |
| Body mass index (kg/m2) | 24.0 ± 3.1 | 23.8 ± 3.4 | 0.75 |
| Menopausal status at diagnosis (premenopause) | 53 (69.7) | 46 (60.5) | 0.23 |
| Comorbidity (yes) b | 32 (42.1) | 25 (32.9) | 0.24 |
| Stage | 0.61 | ||
| I | 13 (17.1) | 15 (19.7) | |
| II | 34 (44.7) | 28 (36.8) | |
| III | 29 (38.1) | 33 (43.4) | |
| Type of surgery (Lumpectomy) | 50 (65.8) | 48 (63.2) | 0.73 |
| Reconstruction surgery (yes) | 6 (7.9) | 6 (7.9) | 0.99 |
| Chemotherapy (neoadjuvant) | 55 (72.4) | 46 (60.5) | 0.12 |
| Dose of radiation therapy (cGy) | 0.06 | ||
| 5000–6000 | 43 (56.6) | 54 (71.1) | |
| 6000–6500 | 33 (43.4) | 22 (29.0) | |
| Hormone therapy (yes, %) | 49 (64.5) | 47 (61.8) | 0.74 |
| Regular PA before diagnosis (yes, %) | 29 (38.2) | 25 (32.9) | 0.79 |
| Plan to perform PA during cancer treatment (yes, %) | 55 (72.4) | 59 (77.6) | 0.58 |
| Motivation for physical activity c | 22.8 ± 4.3 | 22.0 ± 4.0 | 0.29 |
| Self-efficacy d | 28.0 ± 3.1 | 28.0 ± 4.8 | 0.99 |
| Counseling-Only Group (N = 76) | WAT+Counseling Group (N = 76) | p-Value | |
|---|---|---|---|
| Self-reported LTPA (minute/week) | |||
| Before RT | 105.7 ± 166.7 | 119.7 ± 205.4 | 0.64 |
| Immediately after RT a | 181.7 ± 191.0 | 226.4 ± 238.3 | 0.22 |
| 3 months after RT | 202.0 ± 299.1 | 227.3 ± 241.8 | 0.63 |
| 6 months after RT | 194.0 ± 240.9 | 220.0 ± 254.2 | 0.57 |
| P for trend | 0.04 | 0.02 | |
| Relative changes in self-reported LTPA b | |||
| Before RT | Reference | Reference | |
| Immediately after RT a | 57.8 (32.1, 83.5) | 102.8 (72.3, 133.4) | 0.03 |
| 3 months after RT | 86.6 (7.14, 166.0) | 99.0 (50.7, 147,4) | 0.78 |
| 6 months after RT | 56.6 (20.8, 92.4) | 94.5 (56.0, 133.2) | 0.15 |
| Total | Regular PA before Diagnosis | p-Value | ||
|---|---|---|---|---|
| No | Yes | |||
| Before RT (n = 68) | 9351.7 ± 4273.2 | 8336.6 ± 3326.1 | 11,012.4 ± 5025.3 | 0.01 |
| During RT (n = 75) | 11,592.2 ± 3289.8 | 10,858.5 ± 2836.0 | 13,136.0 ± 3692.7 | <0.01 |
| Immediately after RT (n = 74) | 12,240.1 ± 3757.9 | 11,930.9 ± 502.6 | 12,766.3 ± 917.0 | 0.39 |
| p for trend | <0.01 | <0.01 | 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, S.; Lee, J.K.; Kang, D.; Kim, N.; Shim, Y.M.; Park, W.; Choi, D.; Cho, J. Comparing the Effectiveness of a Wearable Activity Tracker in Addition to Counseling and Counseling Only to Reinforce Leisure-Time Physical Activity among Breast Cancer Patients: A Randomized Controlled Trial. Cancers 2021, 13, 2692. https://doi.org/10.3390/cancers13112692
Kong S, Lee JK, Kang D, Kim N, Shim YM, Park W, Choi D, Cho J. Comparing the Effectiveness of a Wearable Activity Tracker in Addition to Counseling and Counseling Only to Reinforce Leisure-Time Physical Activity among Breast Cancer Patients: A Randomized Controlled Trial. Cancers. 2021; 13(11):2692. https://doi.org/10.3390/cancers13112692
Chicago/Turabian StyleKong, Sunga, Jae Kyung Lee, Danbee Kang, Nayeon Kim, Young Mog Shim, Won Park, Dooho Choi, and Juhee Cho. 2021. "Comparing the Effectiveness of a Wearable Activity Tracker in Addition to Counseling and Counseling Only to Reinforce Leisure-Time Physical Activity among Breast Cancer Patients: A Randomized Controlled Trial" Cancers 13, no. 11: 2692. https://doi.org/10.3390/cancers13112692
APA StyleKong, S., Lee, J. K., Kang, D., Kim, N., Shim, Y. M., Park, W., Choi, D., & Cho, J. (2021). Comparing the Effectiveness of a Wearable Activity Tracker in Addition to Counseling and Counseling Only to Reinforce Leisure-Time Physical Activity among Breast Cancer Patients: A Randomized Controlled Trial. Cancers, 13(11), 2692. https://doi.org/10.3390/cancers13112692






